Abstract
Importance
If not promptly recognized, endocrine dysfunction can be life threatening. The incidence and risk of developing such adverse events (AEs) following the use of immune checkpoint inhibitor (ICI) regimens are unknown.
Objective
To compare the incidence and risk of endocrine AEs following treatment with US Food and Drug Administration–approved ICI regimens.
Data Sources
A PubMed search through July 18, 2016, using the following keywords was performed: “ipilimumab,” “MDX-010,” “nivolumab,” “BMS-963558,” “pembrolizumab,” “MK-3475,” “atezolizumab,” “MPDL3280A,” and “phase.”
Study Selection
Thirty-eight randomized clinical trials evaluating the usage of these ICIs for treatment of advanced solid tumors were identified, resulting in a total of 7551 patients who were eligible for a meta-analysis. Regimens were categorized by class into monotherapy with a PD-1 (programmed cell death protein 1) inhibitor, a CTLA-4 (cytotoxic T-lymphocyte-associated protein-4) inhibitor, or a PD-L1 (programmed cell death 1 ligand 1) inhibitor, and combination therapy with PD-1 plus CTLA-4 inhibitors.
Data Extraction and Synthesis
The data were extracted by 1 primary reviewer (R.B.-S.) and then independently reviewed by 2 secondary reviewers (W.T.B. and A.C.G.-C.) following Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. Inferences on the incidence of AEs were made using log-odds random effects models.
Main Outcomes and Measures
Incidence of all-grade hypothyroidism, hyperthyroidism, hypophysitis, primary adrenal insufficiency, and insulin-deficient diabetes.
Results
Overall, 38 randomized clinical trials comprising 7551 patients were included in this systematic review and meta-analysis. The incidence of both hypothyroidism and hyperthyroidism was highest in patients receiving combination therapy. Patients on the combination regimen were significantly more likely to experience hypothyroidism (odds ratio [OR], 3.81; 95% CI, 2.10-6.91, P < .001) and hyperthyroidism (OR, 4.27; 95% CI, 2.05-8.90; P = .001) than patients on ipilimumab. Compared with patients on ipilimumab, those on PD-1 inhibitors had a higher risk of developing hypothyroidism (OR, 1.89; 95% CI, 1.17-3.05; P = .03). The risk of hyperthyroidism, but not hypothyroidism, was significantly greater with PD-1 than with PD-L1 inhibitors (OR, 5.36; 95% CI, 2.04-14.08; P = .002). While patients who received PD-1 inhibitors were significantly less likely to experience hypophysitis than those receiving ipilimumab (OR, 0.29; 95% CI, 0.18-0.49; P < .001), those who received combination therapy were significantly more likely to develop it (OR, 2.2; 95% CI, 1.39-3.60; P = .001). For primary adrenal insufficiency and insulin-deficient diabetes no statistical inferences were made due to the smaller number of events.
Conclusions and Relevance
Our study provides more precise data on the incidence of endocrine dysfunctions among patients receiving ICI regimens. Patients on combination therapy are at increased risk of thyroid dysfunction and hypophysitis.
This systematic review and meta-analysis compares the incidence and risk of endocrine adverse events following treatment with US Food and Drug Administration-approved immune checkpoint inhibitor regimens.
Key Points
Question
What is the incidence and what are the risks of endocrine dysfunction related to different immune checkpoint inhibitor regimens in patients with advanced solid tumors?
Findings
In this meta-analysis of 38 clinical trials including 7551 patients, the incidence of endocrine dysfunction was significantly higher in those treated with combination therapy compared with ipilimumab. Regarding monotherapy, the incidence of thyroid dysfunction and hypophysitis was highest with programmed cell death protein 1 inhibitors and with ipilimumab, respectively.
Meaning
Our study provides more precise data on the incidence of endocrine dysfunctions among patients receiving immune checkpoint inhibitor regimens; such events were higher in patients treated with combination therapy.
Introduction
The recognition that overexpression of immune checkpoint molecules in the tumor microenvironment plays a crucial role in antitumor immunity evasion has revolutionized cancer therapy. Currently, 6 immune checkpoint inhibitors (ICI) have been approved for the treatment of different advanced solid tumors: the CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor ipilimumab; 2 PD-1 (programmed cell death protein 1) inhibitors, nivolumab and pembrolizumab; and 3 PD-L1 (programmed cell death 1 ligand 1) inhibitors, atezolizumab, avelumab, and durvalumab. Additionally, combination therapy of ipilimumab plus nivolumab has been approved for treatment of advanced melanoma. Given the still-growing list of cancer types in which ICI have demonstrated clinical activity, their use is expected to increase in the coming years.
Immune checkpoint molecules have an important function in regulating immune response: after binding to their ligands, these proteins can initiate either inhibitory or stimulatory pathways that modulate T-cell function. Both CTLA-4 and PD-1 play a key role in the maintenance of immunological tolerance to self-antigens, preventing autoimmune disorders. Therefore, at the same time that ICI are able to unleash T cells to fight cancer, they also can trigger autoimmunelike manifestations in different organ systems, generally referred to as immune-related adverse events (irAEs). Endocrine dysfunctions are among the most common irAEs that have been reported in clinical trials with ICI, including hypothyroidism, hyperthyroidism, hypophysitis, primary adrenal insufficiency (PAI), and insulin-deficient diabetes (IDD). However, there has been no report of a systematic review or meta-analysis of the incidence of these dysfunctions across different US Federal Drug Administration (FDA)-approved ICI regimens, or across different tumor subtypes.
