Key Points
Question
Can a negative high-sensitivity troponin assay at 0 and 3 hours following emergency department presentation identify patients at less than 1% risk of 30-day adverse cardiac events?
Findings
In this multicenter evaluation, a single high-sensitivity troponin level less than 6 ng/L ruled out myocardial infarction, while serial levels at 19 ng/L or less identified patients at less than 1% risk of 30-day adverse cardiac events.
Meaning
Identifying a low-risk cohort may permit early emergency department discharge and avoid unnecessary hospitalization.
Abstract
Importance
Physicians need information on how to use the first available high-sensitivity troponin (hsTnT) assay in the United States to identify patients at very low risk for 30-day adverse cardiac events (ACE).
Objective
To determine whether a negative hsTnT assay at 0 and 3 hours following emergency department presentation could identify patients at less than 1% risk of a 30-day ACE.
Design, Setting, and Participants
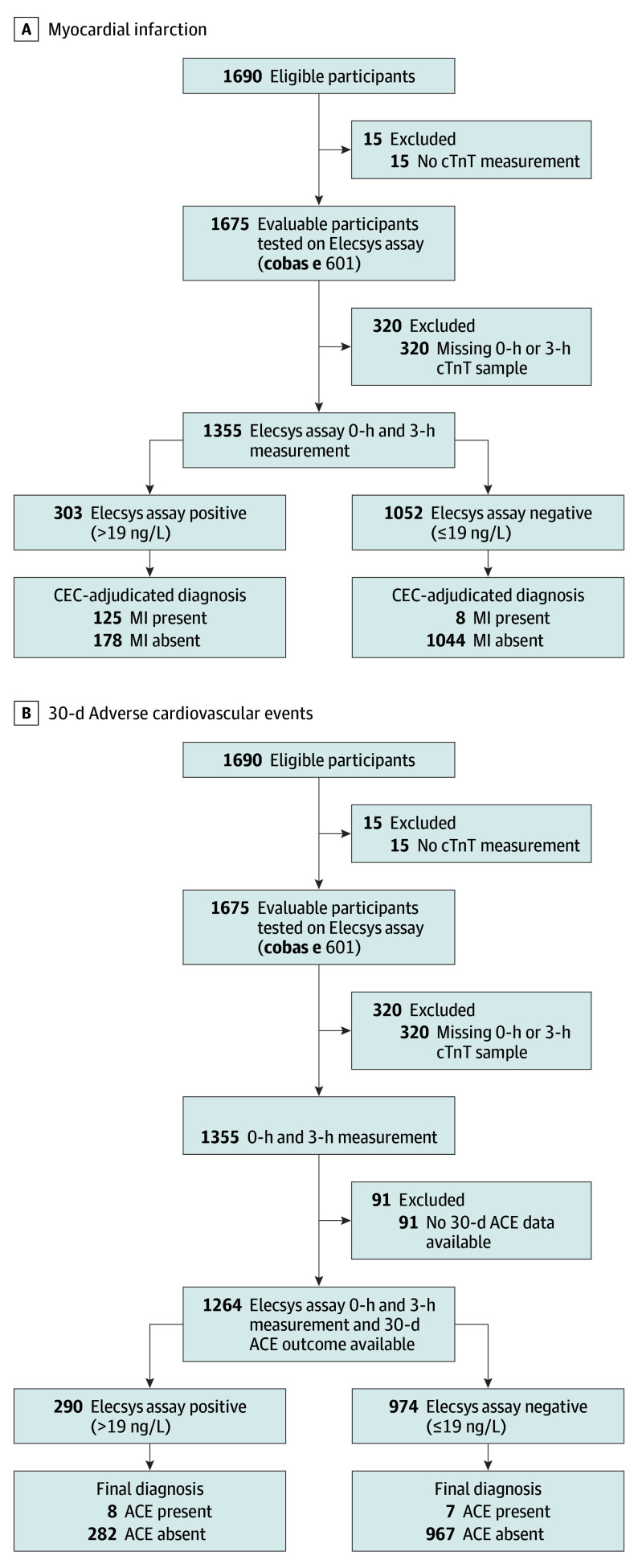
A prospective, observational study at 15 emergency departments in the United States between 2011 and 2015 that included individuals 21 years and older, presenting to the emergency department with suspected acute coronary syndrome. Of 1690 eligible individuals, 15 (no cardiac troponin T measurement) and 320 (missing a 0-hour or 3-hour sample) were excluded from the analyses.
Exposures
Serial hsTnT measurements (fifth-generation Roche Elecsys hsTnT assay).
Main Outcomes and Measures
Serial blood samples from each patient were collected after emergency department presentation (once identified as a potential patient with acute coronary syndrome) and 3 hours, 6 to 9 hours, and 12 to 24 hours later. Adverse cardiac events were defined as myocardial infarction, urgent revascularization, or death. The upper reference level for the hsTnT assay, defined as the 99th percentile, was established as 19 ng/L in a separate healthy US cohort. Patients were considered ruled out for acute myocardial infarction if their hsTnT level at 0 hours and 3 hours was less than the upper reference level. Gold standard diagnoses were determined by a clinical end point committee. Evaluation of assay clinical performance for acute myocardial infarction rule-out was prespecified; the hypothesis regarding 30-day ACE was formulated after data collection.
