Key Points
Question
Is malignant peritoneal mesothelioma associated with anaplastic lymphoma kinase (ALK) rearrangements?
Findings
In a large series of 88 consecutive patients with peritoneal mesothelioma, we identified ALK rearrangements in 3% of cases that (1) present in young women (25% of women younger than 40 years), (2) lack asbestos fibers, (3) have no history of therapeutic radiation, and (4) lack the typical cytogenetic and molecular abnormalities usually present in peritoneal mesothelioma.
Meaning
Identification of clinically actionable ALK rearrangements reveals a novel pathogenetic mechanism of malignant peritoneal mesothelioma with promise for targeted therapy.
This study investigates anaplastic lymphoma kinase rearrangements in a large series of peritoneal mesothelioma tumors.
Abstract
Importance
Malignant peritoneal mesothelioma is a rare, aggressive tumor arising from the peritoneal lining, induced by asbestos, therapeutic radiation, or germline mutations. Nevertheless, the molecular features remain largely unknown.
Objective
To investigate anaplastic lymphoma kinase (ALK) rearrangements in a large series of peritoneal mesothelioma and characterize the mutational landscape of these tumors.
Design, Setting, and Participants
We studied 88 consecutive patients (39 men, 49 women; median age 61, range 17-84 years) with peritoneal mesotheliomas diagnosed at a single institution between 2005 and 2015. We identified ALK-positive mesotheliomas by immunohistochemistry and confirmed ALK rearrangement by fluorescence in situ hybridization (FISH). In ALK-rearranged cases, we characterized the fusion partners using targeted next-generation sequencing of both tumor DNA and RNA. In select cases, we quantified asbestos fibers by combined scanning electron microscopy and x-ray spectroscopy. We also explored ALK rearrangement in a separate series of 205 patients with pleural mesothelioma.
Main Outcomes and Measures
Identification and characterization of novel ALK rearrangements and correlations with clinicopathologic characteristics.
Results
Anaplastic lymphoma kinase was positive by immunohistochemistry in 11 (13%) peritoneal mesotheliomas (focal weak in 8, diffuse strong in 3). In focal weak ALK-positive cases, no ALK rearrangement was detected by FISH or next-generation sequencing. In strong diffuse ALK-positive cases, FISH confirmed ALK rearrangements, and next-generation sequencing identified novel fusion partners ATG16L1, STRN, and TPM1. Patients with ALK-rearranged peritoneal mesotheliomas were women and younger than patients without ALK rearrangement (median age 36 vs 62; Mann-Whitney test, P = .02), but all other clinicopathologic characteristics (size of tumor nodules, histology, treatment, and survival) were not different. No asbestos fibers were detected in ALK-rearranged cases. Furthermore, loss of chromosomal region 9p or 22q or genetic alterations in BAP1, SETD2, or NF2 typically present in peritoneal mesothelioma were absent in the ALK-rearranged cases. All pleural mesotheliomas were ALK-negative by immunohistochemistry.
Conclusions and Relevance
We identified unique ALK rearrangements in a subset of patients with peritoneal mesothelioma, each lacking asbestos fibers, therapeutic radiation, and cytogenetic and molecular alterations typically found in these tumors. Identification of clinically actionable ALK rearrangements may represent a novel pathogenetic mechanism of malignant peritoneal mesothelioma with promise for targeted therapy.
Introduction
Malignant mesothelioma is a rare aggressive neoplasm arising from the pleural, peritoneal, or pericardial lining. Approximately 3000 new cases present annually in the United States. Most mesotheliomas arise from the pleura, but 10% develop in the peritoneum. Despite various treatment modalities (cytoreductive surgery, radiation, and hyperthermic intraoperative/adjuvant chemotherapy), patients with mesothelioma have a poor prognosis. Mesothelioma is associated with exposure to asbestos, less commonly with therapeutic radiation for prior malignant neoplasms and BAP1 tumor predisposition syndrome. However, in cases without an identifiable cause, the pathogenesis remains unknown. Cytogenetically, 40% to 70% of both pleural and peritoneal mesotheliomas harbor loss of 9p including CDKN2A or 22q including NF2. Whereas somatic mutations in CDKN2A, BAP1, NF2, and SETD2 have been commonly identified in pleural mesothelioma, molecular features of peritoneal mesothelioma remain largely unknown.