Given the increasing use of these agents in clinical practice, and the life-threatening nature of endocrine dysfunction if not promptly recognized, we performed a systematic review and meta-analysis of clinical trials with FDA-approved ICI regimens in patients with advanced solid tumors and compared the incidence and risks of endocrine irAEs associated with each regimen.
Methods
Search Methods and Study Selection
We conducted a systematic search of the literature to identify clinical trials of ICI that reported endocrine adverse events (AEs). Original articles that have published results of prospective clinical trials of these ICI regimens for patients with advanced solid tumors, including monotherapy and combination therapy trials, were identified by a PubMed search, and by examining the references of published trials, review articles, editorials, and other relevant articles. The latest manufacturer package inserts were also retrieved. For the PubMed search, the following keywords or corresponding Medical Subject Heading terms were used: “ipilimumab,” “MDX-010,” “nivolumab,” “BMS-963558,” “pembrolizumab,” “MK-3475,” “atezolizumab,” “MPDL3280A,” and “phase.” Articles published online ahead of print were included. The database was searched for articles published on or before July 18, 2016. The search focused on the trials of these 4 agents because at that time, those were the only drugs approved by the FDA.
Our selection criteria included all prospective clinical trials (and/or cohorts) that: (1) investigated the usage of the previously mentioned ICI for treatment of advanced solid tumors; (2) clearly reported an endocrine AE in their safety data, with or without clinical severity grading; and (3) were published in the English language. We excluded trials that: (1) involved combination regimens with other therapies and/or modalities other than ICI (eg, targeted therapy, chemotherapy, radiation therapy, other immunotherapy); (2) did not list endocrine AEs in any arm and/or cohort; and (3) were presented only as meeting abstracts without published full-text original articles. One exception was made for atezolizumab. Given that this agent was recently approved, and there are few clinical trials available, we used unpublished data on phase 1 clinical trial (NCT01375842) that was provided to us by the study sponsor (Genentech Inc). In the event of duplicates, ambiguity, or publications reporting on the same study population, only the most recent, relevant, and/or comprehensive publication was included in the analysis. Any discrepancy in study selection was resolved by consensus.
Data Extraction
The total number of patients treated with ICI, and the number of patients with endocrine dysfunction (hypothyroidism, hyperthyroidism, hypophysitis, PAI, and IDD) for all grades and for grade 3 and higher were collected from the eligible articles. Toxic effects listed as thyroiditis were excluded for 2 reasons: (1) most trials did not report this event and (2) the possible overlapping between its occurrence and both hypothyroidism and hyperthyroidism. The trial phases, tumor types, types of specific agents (ipilimumab, nivolumab, pembrolizumab, or atezolizumab), doses, and frequency of drug administration were recorded. The treatment regimen was classified as CTLA-4 inhibitor, PD-1 inhibitor, or PD-L1 inhibitor when these agents were administered as monotherapy, or as combination therapy when was ipilimumab plus nivolumab was given in a concurrent regimen. The data extraction was performed by 1 primary reviewer (R.B.-S.) and was then independently reviewed by 2 secondary reviewers (W.T.B. and A.C.G.-C.) following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Statistical Analysis
For each clinical trial, the number of patients treated and the number of patients with AEs reported was recorded for each treatment arm and dose level. The observed incidence of each toxic effect is reported by arm with 95% exact confidence intervals. Meta-analysis was performed using random effects models weighted by the number of patients treated. All models were fit using log-odds transformation and restricted maximum likelihood estimation using an offset of 0.5 for all 0 cells. Heterogeneity was evaluated using the Cochran Q statistic and I2 statistics for its proportion of the total variability. Predictors of AEs were explored in multivariable models, using likelihood ratio tests under maximum-likelihood and a 2-sided P value cutoff of .05 was considered statistically significant. Step-down tests contrasting the incidence between monotherapy regimen applied a Bonferroni correction for the 3 pairwise relationships. Sensitivity analyses considered offsets of 0.5 in all and in no cells, fixed-effects models, and models using raw proportions. Results from sensitivity analyses are consistent unless otherwise noted. All analyses were performed using R 3.1.1 (R Project) and the metafor package.