Results
In 1301 healthy volunteers (50.4% women; median age, 48 years), the upper reference level was 19 ng/L. In 1600 patients with suspected acute coronary syndrome (48.4% women; median age, 55 years), a single hsTnTlevel less than 6 ng/L at baseline had a negative predictive value for AMI of 99.4%. In 974 patients (77.1%) with both 0-hour and 3-hour hsTnT levels of 19 ng/L or less, the negative predictive value for 30-day ACE was 99.3% (95% CI, 99.1-99.6). Using sex-specific cutpoints, C statistics for women (0.952) and men (0.962) were similar for acute myocardial infarction.
Conclusions and Relevance
A single hsTnT level less than 6 ng/L was associated with a markedly decreased risk of AMI, while serial levels at 19 ng/L or less identified patients at less than 1% risk of 30-day ACE.
This study determines whether a negative high-sensitivity troponin assay at 0 and 3 hours following emergency department presentation could identify patients at less than 1% risk of a 30-day adverse cardiac event.
Introduction
More than 7 million patients annually present to US emergency departments (EDs) with symptoms suggestive of acute coronary syndrome (ACS).1 Many of these individuals (approximately 90%) will not have an acute myocardial infarction (AMI) and will have no major adverse cardiac events (ACEs), defined as MI, urgent revascularization, or cardiac death, within the next 30 days. However, these patients are commonly hospitalized or kept under observation for an evaluation that is often negative.2 In fact, rule-in rates in some studies approach zero.3,4,5,6 This high rate of negative evaluation occurs because of the nonspecific presentation of ACS and the potential for ACE with inappropriate discharge. There is general consensus that an acceptable ACS risk-stratification strategy for discharge from the ED should identify a cohort with 30-day ACE rates less than 1%.7,8,9 This 1% rate represents the breakpoint between the risk of unnecessary hospitalization resulting in morbidity from a hospital-acquired condition and the potential harm from an inappropriate ED discharge.
Cardiac troponin T and I are well-established biomarkers for AMI diagnosis and outcome prediction.10,11,12 However, conventional troponin assays lack the necessary sensitivity and/or precision to be used reliably for the early identification of patients at risk for 30-day ACE.13,14 Compared with conventional assays, high-sensitivity troponin (hsTnT) assays have significantly greater analytical sensitivity and clinical negative predictive value (NPV) for the diagnosis of AMI.15,16 Furthermore, they may allow the identification of a cohort of ED patients with very low 30-day ACE.15,16 The ability to identify patients at low risk of 30-day ACE could have a significant effect on ACS risk stratification. It could allow for more efficient inpatient resource use as well as improved patient satisfaction and reduced operational costs. Safe and rapid ED discharge could contribute to reducing pressure on EDs and decreasing wait times, thus allowing greater attention to other ED patients.
The first hsTnT assay in the United States became available in January 2017 (the fifth-generation Elecsys assay). Our purpose was to evaluate the ability of this assay to identify ED patients with suspected ACS who are at very low risk for 30-day ACE.
Methods
This was a prospective, observational study of patients with suspected ACS enrolled at 15 US EDs from 2011 to 2015 (Charleston Area Medical Center, Charleston, West Virginia; Cleveland Clinic Foundation, Cleveland, Ohio; Cooper University Hospital, Camden, New Jersey; Duke University, Durham, North Carolina; Health Science Center of Houston Medical School, Houston, Texas; Henry Ford Health System, Detroit, Michigan; Indianapolis University, Indianapolis, Indiana; Mayo Clinic, Rochester, Minnesota; Minneapolis Heart Institute Foundation, Minneapolis, Minnesota; Newton Wellesley Hospital, Newton, Massachusetts; Prince George's Hospital, Cheverly, Maryland; San Francisco General Hospital, San Francisco, California; South Shore Hospital, Weymouth, Massachusetts; Thomas Jefferson University Hospital, Philadelphia, Pennsylvania; University of North Carolina, Chapel Hill; eFigure 1 in the Supplement). Roche Diagnostics, in collaboration with the clinical investigators, collected data as part of a US Food and Drug Administration (FDA) submission to evaluate the hsTnT Fifth Generation Elecsys TnT Short Turn Around Time (hsTnT Gen 5 STAT) assay (Roche Inc). All authors could request any analysis from the database and assumed responsibility for the complete manuscript and data integrity. Prior to study start, ethics approval was obtained from all relevant institutional review boards. The studies were conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization guidelines for Good Clinical Practice, and the Code of Federal Regulations 21, Part 50.
After written informed consent, patients 21 years and older presenting to the ED with symptoms of ACS were prospectively enrolled. Inclusion criteria were symptoms suggestive of ACS and ability to provide a baseline blood sample within 24 hours of symptom onset. Exclusion criteria were AMI within the last 3 months, transfer from another medical facility, surgery (including percutaneous coronary intervention) or hospitalization within the last 3 months, recent cardioversion or defibrillation, acute noncardiac primary illness prior to enrollment (eg, severe sepsis), cardiogenic shock, and pregnancy.
Serial blood samples from each patient were collected after ED presentation (once identified as a potential patient with ACS) and 3 hours, 6 to 9 hours, and 12 to 24 hours later. Clinical care was otherwise dictated by local care standards.
An independent clinical events committee (CEC), made up of 2 cardiologists and 1 emergency physician, adjudicated the rule-in AMI diagnosis for each patient per the Third Universal Definition of AMI criteria.13 The CEC had access to all clinical data (including the local troponin assay results), but was blinded to hsTnT Gen 5 STAT results and the local diagnosis. Any discrepancies between the assigned cardiologist and emergency physician (15% of cases) were resolved by discussion or case review by the full committee. The performance of the hsTnT Gen 5 STAT assay was determined by comparison of the hsTnT assay results with the CEC-adjudicated diagnosis. Patients were followed up for 30 days to determine ACE, defined as all postdischarge death, AMI, or urgent myocardial revascularization. Follow-up was conducted via medical record review and telephone contact.