After encountering 1 index case of peritoneal mesothelioma harboring ALK rearrangement, we systematically investigated the presence of ALK alterations in a large series of 88 patients with peritoneal mesothelioma and characterized the mutational landscape of these tumors.
Methods
This study was approved by the institutional review board of the Brigham and Women’s Hospital. We reviewed clinicopathologic characteristics and performed immunohistochemistry for ALK in all peritoneal mesotheliomas retrieved from the archives of Brigham and Women’s Hospital (eTable 1 in the Supplement). In ALK-positive cases, ALK rearrangement was confirmed by FISH (eMethods in the Supplement). Anaplastic lymphoma kinase fusion partners were identified using 2 next generation sequencing platforms targeting tumor DNA (OncoPanel) and RNA (AMP translocation) (eMethods in the Supplement). In select cases, we quantified asbestos fibers by combined scanning electron microscopy and x-ray spectroscopy (eMethods in the Supplement). Immunohistochemistry for ALK was also performed in 205 pleural mesotheliomas (eMethods in the Supplement).
Results
Identification and Characterization of ALK Rearrangements
The characteristics of the study group are detailed in eResults in the Supplement. Screening the cohort using immunohistochemistry for ALK, we identified 3 cases of peritoneal mesothelioma with strong and diffuse cytoplasmic expression (eFigure 1A in the Supplement), 8 cases with focal and weak expression (eFigure 1B in the Supplement), and the remaining 77 cases were lacking ALK expression. In contrast, all pleural mesotheliomas were ALK-negative, consistent with published sequencing results. We explored the molecular features of the ALK-positive peritoneal mesotheliomas.
In all 3 cases with diffuse strong ALK expression, FISH confirmed the presence of ALK rearrangements (eFigure 1C in the Supplement). In contrast, in the cases with focal weak ALK expression (7 cases with available material), no bona fide ALK rearrangements were found, by FISH (4 cases) or targeted next generation sequencing (OncoPanel, 3 cases). Instead, peritoneal mesotheliomas with focal weak ALK expression displayed an abnormal FISH pattern indicative of polysomy for the corresponding region of 2p23 (eFigure 1D in the Supplement). The ALK-negative cases had no ALK rearrangements.
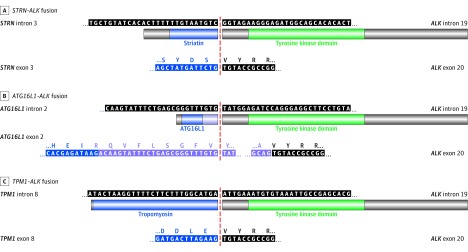
Using targeted next generation sequencing of both tumor DNA and RNA, we identified novel ALK fusion partners: STRN, ATG16L1, and TPM1 in 1 case each of the ALK-rearranged peritoneal mesotheliomas (Figure 1). The presence of STRN-ALK translocation has been rarely reported in carcinomas. To our knowledge, ATG16L1-ALK fusion has not been described to date; whereas TPM1-ALK fusion has been uncommonly described in bladder carcinomas. The ALK breakpoint was mapped to intron 19 in all 3 cases, the breakpoint of STRN to intron 3 (Figure 1A), the breakpoint of ATG16L1 to intron 2 (Figure 1B), and the breakpoint of TPM1 to the beginning of exon 9 (Figure 1C). Both STRN-ALK and TPM1-ALK fusions were in-frame alterations, predicted by DNA sequencing and confirmed by RNA sequencing (Figures 1A and C). For the ATG16L1-ALK fusion, RNA sequencing demonstrated a 122-base-pair insertion leading to an in-frame fusion between ATG16L1 and ALK (Figure 1B). In this case, karyotyping demonstrated t(2;2)(p23;q35), confirming rearrangement of ALK at 2p23 (eFigure 2 in the Supplement).
Figure 1. DNA and Amino Acid Sequences of the 3 ALK Fusion Proteins in Peritoneal Mesothelioma .