Results
Eligible Studies and Characteristics
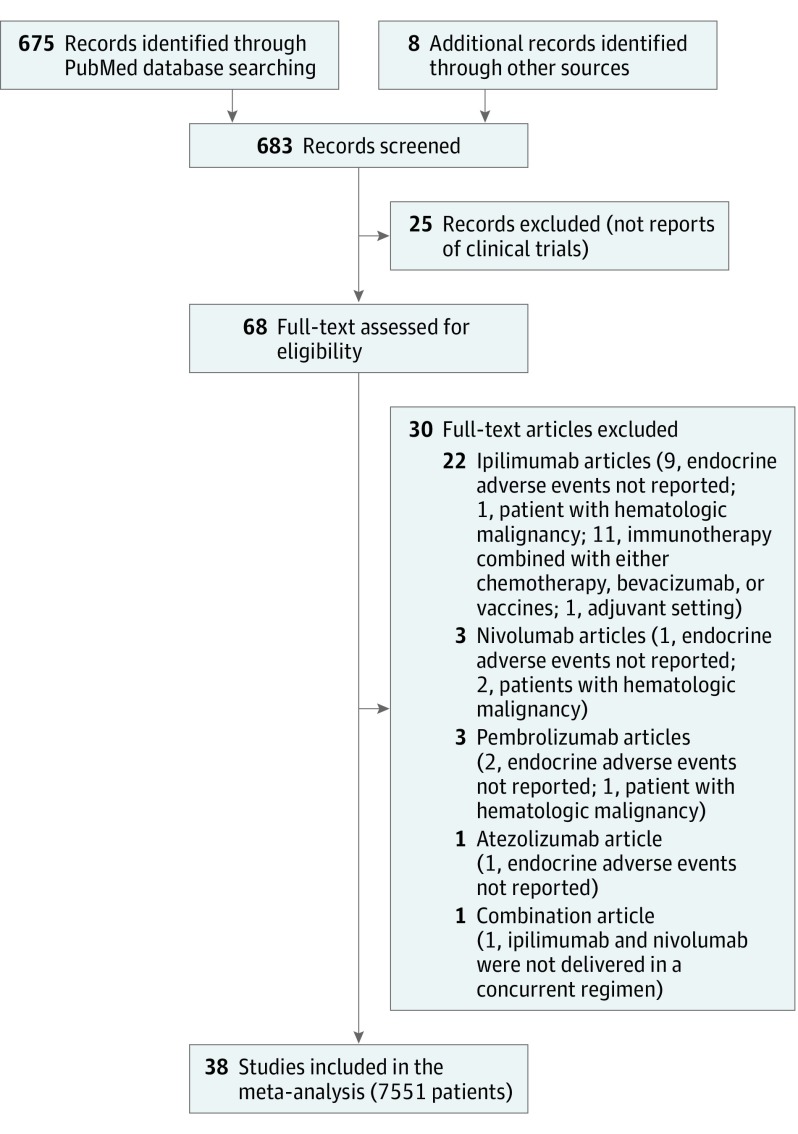
The PubMed search and the review of reference lists identified a total of 683 records (Figure 1). After screening and eligibility assessment, we identified a total of 38 clinical trials that are represented in the data set (eTable 1 in the Supplement). This includes 8 phase 3 studies; 1, phase 2/3; 14, phase 2; 2, phase 1/2; and 13, phase 1. Within these studies, the following cohorts were excluded from analysis: 7 cohorts treated with chemotherapy alone, 1 cohort that included everolimus, and 3 cohorts with included vaccines. This left a total of 71 cohorts of patients to evaluate the incidence of endocrine AEs with ICI. The number of patients per cohort with safety data ranged from 3 to 558 (median, 54 patients), with a total of 7551 patients with AE data among the 7657 total enrolled across studies (98.6%). The most common disease types were melanoma (25 cohorts; n = 3346 patients), non–small-cell lung cancer (10 cohorts; n = 1906 patients), and renal cell carcinoma (6 cohorts; n = 664 patients).
Figure 1. Flow Diagram of Study Selection.
We categorized the regimens by class as monotherapy with a PD-1 inhibitor (48 cohorts; n = 4953 patients), a CTLA-4 inhibitor (12 cohorts; n = 1013 patients), a PD-L1 inhibitor (3 cohorts; n = 1010 patients), and combination therapy with PD-1 (nivolumab) plus CTLA-4 inhibitor (ipilimumab) (8 cohorts; n = 575 patients). Specific PD-1 inhibitors include nivolumab (26 cohorts; n = 2494 patients) and pembrolizumab (22 cohorts; n = 2459 patients). All cohorts of CTLA-4 inhibitor and PD-L1 inhibitor included, respectively, ipilimumab and atezolizumab.
Dose information was abstracted for each cohort; for ipilimumab, pembrolizumab, and nivolumab, we used a threshold of 10 mg/kg to indicate high vs low dose. Only 1 cohort received high-dose ipilimumab, and only 3 cohorts received high-dose nivolumab, preventing us from making cross-study comparisons. With pembrolizumab, 13 of 22 cohorts received a high dose (n = 1804 patients); and 9 cohorts received a low dose (n = 655 patients); a dose effect was explored with this agent (eTable 1 in the Supplement).
Incidence of Hypothyroidism
Across all study arms, 472 cases of any-grade hypothyroidism were observed among the 7551 patients enrolled in 38 studies (eTable 1 in the Supplement). One study did not report events by grade, but across other studies only 9 cases of grade 3 or higher hypothyroidism were reported (0.12% of patients).