Samples were collected in lithium heparin tubes, centrifuged for 15 minutes, and stored at –70°C. Troponin T concentrations were measured by laboratory personnel blinded to sample origin identity, using the hsTnT Gen 5 STAT assay on the cobas e 601 analyzer at 1 of 4 sites. The assay is an electrochemiluminescence sandwich immunoassay, which uses both ruthenium-labeled and biotin-labeled antibodies to form a sandwich complex with troponin T. It has a specified range of 6 ng/L to 10 000 ng/L (all platforms) and a limit of quantification (LoQ) of 6 ng/L. Although the limit of detection for the hsTnT used in this analysis has been reported to be 3 ng/L, results less than the LoQ are not reported, per FDA regulations. Finally, no changes have occurred in the manufacture of this assay since the start of this investigation.
Reference Range Evaluation
Prior to this study, we performed a prospective, 4-center evaluation to identify the reference range for the hsTnT assay. After written informed consent was obtained, healthy individuals 21 years and older were enrolled, and blood samples were obtained to determine the 99th percentile upper reference level (URL). Inclusion criteria were no current diagnosis of cancer and no history of chronic disease (heart, cancer, renal, thyroid, respiratory [excluding asthma], autoimmune, or diabetes). Exclusion criteria included a history of ACS, pregnancy or birth within 6 weeks, currently taking prescription drugs for chronic disease, hospitalization within the previous 3 months, or blood pressure greater than 140/90 mm Hg. The 99th percentile hsTnT concentration from this cohort was subsequently used as the URL for the evaluation of diagnostic performance.
Data Analyses
Statistical analyses were performed using R, version 3.2.2 (R Foundation)17 and SAS, version 9.4 (SAS Institute) software. The CEC adjudication diagnosis was entered into a Code of Federal Regulations Part 11–compliant database. Clinical sensitivity, specificity, NPV, and positive predictive value (PPV) are expressed as point estimates and 95% Clopper-Pearson confidence intervals for each sample time. We also evaluated diagnostic test characteristics for the 99th percentile URL cutoff overall and for sex-specific cutoffs.18 Cutoff-independent receiver operator characteristic curves were plotted, and the C statistic was calculated.
A post hoc analysis of serial hsTnT concentrations was performed and the NPV for 30-day ACE determined. We secondarily evaluated the effect of different 99th percentile URL cutoffs (19 ng/L vs 14 ng/L).
Because the FDA restricts results less than the LoQ from being reported, an analysis of the following hsTnT results at time 0 hours and 3 hours, respectively, was performed in patients with hsTnT results of 19 ng/L or less: less than 6 ng/L and less than 6 ng/L, less than 6 ng/L and between 6 and 19 ng/L inclusive, between 6 and 19 ng/L inclusive and less than 6 ng/L, and between 6 and 19 ng/L inclusive and between 6 and 19 ng/L inclusive.
Results
Reference Range Evaluation
A total of 1312 healthy individuals were enrolled in the reference range analyses, of whom 11 were excluded (blood not obtainable in 7 and inadequate sample in 4), leaving 1301 for analysis. Of these, 656 were women (50.4%); 891 were white (68.5%), 242 were African American (18.6%), 86 were Asian (6.6%), and 77 were classed as “other” race/ethnicity (6.3%). The median age was 48 years (interquartile range [IQR], 33-55 years) and did not differ between women and men (48 years; IQR, 33-55 years and 47 years; IQR, 32-54 years, respectively). The overall 99th percentile URL hsTnT concentration was 19 ng/L. Sex-specific values were 22 ng/L and 14 ng/L in men and women, respectively. The coefficient of variation for imprecision at the URL was less than 10%.
Diagnostic Performance in Patients With Suspected ACS
A total of 1690 eligible patients with ACS symptoms were enrolled (Figure 1). Of these, 1679 had hsTnT measured at 1 or more points. Follow-up information (30-day) was available for 1678 patients: 1564 were followed up by telephone (n = 1054) and/or medical records review (n = 1618), 113 were lost to follow-up, and for 1 patient, follow-up was not applicable. Patient characteristics are summarized in Table 1. Median time from symptom onset to presentation and presentation to baseline blood draw was 2.9 hours (IQR, 1.4-6.6 hours) and 2.9 hours (IQR, 0.8-3.4 hours), respectively. Overall, 173 patients (10.3%) had an adjudicated AMI diagnosis. The sensitivities, specificities, PPVs, and NPVs for the diagnosis of AMI, using the overall and sex-specific URL, are presented in Table 2. Diagnostic performance of the assay using 99th percentile sex-specific cutoffs (14 ng/L in women and 22 ng/L in men) was comparable with that based on the overall cutoff, with an NPV of 99.3% at 3 hours for both sexes. Because we used contemporary troponin gold standard, further research will be necessary to determine the need for sex-specific cutpoints. Overall, the C statistic for AMI at 3 hours was 0.958, with little difference between men and women (0.952 and 0.962, respectively).
Figure 1. Standards for Reporting Diagnostic Accuracy Studies Diagrams .
A, Myocardial infarction (MI); B, Thirty-day adverse cardiovascular events (ACE; myocardial infarction, urgent revascularization, or cardiac death). CEC indicates clinical events committee; cTnT, cardiac troponin T.