A, Peritoneal mesothelioma with STRN-ALK fusion, with (top) DNA sequencing mapping breakpoints to intron 3 of STRN and intron 19 of ALK, and (bottom) RNA sequencing confirming in-frame fusion of exon 3 of STRN to exon 20 of ALK. B, Peritoneal mesothelioma with ATG16L1-ALK fusion, with (top) DNA sequencing mapping breakpoints to intron 2 of ATG16L1 and intron 19 of ALK, and (bottom) RNA sequencing showing insertion of 122 base pairs between the ATG16L1 exon 2 and ALK exon 20 secondary to alternative splicing, leading to in-frame fusion of exon 2 of ATG16L1 followed by additional 40 amino acids to exon 20 of ALK. C, Peritoneal mesothelioma with TPM1-ALK fusion, with (top) DNA sequencing mapping breakpoints to intron 8/exon 9 of TPM1 and intron 19 of ALK, and (bottom) RNA sequencing confirming in-frame fusion of exon 8 of TPM1 to exon 20 of ALK.
Clinicopathologic Characteristics of Peritoneal Mesotheliomas With ALK Rearrangements
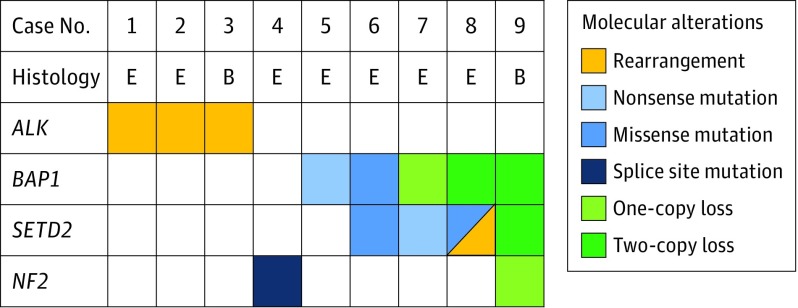
All patients with ALK-rearranged peritoneal mesotheliomas were women and significantly younger than patients without ALK rearrangement (median age, 36 vs 62 years; Mann-Whitney test, P = .02) (eTable 1 in the Supplement). All other clinicopathologic characteristics including tumor size, histology, treatment, and survival were not different between cases with and without ALK rearrangement (eTable 1 in the Supplement), although the small number of cases with ALK rearrangement precluded definitive comparison. Each ALK-rearranged peritoneal mesothelioma lacked a history of therapeutic radiation, had multiple confluent peritoneal nodules (eFigure 3A in the Supplement), showed a tubulopapillary or solid histology (eFigure 3 in the Supplement), expressed mesothelial markers (keratin AE1/AE3, calretinin, and WT1), and retained BAP1 expression (eFigure 4 and eTable 2 in the Supplement). Ultrastructural studies in 2 available cases demonstrated apical microvilli and numerous desmosomes, confirming a mesothelial phenotype (eFigure 5 in the Supplement). No asbestos fibers were detected in any of the ALK-rearranged cases by combined scanning electron microscopy and x-ray spectroscopy. Furthermore, the typical loss of chromosomal region 9p or 22q was not detected in any of ALK-rearranged cases using FISH or karyotyping. All 3 ALK-rearranged peritoneal mesotheliomas lacked molecular alterations in BAP1, SETD2, or NF2 that are typically present in other peritoneal mesotheliomas (Figure 2).
Figure 2. Genetic Alterations in Peritoneal Mesothelioma.
Heat map comparing the distribution of recurrent single nucleotide and copy number variations in ALK-rearranged peritoneal mesotheliomas (cases 1-3) and peritoneal mesotheliomas with no ALK rearrangement (cases 4-9) using targeted next generation DNA sequencing. Peritoneal mesotheliomas with ALK rearrangements comprise a distinct genetic cluster lacking the most common molecular alterations typically found in peritoneal mesotheliomas (BAP1, SETD2, and NF2). E indicates epithelioid mesothelioma; B, biphasic mesothelioma.