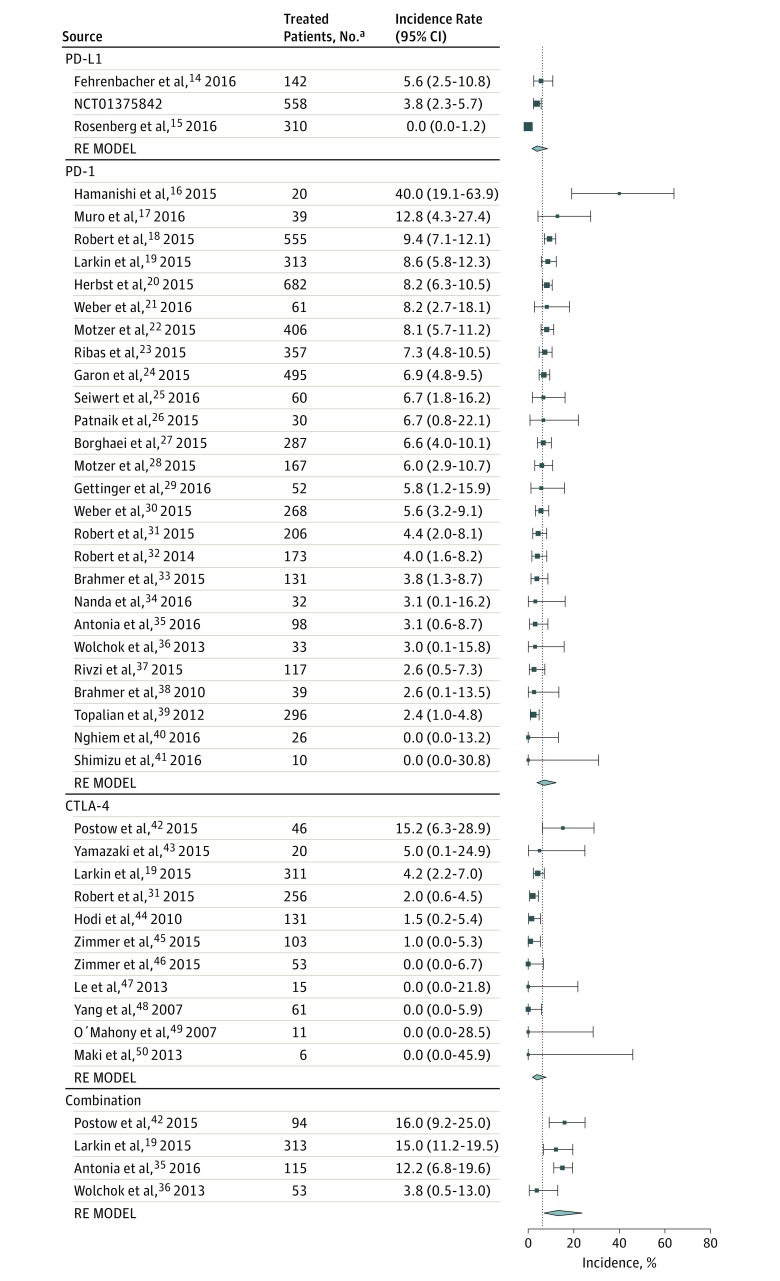
Using the mixed-effects model, the overall incidence of hypothyroidism was estimated to be 6.6% (95% CI, 5.5%-7.8%), and a statistically significant difference was observed among the classes of ICI regimens (P < .001) (Figure 2). The predicted incidence of hypothyroidism ranged from 3.8% (95% CI, 1.9%-7.8%) with ipilimumab to 13.2% (95% CI, 6.9%-23.8%) with combination therapy.
Figure 2. Incidence of All-Grade Hypothyroidism During Treatment With Different Immune Checkpoint Inhibitor Regimens.
Incidence rates are represented by boxes and whiskers indicate binomial exact 95% CIs. Model results are shown for each class of immunotherapy as a polygon using the fitted value and 95% prediction interval. For the phase 1 clinical trial NCT01375842 the study sponsor (Genentech Inc) provided data (unpublished). Combination indicates nivolumab plus ipilimumab; CTLA-4, cytotoxic T-lymphocyte associated protein 4; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; and RE MODEL, random effects model.
aIncludes the number of patients eligible for safety analysis.
Both patients who received PD-1 inhibitors (odds ratio [OR], 1.89; 95% CI, 1.17-3.05; adjusted P = .03), as well as those treated with the combination regimen (OR, 3.81; 95% CI, 2.10-6.91; unadjusted P < .001) were significantly more likely to experience any grade hypothyroidism than those treated with ipilimumab monotherapy (eTable 2 in the Supplement). The difference in risk of hypothyroidism between PD-1 and PD-L1 inhibitors did not reach statistical significance (OR, 0.53; 95% CI, 0.29-0.96; adjusted P = .11). The odds of experiencing hypothyroidism were not significantly different between patients treated with the PD-L1 inhibitor and ipilimumab. The test of residual heterogeneity among studies was highly significant (Q = 98, P = .01, I2 = 33.9%) (eFigure 1 in the Supplement); however, no other study-level factors were identified to be associated with hypothyroidism.
In addition, no substantial difference in rates of hypothyroidism was observed among PD-1 inhibitors (nivolumab, 6.5% vs pembrolizumab, 7.9%) or in dose of pembrolizumab (7.6% with <10 mg/kg vs 8.2% with 10 mg/kg). Furthermore, the tumor type was not significantly associated with the incidence of hypothyroidism with PD-1 inhibitors. Moreover, when we restricted the analysis to the subset of trials that included only patients with melanoma, the differences between combination therapy and monotherapy with CTLA-4 or PD-1 inhibitors were of similar magnitude and remained statistically significant.
Incidence of Hyperthyroidism
The incidence of hyperthyroidism for all grades and for grade 3 and higher in 37 studies is described in eTable 1 in the Supplement. One study did not report events; across all other study cohorts, 194 cases of any-grade hyperthyroidism were observed among the 7531 patients. Only 7 cases of grade 3 or higher hyperthyroidism (0.10%) were reported.