Table 1. Characteristics of the Clinical Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All Patients (N = 1679) |
MI (n = 173) |
Non-MI (n = 1506) |
|
| Age, median (IQR), y | 55 (47, 64) | 59 (52, 69)a | 54 (46, 64) |
| Male | 866 (51.6) | 107 (61.8)b | 759 (50.4) |
| BMI, median (IQR) | 29.9 (25.9-35.4) | 29.5 (26-34.4) | 29.9 (25.9-35.5) |
| Current smoker | 509 (30.5) | 63 (36.4) | 446 (29.9) |
| History of smoking | 489 (29.3) | 54 (31.2) | 435 (29.1) |
| Heart rate, bpm, median (IQR) | 80 (69, 91) | 82 (69, 99)b | 79.5 (69, 90) |
| Systolic BP, median (IQR), mm Hg | 142 (125-158) | 145 (124-166)b | 142 (125-157) |
| Diastolic BP, median (IQR), mm Hg | 81 (71-91) | 84 (72-96) | 81 (71-91) |
| Prior MI | 308 (18.6) | 56 (33.7)b | 252 (16.9) |
| Angina pectoris | 155 (9.4) | 22 (13.1) | 133 (9.0) |
| Coronary intervention | 374 (22.5) | 56 (32.6)b | 318 (21.3) |
| CAD | 436 (26.5) | 67 (40.1)a | 369 (24.9) |
| Cerebrovascular disease | 110 (6.8) | 13 (7.6) | 97 (6.7) |
| Congestive heart failure | 142 (8.5) | 28 (16.2)a | 114 (7.6) |
| Cardiomyopathy | 101 (6.3) | 20 (12.0)b | 81 (5.6) |
| Myocarditis | 4 (0.2) | 0 (0) | 4 (0.3) |
| Hypertension | 1108 (66.2) | 135 (78)a | 973 (64.9) |
| Valvular heart disease | 82 (5.1) | 13 (7.8) | 69 (4.8) |
| Prosthetic heart disease | 6 (0.4) | 0 (0) | 6 (0.4) |
| Diabetes | 437 (26.1) | 59 (34.1)b | 378 (25.2) |
| Hyperlipidemia | 834 (50.1) | 103 (59.5)b | 731 (49.0) |
| Autoimmune disease | 113 (7.0) | 15 (8.8) | 98 (6.8) |
| Left bundle branch block | 34 (2.0) | 8 (4.6)b | 26 (1.7) |
| ST elevation | 61 (3.7) | 16 (9.4)a | 45 (3.1)c |
| ST-segment depression | 119 (7.3) | 40 (23.8)a | 79 (5.4) |
| T-wave inversions | 242 (14.8) | 51 (30.0)a | 191 (13.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; CAD, coronary artery disease; IQR, interquartile range; MI, myocardial infarction.
P < .001 for the comparison between patients with MI and patients without MI (Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables).
P < .05 for the comparison between patients with MI and patients without MI (Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables).
ST elevation owing to causes other than MI (eg, left ventricular hypertrophy).
Table 2. Diagnostic Performance (95% CI) for AMI of the hsTnT Fifth-Generation Elecsys TnT by Time and Stratified by Overall (<19 ng/L) and Sex-Defined URLs.
| Sample Time | No. | Performance (95% CI) | |||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | ||
| Overall URL (19 ng/L) | |||||
| 0 h | |||||
| All | 1600 | 86.0 (79.7-90.9) | 88.0 (86.2-89.6) | 44.9 (39.3-50.6) | 98.2 (97.3-98.9) |
| Male | 829 | 88.1 (80.2-93.7) | 84.1 (81.2-86.7) | 43.4 (36.5-57.5) | 98.1 (97.0-99.0) |
| Female | 771 | 82.5 (70.9-90.9) | 91.9 (89.7-93.8) | 47.7 (38.1-57.5) | 98.3 (97.0-99.2) |
| 3 h | |||||
| All | 1415 | 94.3 (89.1-97.5) | 86.6 (84.6-88.4) | 43.6 (37.9-49.4) | 99.3 (98.6-99.7) |
| Male | 733 | 95.6 (89.1-98.8) | 83.0 (79.9-85.8) | 44.4 (37.3-51.6) | 99.3 (98.1-99.8) |
| Female | 682 | 91.8 (80.4-97.7) | 90.2 (87.6-92.4) | 42.1 (32.6-52.0) | 99.3 (98.2-99.8) |
| 6-9 h | |||||
| All | 1158 | 94.9 (89.8-97.9) | 85.3 (83.0-87.4) | 46.6 (40.7-52.6) | 99.2 (98.4-99.7) |
| Male | 622 | 96.7 (90.8-99.3) | 80.2 (76.5-83.5) | 45.9 (38.7-53.2) | 99.3 (98.0-99.9) |
| Female | 536 | 91.3 (79.2-97.6) | 90.8 (87.9-93.2) | 42.1 (32.6-52.0) | 99.3 (98.2-99.8) |
| 12-24 h | |||||
| All | 872 | 91.9 (85.2-96.2) | 80.6 (77.6-83.3) | 40.8 (34.6-47.2) | 98.6 (97.3-99.3) |
| Male | 473 | 94.4 (86.4-98.5) | 76.3 (71.8-80.4) | 41.7 (34.1-49.7) | 98.7 (96.7-99.6) |
| Female | 399 | 87.2 (72.6-95.7) | 85.3 (81.2-88.8) | 39.1 (28.8-50.1) | 98.4 (96.3-99.5) |
| Male URL (22 ng/L) | |||||
| 0 h | 829 | 85.1 (76.7-91.4) | 87.2 (84.6-89.6) | 48.0 (40.5-55.6) | 97.7 (96.2-98.7) |
| 3 h | 733 | 95.6 (89.1-98.8) | 86.3 (83.4-88.9) | 49.7 (42.1-57.4) | 99.3 (98.2-99.8) |
| 6-9 h | 622 | 93.5 (86.3-97.6) | 82.3 (78.7-85.4) | 47.8 (40.3-55.3) | 98.6 (97.1-99.5) |
| 12-24 h | 473 | 94.4 (86.4-98.5) | 80.0 (75.8-83.9) | 45.9 (37.7-54.3) | 98.8 (96.9-99.7) |
| Female URL (14 ng/L) | |||||
| 0 h | 771 | 85.7 (74.6-93.3) | 88.1 (85.5-90.4) | 39.1 (30.9-47.8) | 98.6 (97.3-99.3) |
| 3 h | 682 | 91.8 (80.4-97.7) | 86.9 (84.0-89.4) | 35.2 (26.9-44.1) | 99.3 (98.2-99.8) |
| 6-9 h | 536 | 91.3 (79.2-97.6) | 86.5 (83.2-89.4) | 38.9 (29.7-48.7) | 99.1 (97.6-99.7) |
| 12-24 h | 399 | 92.3 (79.1-98.4) | 81.4 (77.0-85.3) | 35.0 (25.8-45.0) | 99.0 (97.1-99.8) |
Abbreviations: hsTnT, high-sensitivity troponin; NPV, negative predictive value; PPV, positive predictive value; TnT, troponin T; URL, upper reference level.