No significant difference in overall survival was noted with age, history of therapeutic radiation, tumor histology, or BAP1 expression (eResults, eTable 3, and eFigure 6 in the Supplement). Although overall survival was not affected by the presence of ALK rearrangement (present vs absent; HR, 0.5; 95% CI, 0.1-1.8; P = .29) or ALK protein expression (present vs absent; HR, 1.5; 95% CI, 0.6-4.2; P = .40), patients with weak focal ALK expression have a shorter overall survival than patients with absent or diffuse strong ALK expression (median survival, 4.8, 38.4, and 79.2 months, respectively; 3-way analysis, P = .03) (eResults and eFigure 7 in the Supplement).
Discussion
Oncogenic fusions of ALK to diverse partners have been reported in carcinomas, mesenchymal tumors, and lymphomas. In some ALK-rearranged tumors, ALK is a pivotal therapeutic target. However, the ALK status in mesotheliomas remains unexplored. Recently, ALK rearrangement has been described in 1 pediatric peritoneal mesothelioma, although the fusion partner was not investigated.
Limitations
Although most studies have not found ALK rearrangement in mesotheliomas, this may be owing to the tumor location (pleural vs peritoneal) and the magnitude of the study cohort. Our study was limited by the low incidence of peritoneal mesothelioma and the low prevalence of patients with ALK rearrangement. Larger multiinstitutional prospective studies are needed to confirm our correlative findings and explore the efficacy of ALK-targeted therapy. Genome-wide analysis of 216 pleural mesotheliomas has found no ALK-rearranged cases. Indeed, our study did not identify any ALK-positive pleural mesotheliomas. In contrast, in peritoneal mesothelioma, we identified ALK rearrangements in 3 of 88 (3%) patients, including 2 of 8 (25%) women under 40, suggesting that ALK rearrangement may be more prevalent in young women with peritoneal mesothelioma. We identified (1) the precise ALK breakpoints by DNA sequencing, (2) the in-frame mutant ALK fusion transcripts by RNA sequencing, and (3) ALK protein overexpression by immunohistochemistry, which together strongly suggest that the ALK alterations uncovered in this study represent functional and clinically-actionable alterations (Figure 1) (eFigure 1A and eFigure 8 in the Supplement). Furthermore, ALK-rearranged cases lacked asbestos fibers, a history of therapeutic radiation, and loss of chromosomal region 9p or 22q or the genetic alterations in BAP1, SETD2, or NF2 that are typically present in peritoneal mesothelioma (Figure 2). Our findings suggest that oncogenic ALK fusion may represent a novel causation mechanism for a subset of patients with peritoneal mesothelioma. These patients can be readily identified using ALK immunohistochemistry and triaged toward a personalized targeted treatment.
Conclusions
We identified ALK rearrangements in 3% of patients with peritoneal mesotheliomas that (1) present in younger women, (2) lack asbestos fibers, (3) have no history of radiation exposure, and (4) lack the typical genetic abnormalities present in peritoneal mesothelioma. We describe clinically actionable ALK rearrangements as a novel pathogenetic mechanism in a subset of peritoneal mesotheliomas with promise for targeted therapy.