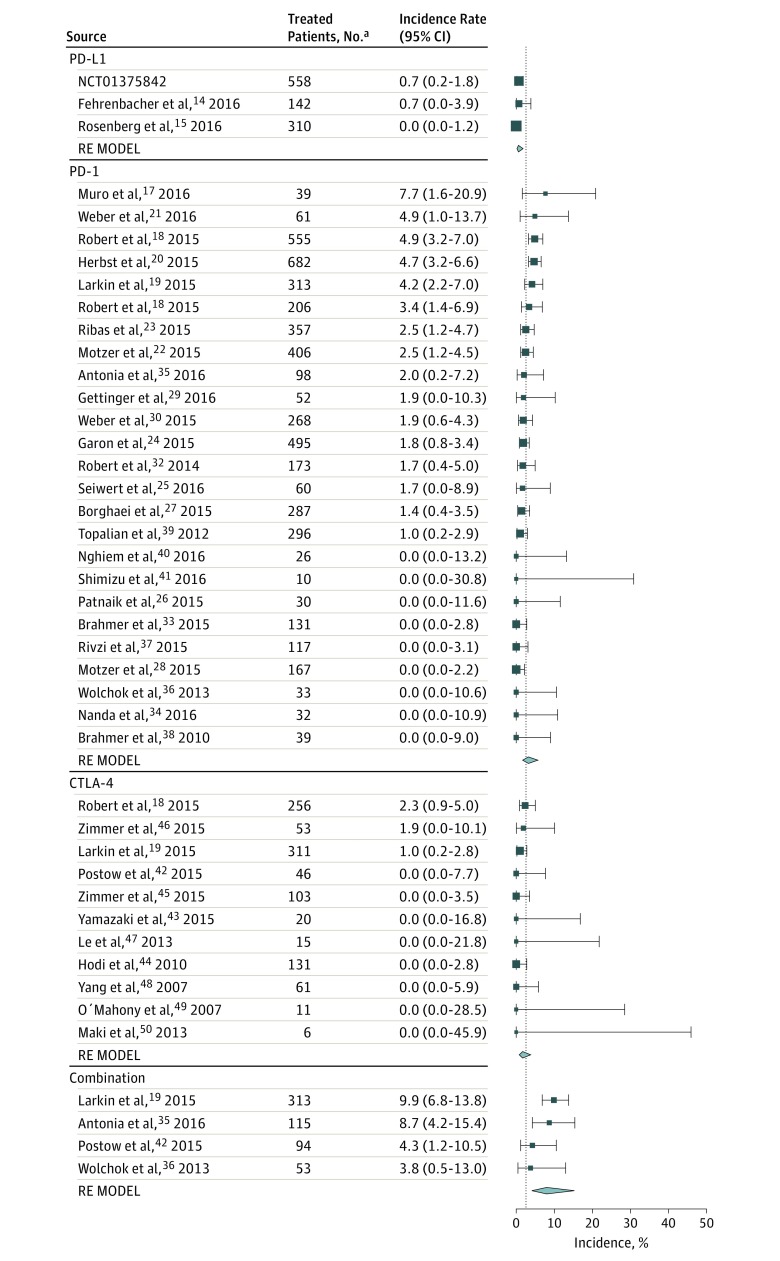
Based on the mixed-effects model, the overall incidence of hyperthyroidism was estimated to be 2.9% (95% CI, 2.4%-3.7%), and a statistically significant difference was observed among the classes of immunotherapy (P < .001) (Figure 3). The predicted incidence in hyperthyroidism ranged from 0.6% (95% CI, 0.2%-1.8%) with the PD-L1 inhibitor to 8.0% (95% CI, 4.1%-15.3%) with combination therapy. Patients treated with the combination regimen were significantly more likely to experience any grade hyperthyroidism than those treated with ipilimumab monotherapy (OR, 4.27; 95% CI, 2.05-8.90; P = .001) (eTable 2 in the Supplement); whereas increased incidence in patients who received PD-1 inhibitors failed to reach statistical significance after correcting for multiple comparisons (OR, 1.89; 95% CI, 1.02-3.52; adjusted P = .13). The risk of hyperthyroidism was significantly greater with PD-1 inhibitors than with PD-L1 inhibitors (OR, 5.36; 95% CI, 2.04-14.08; adjusted P = .002). The odds of experiencing hyperthyroidism were not significantly different between patients treated with PD-L1 inhibitors and ipilimumab. The test of residual heterogeneity among studies was not significant (Q = 61, P = .62, I2 = 18.3%) (eFigure 1 in the Supplement).
Figure 3. Incidence of All-Grade Hyperthyroidism During Treatment With Different Immune Checkpoint Inhibitor Regimens.
Incidence rates are represented by boxes and whiskers indicate binomial exact 95% CIs. Model results are shown for each class of immunotherapy as a polygon using the fitted value and 95% prediction interval. For the phase 1 clinical trial NCT01375842 the study sponsor (Genentech Inc) provided data (unpublished). Combination indicates nivolumab plus ipilimumab; CTLA-4, cytotoxic T-lymphocyte associated protein 4; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; and RE MODEL, random effects model.
aIncludes the number of patients eligible for safety analysis.
Regarding the PD-1 inhibitors, there was a significant difference in rates of hyperthyroidism observed between nivolumab and pembrolizumab (respectively, 2.5% [95% CI, 1.3%-4.6%] vs 3.8% [95% CI, 2.1%-6.9%], P = .04), but no significant difference between low- and high-dose pembrolizumab that could account for the difference between the 2 agents was noted. Furthermore, tumor type was not significantly associated with the incidence of hyperthyroidism with PD-1 inhibitors. The difference in hyperthyroidism incidence among classes of ICI remained statistically significant within the subset of melanoma trials.