The sensitivity and NPV peaked/plateaued at the 3-hour sampling time. A single hsTnT measurement using the URL at baseline had inadequate performance for clinical decision making. Based on a single 3-hour sample, the sensitivity, NPV, specificity, and PPV measurements for AMI were 94.3% (95% CI, 89.1%-97.5%), 99.3% (95% CI, 98.6%-99.7%), 86.6% (95% CI, 84.6%-88.4%), and 43.6% (95% CI, 37.9%-49.4%), respectively. Unfortunately, based on hsTnT alone, there were 8 false-negative results at 3 hours (Table 2); none of these patients experienced an additional ACE during the 30-day follow-up.
Overall, of 1355 patients with serial hsTnT results at 0 hours and 3 hours (Figure 2A), 1052 had a non-AMI CEC diagnosis. There were 1264 patients with hsTnT results at 0 hours and 3 hours and who had 30-day follow-up information (Figure 2B), of whom 290 (22.9%) had a hsTnT level exceeding the URL (>19 ng/L) at either point. This non–rule-out cohort had a 30-day ACE rate of 2.8% (n = 8) vs 0.7% (n = 7) in the 974 patients (77.1%) with both serial hsTnT measurements that were less than the URL. Serial hsTnT levels less than the URL resulted in a 99.3% NPV for 30-day ACE (Figure 2B). None of the 7 patients with serial 3-hour time false-negative results experienced AMI or death within the subsequent 30 days.
Figure 2. Subgroup Analysis of Patients With Baseline and 3-Hour High-Sensitivity Troponin (hsTnT) Level of 19 ng/L or Less .
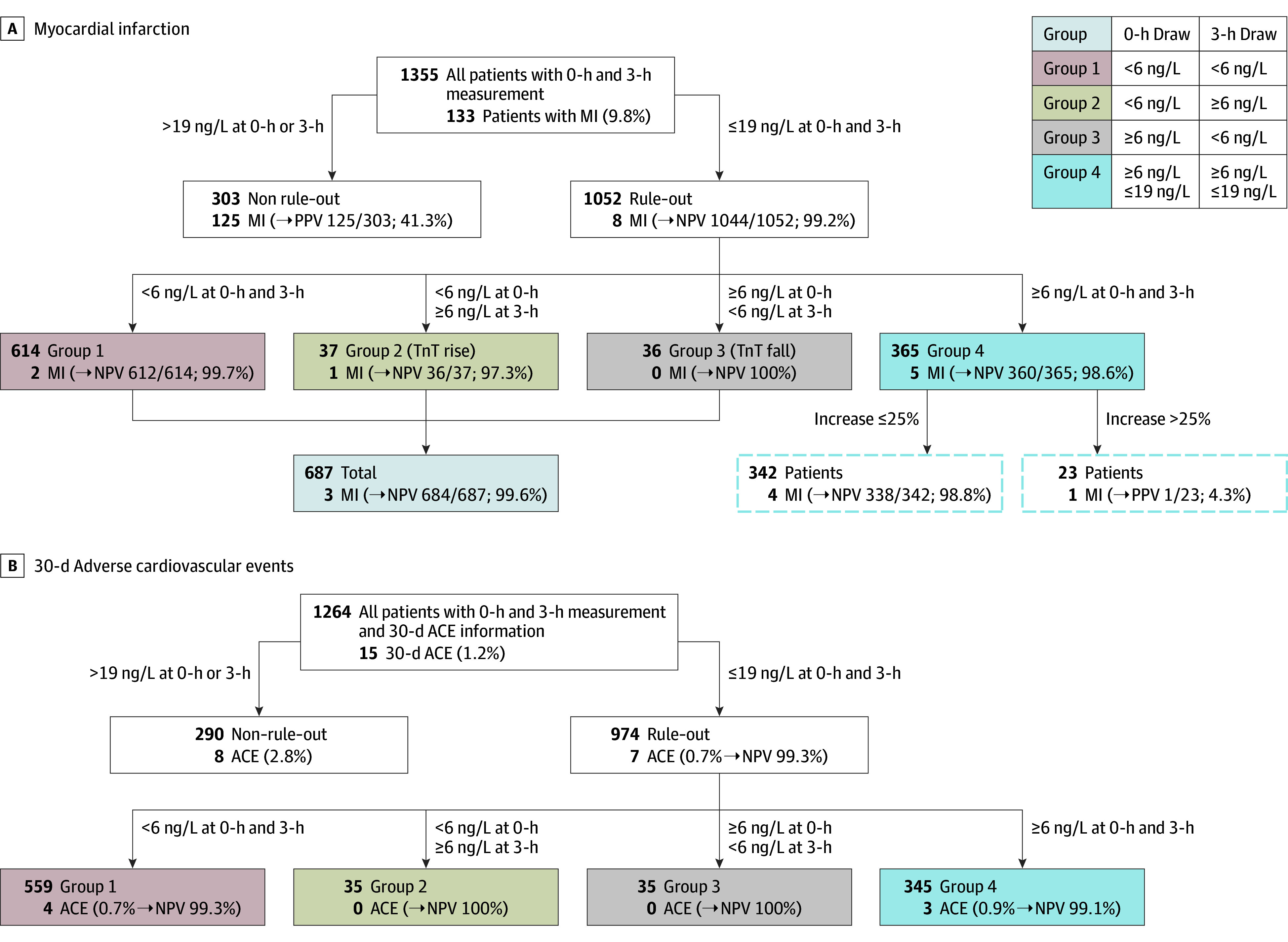
Negative predictive values (NPVs) are presented for clinical events, committee-adjudicated myocardial infarction (MI) diagnosis (A), and the occurrence of cardiac events or death within 30 days (B). PPV indicates positive predictive value.