eMethods
eResults
eTable 1. Clinicopathologic characteristics of patients with diffuse malignant peritoneal mesothelioma
eTable 2. Immunophenotypic and cytogenetic characteristics of diffuse malignant peritoneal mesothelioma with and without ALK rearrangement
eTable 3. Univariate analysis of overall survival in relation to clinicopathologic characteristics among patients with diffuse malignant peritoneal mesothelioma
eFigure 1. Immunohistochemistry for ALK in peritoneal mesotheliomas
eFigure 2. Karyotype of a case of diffuse malignant peritoneal mesothelioma demonstrates rearrangement at chromosome 2p23
eFigure 3. Gross photograph and photomicrographs of diffuse malignant peritoneal mesothelioma with ALK rearrangement
eFigure 4. Immunophenotype of diffuse malignant peritoneal mesothelioma with ALK rearrangement
eFigure 5. Electron micrographs of diffuse malignant peritoneal mesothelioma with ALK rearrangement
eFigure 6. Overall survival of patients with diffuse malignant peritoneal mesothelioma
eFigure 7. Overall survival of patients with diffuse malignant peritoneal mesothelioma according to ALK rearrangement and ALK expression status
eFigure 8. Structural schematics of the ALK fusion proteins in diffuse malignant peritoneal mesothelioma
eReferences
References
- 1.Mazurek JM, Syamlal G, Wood JM, Hendricks SA, Weston A. Malignant mesothelioma mortality—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009;20(6):935-944. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Yan TD, Stuart OA, Yoo D. Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32(6):686-691. [DOI] [PubMed] [Google Scholar]
- 4.Magge D, Zenati MS, Austin F, et al. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol. 2014;21(4):1159-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone M, Emri S, Dogan AU, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7(2):147-154. [DOI] [PubMed] [Google Scholar]
- 6.Taylor S, Carpentieri D, Williams J, Acosta J, Southard R. Malignant Peritoneal Mesothelioma in an Adolescent Male With BAP1 Deletion. J Pediatr Hematol Oncol. 2015;37(5):e323-e327. [DOI] [PubMed] [Google Scholar]
- 7.Chirieac LR, Barletta JA, Yeap BY, et al. Clinicopathologic characteristics of malignant mesotheliomas arising in patients with a history of radiation for Hodgkin and non-Hodgkin lymphoma. J Clin Oncol. 2013;31(36):4544-4549. [DOI] [PubMed] [Google Scholar]
- 8.Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48(4):407-416. [DOI] [PubMed] [Google Scholar]
- 9.Singhi AD, Krasinskas AM, Choudry HA, et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol. 2016;29(1):14-24. [DOI] [PubMed] [Google Scholar]
- 10.Kelly LM, Barila G, Liu P, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A. 2014;111(11):4233-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cramer DW, Welch WR, Berkowitz RS, Godleski JJ. Presence of talc in pelvic lymph nodes of a woman with ovarian cancer and long-term genital exposure to cosmetic talc. Obstet Gynecol. 2007;110(2 Pt 2):498-501. [DOI] [PubMed] [Google Scholar]
- 13.Mariño-Enríquez A, Dal Cin P. ALK as a paradigm of oncogenic promiscuity: different mechanisms of activation and different fusion partners drive tumors of different lineages. Cancer Genet. 2013;206(11):357-373. [DOI] [PubMed] [Google Scholar]
- 14.Cagle PT, Chirieac LR. Advances in treatment of lung cancer with targeted therapy. Arch Pathol Lab Med. 2012;136(5):504-509. [DOI] [PubMed] [Google Scholar]
- 15.Loharamtaweethong K, Puripat N, Aoonjai N, Sutepvarnon A, Bandidwattanawong C. Anaplastic lymphoma kinase (ALK) translocation in paediatric malignant peritoneal mesothelioma: a case report of novel ALK-related tumour spectrum. Histopathology. 2016;68(4):603-607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eResults
eTable 1. Clinicopathologic characteristics of patients with diffuse malignant peritoneal mesothelioma
eTable 2. Immunophenotypic and cytogenetic characteristics of diffuse malignant peritoneal mesothelioma with and without ALK rearrangement
eTable 3. Univariate analysis of overall survival in relation to clinicopathologic characteristics among patients with diffuse malignant peritoneal mesothelioma
eFigure 1. Immunohistochemistry for ALK in peritoneal mesotheliomas
eFigure 2. Karyotype of a case of diffuse malignant peritoneal mesothelioma demonstrates rearrangement at chromosome 2p23
eFigure 3. Gross photograph and photomicrographs of diffuse malignant peritoneal mesothelioma with ALK rearrangement
eFigure 4. Immunophenotype of diffuse malignant peritoneal mesothelioma with ALK rearrangement
eFigure 5. Electron micrographs of diffuse malignant peritoneal mesothelioma with ALK rearrangement
eFigure 6. Overall survival of patients with diffuse malignant peritoneal mesothelioma
eFigure 7. Overall survival of patients with diffuse malignant peritoneal mesothelioma according to ALK rearrangement and ALK expression status
eFigure 8. Structural schematics of the ALK fusion proteins in diffuse malignant peritoneal mesothelioma
eReferences