Incidence of Hypophysitis
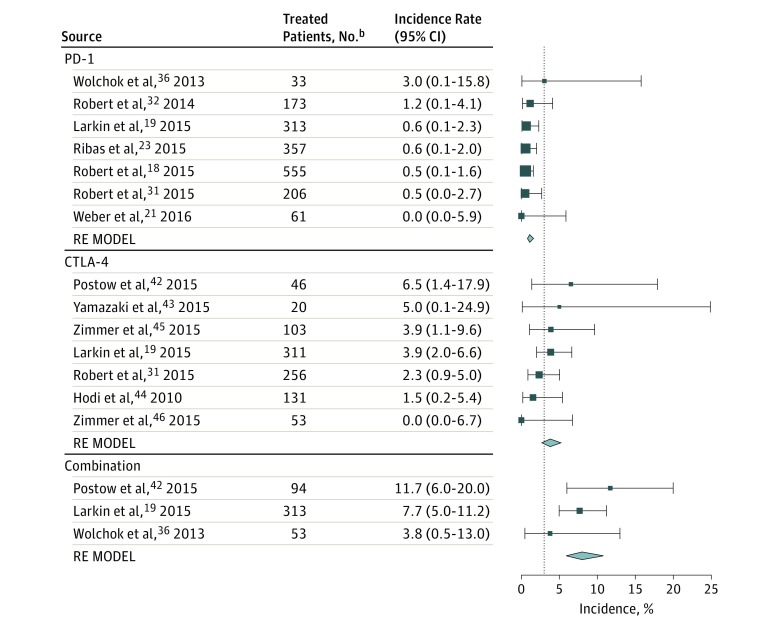
The incidence of hypophysitis for all grades and for grade 3 and higher in the 34 studies that reported this toxic effect is described in eTable 1 in the Supplement. A total of 85 cases of any grade hypophysitis were observed among 6472 patients. Among these cases, 76 occurred in patients with melanoma. A total of 34 cases of grade 3 or higher hypophysitis were reported (0.5%). Overall, the observed incidence of hypophysitis was greatest with combination therapy at 6.4%; 3.2%, CTLA-4 inhibitors; 0.4%, PD-1 inhibitors; and less than 0.1%, PD-L1 inhibitors (eFigure 2 in the Supplement). Due to the low incidence in other diseases (9 of 3394 cases treated [0.3%]), meta-analysis models of drug-specific effects were restricted to studies in advanced melanoma that reported the incidence of hypophysitis (76 of 3078 cases treated [2.5%]) (Figure 4). Compared with patients who received ipilimumab, patients who received PD-1 inhibitors were significantly less likely to experience any grade hypophysitis (OR, 0.29; 95% CI, 0.18-0.49; P < .001); those who received combination therapy were significantly more likely to develop this irAE (OR, 2.2; 95% CI, 1.39-3.60; P = .001) (eTable 3 in the Supplement). Among the melanoma-specific trials included in this analysis, there were no patients treated with the PD-L1 inhibitor atezolizumab.
Figure 4. Incidence of All-Grade Hypophysitis During Treatment With Different Immune Checkpoint Inhibitor Regimensa.
Incidence rates are represented by boxes and whiskers indicate binomial exact 95% CIs. Model results are shown for each class of immunotherapy as a polygon using the fitted value and 95% prediction interval. Combination indicates nivolumab plus ipilimumab; CTLA-4, cytotoxic T-lymphocyte associated protein 4; PD-1, programmed cell death-1; and RE MODEL, random effects model.
aThis analysis was restricted to melanoma-specific studies.
bIncludes the number of patients eligible for safety analysis.
Incidence of Primary Adrenal Insufficiency and Insulin Deficient Diabetes
The incidence of all grade PAI and IDD following the use of ICI is described in eTable 1 in the Supplement. For these AEs, no statistical inferences were made due to the smaller number of events. Four studies did not report events; across the other 62 study cohorts, 43 cases of any-grade PAI were reported among 5831 patients (0.7%), 14 of which (0.2%) were graded 3 or higher. Importantly, among the 262 patients who received combination therapy and had data on adrenal insufficiency reported, 11 experienced PAI, reflecting a reported incidence of 4.2% in this group. Regarding IDD, only 13 cases of any-grade were reported (0.2%), with 6 cases indicated as grade 3 or higher (0.1%). Notably, all but 1 case was observed in patients treated with PD-1 inhibitors.
Discussion
This meta-analysis shows that the incidence of different endocrine AEs following ICIs is significantly higher in patients on combination therapy compared with patients treated with monotherapy. Among patients on monotherapy regimens, the incidence of thyroid dysfunction was higher in those treated with anti–PD-1 agents; conversely, the incidence of hypophysitis was highest in those treated with ipilimumab. Furthermore, there was no association between the tumor type and the incidence of ICI-induced thyroid dysfunctions. To our knowledge, this is the most comprehensive systematic review and meta-analysis on the incidence of endocrine dysfunction following the use of FDA-approved ICI regimens in patients with advanced solid tumors. Although a previous meta-analysis showed that, overall, ICIs are associated with increased risk of hypothyroidism or hyperthyroidism, hypophysitis, and adrenal insufficiency compared with placebo or chemotherapy, our study is the first that we know of to compare the incidence and risks of such irAEs associated with each different regimen, and the first that we know of to also include trials with PD-L1 inhibitors or combination therapy.