Troponin trajectory was evaluated in greater detail in 974 patients with an hsTnT level of 19 ng/L or less at 0 and 3 hours. For most of these patients, the hsTnT result did not cross the LoQ margin during the 3 hours but had either both measures less than (n = 559; 57.4%) or both greater than (n = 345; 35.4%) the LoQ (6 ng/L). Their NPV for 30-day ACE was 99.3 and 99.1%, respectively. Only 70 patients had their hsTnT result rise (n = 35) or fall (n = 35) across the LoQ during the 0-hour and 3-hour sampling. Both of these cohorts had a 100% NPV for 30-day ACE (Figure 2B).
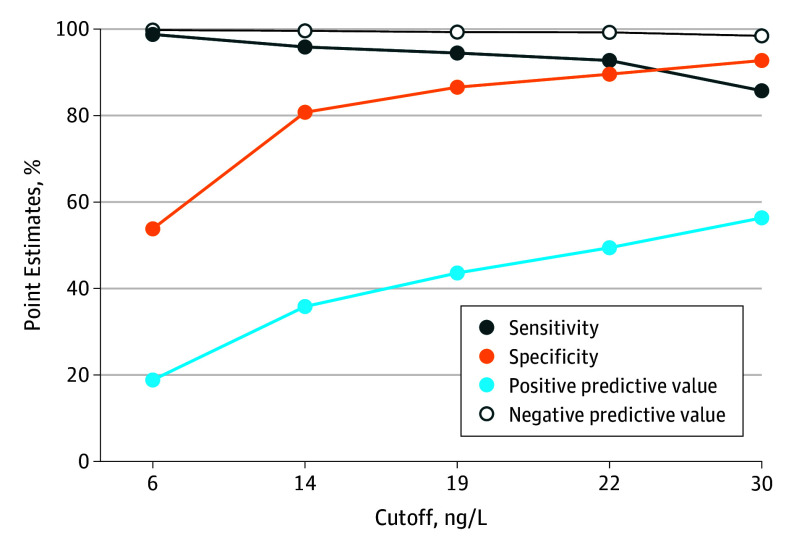
Figure 3 presents the assay’s diagnostic performance based on various cutoffs. Using a cutoff of less than 6 ng/L for baseline hsTnT concentrations (n = 1600) provided an AMI NPV of 99.4% (95% CI 98.6-99.8) and ruled out AMI in 50.3%. At 3 hours, the less than 6 ng/L cutoff (n = 1415) gave an NPV of 99.7% (95% CI, 99.0-100.0) for AMI rule-out in 48.7%.
Figure 3. Influence of the Cutoff Point on Diagnostic Performance.
Because clinicians were blinded to hsTnT, we evaluated index visit hospitalization (defined as length of stay greater than 24 hours) and coronary revascularization rates in patients with a 0-hour and 3-hour hsTnT levels of 19 ng/L or less (57.5% and 3.6%, respectively) vs the cohort with hsTnT levels greater than 19 ng/L (89.2% and 21.8%, respectively). Because of the time difference between presentation and hsTnT measurement, a sensitivity analysis was performed at the single baseline hsTnT before and after 6 hours following symptom onset. While there were no statistical differences in performance, improvements in sensitivity (82%-90.7%) and NPV (97.6%-98.9%) occurred at the cost of specificity (88.8%-86.9%) and PPV (47.4%-42.8%), with longer times.
Because the rest of the world uses a lower URL (14 ng/L) than the United States, an analysis of the diagnostic reclassification occurring between URLs was performed. This analysis found only 2 of 140 CEC-confirmed AMIs having hsTnT values between the 2 URLs at the 3-hour sampling point (eFigure 2 in the Supplement).
Discussion
We report that, in patients presenting to US EDs with suspected ACS, hsTnT levels identified a large proportion at very low 30-day ACE risk. Identifying a low-risk cohort may permit early ED discharge and avoid unnecessary hospitalization. Rapid turnaround of low-risk patients could translate into a reduction in ED volumes, which would benefit patients (shorter waiting times, increased satisfaction, improved outcomes, and saved costs), clinicians (decreased diagnostic ambiguity and medicolegal burden), and hospitals (by providing cost-saving benefits).19,20,21
In this analysis, we used a hsTnT URL cutoff level of 19 ng/L. Derived from a healthy cohort, it represents the 99th percentile in the United States, consistent with the Third Universal Definition of AMI.13 This cutpoint exceeds the 14 ng/L used globally and may be explained by geographically dependent cardiovascular risk.22,23 When applied in clinical practice, the clinician must consider the similarities of their population to that used for the derivation of this cutpoint because the 99th percentile is entirely dependent on the reference study population. We also evaluated sex-specific URLs (14 ng/L for women and 22 ng/L for men) and found diagnostic performance was largely unchanged, in contrast to a prior report with high-sensitive troponin I reporting a doubling of the female AMI rate with sex-specific URLs.24,25
Based on the 19 ng/L cutoff and compared with a CEC-adjudicated clinical diagnosis, the hsTnT assay had excellent sensitivity and NPV for the diagnosis of AMI and 30-day ACE. Values for sensitivity and NPV at the 3-hour sampling point exceeded 99%, such that a 3-hour point may be adequate for the early rule out of AMI using the 99th percentile URL. We do note that the high sensitivity and NPV were achieved at the cost of a lower specificity and PPV.
Importantly, we demonstrate a very low rate of false-negative results, associated with only limited clinical consequences. None of the seven 3-hour time false-negative results were associated with AMI or death in the subsequent 30 days. We also evaluated the false-negative results between 0 and 3 hours to determine whether the magnitude of a serial hsTnT change (ie, the Δ) could be predictive. Of the 8 false-negative results, 6 did not have a hsTnT change greater than 22 ng/L, thus limiting the clinically utility of applying a serial change (Δ).
An ACS diagnostic strategy with NPV of 99.3% for 30-day ACE is clinically useful and acceptable. In this cohort, the NPV for 30-day ACE exceeded 99% in all subgroups with hsTnT levels of 19 ng/L or less at 0 and 3 hours. A rule-out strategy based on a single hsTnT at presentation and using lower cutoffs has been proposed by others.26,27 However, in our study, a single hsTnT level of 19 ng/L or less is probably inadequate because it provided an AMI NPV of only 98.2% at baseline presentation. Conversely, use of the LoQ cutoff (6 ng/L) may represent a potential option because it demonstrated an AMI NPV of 99.4%.
The median time from symptom onset to ED presentation was 2.9 hours, while time from symptom onset to baseline hsTnT was 5.8 hours. Clinicians should consider that the performance characteristics of hsTnT may not be duplicated at earlier times.
Limitations
This study has several limitations in the clinical application of its results. First, as an observational analysis with the results blinded to the clinician, no clinical decision making occurred as a result of the hsTnT results, although fewer patients were hospitalized in the rule-out cohort (57.5%) vs the rule-in cohort (89.2%). Some patients with a negative hsTnT were hospitalized and may have derived clinical benefit. Ergo, we cannot comment on how knowledge of the hsTnT results may have affected clinical outcomes. Second, patients likely to have non-ACS elevations of hsTnT (eg, recent prior AMI or renal insufficiency) were excluded from enrollment in this trial; thus, the patients enrolled in this study should be considered when making clinical decisions. Third, these data are not applicable to environments that do not use the identical assay because hsTnT and hsTnI testing results are not interchangeable. Fourth, our event rate was low, as is consistent with contemporary ED practice. Fifth, by the constraints of a study, median time to the baseline hsTnT draw was 5.8 hours. While this did not statistically change the performance of the assay, physicians should consider that shorter times between symptoms and hsTnT measurement result are likely to result in a deterioration of NPV. Additionally, we did not use a clinical risk-stratification tool (eg, Emergency Department Assessment of Chest Pain Score [EDACS]; History, ECG, Age, Risk Factors, and Troponin [HEART] Score; or Thrombolysis In Myocardial Infarction [TIMI] Score). This has the advantage of avoiding reliance on subjectively gathered information; however, the potential for risk stratification tools to affect a “one-and-done” single troponin strategy requires additional investigation. Finally, because of the blinded nature of the hsTnT, we cannot present the additional diagnostic effect of hsTnT against standard clinical criteria.
Strengths of the study include that the reference range population was representative of the US population (with the caveat that most of participating hospitals were larger academic centers) and generally in line with National Hospital Ambulatory Medical Care Survey data for patients receiving cardiac biomarker testing in the ED, more than double the size of the International Federation of Clinical Chemistry criteria,28,29 and is relevant to the region where the test is used most. Furthermore, the precision criteria of the assay at the cutoff met the criteria defined in the third universal definition of AMI.13 Finally, we focused on a clinically relevant outcome (NPV) to identify patients potentially safe for early ED discharge.
Conclusions
In this multicenter evaluation, we demonstrated that a 0-hour and 3-hour hsTnT assay using less than 6 ng/L provides a safe and early rule-out for AMI and identified a large cohort of patients with suspected ACS at very low risk of 30-day ACE.
eFigure 1. Distribution of Study Sites in the United States
eFigure 2. The Influence of US 19 ng/L vs Global 14 ng/L URL on AMI Diagnosis at 3-Hour Sampling Point.