Thyroid disorders have emerged as one of the most common AEs associated with ICI therapy. Notably, ICI may rarely induce life-threatening thyroid storm. In our data set, there were rare grade 3 hypothyroidism and hyperthyroidism events (0.2%). Our study demonstrated a higher incidence of hypothyroidism among patients who received combination therapy or PD-1 inhibitors compared with those who received ipilimumab. There was no statistically significant difference in the risk of hypothyroidism between PD-L1 inhibitors and PD-1 inhibitors, or between PD-L1 inhibitors and ipilimumab. Multivariable analysis also showed no relationship between hypothyroidism and tumor type, type of PD-1 inhibitor, or dose of pembrolizumab. Furthermore, compared with those on ipilimumab, patients on combination therapy were more likely to develop hyperthyroidism, whereas increased incidence in patients who received PD-1 inhibitors failed to reach statistical significance in the adjusted analysis. Additionally, there was a higher incidence of hyperthyroidism among patients receiving PD-1 inhibitors compared with those receiving PD-L1 inhibitors, and among those treated with pembrolizumab compared with nivolumab.
A precise explanation for these observed differences is unknown. However, based on prior clinical studies, and rarity of Graves disease following the use of ICIs, we can hypothesize that, in most cases, both hypothyroidism and hyperthyroidism following the use of these agents are different manifestations of the same pathological entity: a destructive thyroiditis mediated by cytotoxic T cells against the thyroid gland. Thus, a possible explanation for the observed differences across ICI regimens may be the fact that monotherapy may be associated with a milder and more transient thyrotoxicosis phase, making it possible to miss in nonscheduled thyroid function tests. Moreover, it is also unclear why there are higher rates of thyroid dysfunction in patients on PD-1 inhibitors compared with those on PD-L1 inhibitors or on ipilimumab. One hypothesis for this is that there is a role for PD-L2 (programmed cell death 1 ligand 2) blockade in the pathogenesis of thyroid dysfunction, as there is now data that normal thyroid tissue expresses both PD-L1 and PD-L2 molecules. Unfortunately, none of the 38 studies included in this analysis specify the etiology of hyperthyroidism.
Hypophysitis has emerged as the most common endocrine dysfunction induced during ipilimumab therapy, and, if unrecognized, can be life threatening. In our study, given the low incidence of hypophysitis in patients with nonmelanoma tumors, we restricted our analysis to include only studies of melanoma. In this subset, the incidence of hypophysitis was statistically higher among patients on combination therapy, followed by those on ipilimumab alone, and rarely reported among patients treated with PD-1 inhibitors alone.
Although the pathogenesis of hypophysitis secondary to CTLA-4 blockade remains unknown, previous studies suggest an autoimmune-based mechanism. Iwama et al showed that CTLA-4 protein is expressed in murine and human pituitary glands, and that repeated injections of an anti–CTLA-4 monoclonal antibody induce a mouse model of complement-mediated hypophysitis. Iwama et al also showed the presence of antithyrotrophs, corticotrophs, and gonadotrophs antibodies in the serum of patients with ipilimumab-induced hypophysitis. More recently, an autopsy study, performed in a subject who developed severe hypophysitis while on anti–CTLA-4 therapy, showed findings suggestive of type II (mediated by immunoglobulin G or immunoglobulin M antibodies directed against antigen on cells or extracellular materials) and IV (T lymphocytes dependent) hypersensitivity reactions, and showed strong CTLA-4 expression in the pituitary. The higher rates of hypophysitis seen with that combination therapy reinforces the nonredundant roles of different checkpoint molecules in regulation of immune responses.
Previous studies suggest that frequency and severity of irAEs with ipilimumab are dose dependent. In our data set, among patients treated with ipilimumab monotherapy, only 1 cohort received it at the dose of 10 mg/kg, preventing us from evaluating any dose-dependent effect on the incidence of endocrine dysfunction. Recently, a randomized phase 3 trial compared the efficacy and safety of ipilimumab 10 mg/kg or 3 mg/kg (every 3 weeks for 4 doses) in patients with advanced melanoma. Although the higher dose improved overall survival of patients, it was also associated with increased rates of any-grade irAEs compared with the lower dose, as well as hypophysitis (6.6% vs 3.3%), hypothyroidism (2.2% vs 1.9%) and thyroiditis (1.4% vs 0.6%).
Less is known about ICI-induced PAI and IDD. Although our study found only 43 cases of any grade PAI reported among 5831 patients (0.7%), the reported rate of PAI among patients on combination therapy was 4.2%. Likewise, among the 13 cases of any-grade IDD reported, 12 occurred on anti–PD-1 therapy and 1 on ipilimumab. For these AEs, no statistical inferences were made due to the small number of events. To date, few case reports have been published on these types of irAEs.
Limitations
Our study has several limitations. We conducted our meta-analysis at the study level; therefore, variables at the patient level were not available for this analysis. Thus, we could not establish additional potential risk factors possibly associated with the development of endocrine AEs, including the role of sex, baseline thyroid or pituitary functions, or presence of thyroid autoantibodies. In addition, the use of meta-analytic techniques to pool published summary data are associated with the possibility of missing studies, heterogeneity of included studies, and the use of aggregated patient data, which can restrict the ability to check for uniform definitions of outcome variables. Importantly, the studies included in this analysis had different rules to monitor patients for endocrine dysfunctions, which could have influenced the reported incidence of these AEs across these various protocols.