References
- 1.CDC . National hospital ambulatory medical care survey: 2010 emergency department summary tables. Washington, DC: Centers for Disease Control and Prevention, Ambulatory and Hospital Care Statistics Branch; 2010. [Google Scholar]
- 2.Ekelund U, Nilsson HJ, Frigyesi A, Torffvit O. Patients with suspected acute coronary syndrome in a university hospital emergency department: an observational study. BMC Emerg Med. 2002;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell AM, Garvey JL, Chandra A, Diercks D, Pollack CV, Kline JA. Prospective multicenter study of quantitative pretest probability assessment to exclude acute coronary syndrome for patients evaluated in emergency department chest pain units. Ann Emerg Med. 2006;47(5):447. [DOI] [PubMed] [Google Scholar]
- 4.Reilly BM, Evans AT, Schaider JJ, Wang Y. Triage of patients with chest pain in the emergency department: a comparative study of physicians’ decisions. Am J Med. 2002;112(2):95-103. [DOI] [PubMed] [Google Scholar]
- 5.Freas GC. Medicolegal aspects of acute myocardial infarction. Emerg Med Clin North Am. 2001;19(2):511-521. [DOI] [PubMed] [Google Scholar]
- 6.Pilote L, Granger C, Armstrong PW, Mark DB, Hlatky MA. Differences in the treatment of myocardial infarction between the United States and Canada: a survey of physicians in the GUSTO trial. Med Care. 1995;33(6):598-610. [DOI] [PubMed] [Google Scholar]
- 7.Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department? a clinical survey. Int J Cardiol. 2013;166(3):752-754. [DOI] [PubMed] [Google Scholar]
- 8.Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091-2098. [DOI] [PubMed] [Google Scholar]
- 9.Christenson J, Innes G, McKnight D, et al. A clinical prediction rule for early discharge of patients with chest pain. Ann Emerg Med. 2006;47(1):1-10. [DOI] [PubMed] [Google Scholar]
- 10.Mair J, Artner-Dworzak E, Lechleitner P, et al. Cardiac troponin T in diagnosis of acute myocardial infarction. Clin Chem. 1991;37(6):845-852. [PubMed] [Google Scholar]
- 11.Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997;337(23):1648-1653. [DOI] [PubMed] [Google Scholar]
- 12.Morrow DA, Cannon CP, Jesse RL, et al. ; National Academy of Clinical Biochemistry . National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. [DOI] [PubMed] [Google Scholar]
- 14.Hickman PE, Lindahl B, Cullen L, Koerbin G, Tate J, Potter JM. Decision limits and the reporting of cardiac troponin: meeting the needs of both the cardiologist and the ED physician. Crit Rev Clin Lab Sci. 2015;52(1):28-44. [DOI] [PubMed] [Google Scholar]
- 15.Lipinski MJ, Baker NC, Escárcega RO, et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: a collaborative meta-analysis. Am Heart J. 2015;169(1):6-16.e6. [DOI] [PubMed] [Google Scholar]
- 16.Pickering JW, Than MP, Cullen L, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin t measurement below the limit of detection: a collaborative meta-analysis. Ann Intern Med. 2017;166(10):715-724. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team . R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2015.
- 18.Hahn GJ, Meeker WQ. Statistical Intervals: A Guide for Practitioners. New York, NY: John Wiley & Sons; 1991. [Google Scholar]
- 19.Pines JM, Pollack CV Jr, Diercks DB, Chang AM, Shofer FS, Hollander JE. The association between emergency department crowding and adverse cardiovascular outcomes in patients with chest pain. Acad Emerg Med. 2009;16(7):617-625. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein SL, Aronsky D, Duseja R, et al. ; Society for Academic Emergency Medicine, Emergency Department Crowding Task Force . The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. [DOI] [PubMed] [Google Scholar]
- 21.Aldridge ES, Rogers IR, Bailey PM, Rogers JR. Emergency department “undercrowding” is associated with decreased waiting times. Emerg Med Australas. 2016;28(3):268-272. [DOI] [PubMed] [Google Scholar]
- 22.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(2):254-261. [DOI] [PubMed] [Google Scholar]
- 23.Saenger AK, Beyrau R, Braun S, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011;412(9-10):748-754. [DOI] [PubMed] [Google Scholar]
- 24.Shah AS, Griffiths M, Lee KK, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah AS, Newby DE, Mills NL. High sensitivity cardiac troponin in patients with chest pain. BMJ. 2013;347:f4222. [DOI] [PubMed] [Google Scholar]
- 26.Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58(13):1332-1339. [DOI] [PubMed] [Google Scholar]
- 27.Zhelev Z, Hyde C, Youngman E, et al. Diagnostic accuracy of single baseline measurement of Elecsys Troponin T high-sensitive assay for diagnosis of acute myocardial infarction in emergency department: systematic review and meta-analysis. BMJ. 2015;350:h15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apple FS, Jaffe AS, Collinson P, et al. ; International Federation of Clinical Chemistry (IFCC) Task Force on Clinical Applications of Cardiac Bio-Markers . IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem. 2015;48(4-5):201-203. [DOI] [PubMed] [Google Scholar]
- 29.Makam AN, Nguyen OK. Use of cardiac biomarker testing in the emergency department. JAMA Intern Med. 2015;175(1):67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Distribution of Study Sites in the United States
eFigure 2. The Influence of US 19 ng/L vs Global 14 ng/L URL on AMI Diagnosis at 3-Hour Sampling Point.