Conclusions
Our study provides more precise data on the incidence of endocrine irAEs induced by different ICI regimens. Although combination therapy with ICIs has strong preclinical rationale and showed an improvement in progression-free survival in patients with melanoma, this approach increases the risk of endocrine dysfunction following its use. While all 4 drugs included in our study block immune checkpoint molecules, the differences in the incidence of the above discussed endocrine AEs suggest that the CTLA-4 and PD-1 or PD-L1 or PD-L2 axes have distinct importance in the maintenance of immune tolerance against the thyroid, pituitary, and adrenal glands. Based on our results, we do recommend monitoring of thyroid-stimulating hormone and free thyroxine levels before each ICI infusion for at least the first 5 cycles of therapy.
eTable 1. Incidence of endocrine dysfunctions in all studies included in the meta-analysis
eTable 2. Meta-regression model results for all grade hypothyroidism and hyperthyrodism
eTable 3. Meta-regression model results for any grade hypophisitis in patients with melanoma
eFigure 1. Funnel plots for ICI studies for all grades hypothyroidism, hyperthyroidism and hypophysitis
eFigure 2. Forest plots of the incidence of all grade hypophysitis during treatment with different ICI regimens
eReferences.
References
- 1.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734-1736. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD. PD-1 Blockers. Cell. 2015;162(5):937. [DOI] [PubMed] [Google Scholar]
- 5.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985-988. [DOI] [PubMed] [Google Scholar]
- 6.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541-547. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141-151. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319-322. [DOI] [PubMed] [Google Scholar]
- 9.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. [DOI] [PubMed] [Google Scholar]
- 10.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 12.R core team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014. http://www.R-project.org/. Accessed August 2, 2017.
- 13.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. [Google Scholar]
- 14.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015-4022. [DOI] [PubMed] [Google Scholar]
- 17.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717-726. [DOI] [PubMed] [Google Scholar]
- 18.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. [DOI] [PubMed] [Google Scholar]
- 19.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. [DOI] [PubMed] [Google Scholar]
- 21.Weber J, Gibney G, Kudchadkar R, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. 2016;4(4):345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. [DOI] [PubMed] [Google Scholar]
- 25.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. [DOI] [PubMed] [Google Scholar]
- 26.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286-4293. [DOI] [PubMed] [Google Scholar]
- 27.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384. [DOI] [PubMed] [Google Scholar]
- 31.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330. [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109-1117. [DOI] [PubMed] [Google Scholar]
- 33.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883-895. [DOI] [PubMed] [Google Scholar]
- 36.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu T, Seto T, Hirai F, et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest New Drugs. 2016;34(3):347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamazaki N, Kiyohara Y, Uhara H, et al. Phase II study of ipilimumab monotherapy in Japanese patients with advanced melanoma. Cancer Chemother Pharmacol. 2015;76(5):997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmer L, Eigentler TK, Kiecker F, et al. Open-label, multicenter, single-arm phase II DeCOG-study of ipilimumab in pretreated patients with different subtypes of metastatic melanoma. J Transl Med. 2015;13:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS One. 2015;10(3):e0118564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Mahony D, Morris JC, Quinn C, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13(3):958-964. [DOI] [PubMed] [Google Scholar]
- 50.Maki RG, Jungbluth AA, Gnjatic S, et al. A pilot study of anti-CTLA4 antibody ipilimumab in patients with synovial sarcoma. Sarcoma. 2013;2013:168145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel-Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta-analysis. Future Oncol. 2016;12(3):413-425. [DOI] [PubMed] [Google Scholar]
- 52.Morganstein DL, Lai Z, Spain L, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017;86(4):614-620. [DOI] [PubMed] [Google Scholar]
- 53.McMillen B, Dhillon MS, Yong-Yow S. A rare case of thyroid storm. BMJ Case Rep. 2016;2016:1136–, bcr-2016-bcr-214603.. doi: 10.1136/bcr-2016-214603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;100(5):1738-1741. [DOI] [PubMed] [Google Scholar]
- 55.Azmat U, Liebner D, Joehlin-Price A, Agrawal A, Nabhan F. Treatment of ipilimumab induced Graves’ disease in a patient with metastatic melanoma. Case Rep Endocrinol. 2016;2016:2087525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164(2):303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamauchi I, Sakane Y, Fukuda Y, et al. Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid. 2017;27(7):894-901. [DOI] [PubMed] [Google Scholar]
- 58.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. [DOI] [PubMed] [Google Scholar]
- 61.Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186(12):3225-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res. 2013;19(14):3977-3986. [DOI] [PubMed] [Google Scholar]
- 63.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611-622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Incidence of endocrine dysfunctions in all studies included in the meta-analysis
eTable 2. Meta-regression model results for all grade hypothyroidism and hyperthyrodism
eTable 3. Meta-regression model results for any grade hypophisitis in patients with melanoma
eFigure 1. Funnel plots for ICI studies for all grades hypothyroidism, hyperthyroidism and hypophysitis
eFigure 2. Forest plots of the incidence of all grade hypophysitis during treatment with different ICI regimens
eReferences.