Key Points
Question
Based on data from the United Kingdom Collaborative Trial of Ovarian Cancer Screening, is multimodal screening (MMS) with serum cancer antigen 125 (CA-125) interpreted using a risk algorithm cost-effective?
Findings
Multimodal screening is both more expensive and more effective in reducing ovarian cancer mortality than no screening. After accounting for uncertainty in the parameters, MMS reduced mortality by 15% with an incremental cost-effectiveness ratio ranging from $106 187 to $155 256.
Meaning
Ovarian cancer screening is potentially cost-effective in the United States depending on final significance of mortality reduction and cost of the CA-125 risk algorithm.
Using a Markov simulation model based on the United Kingdom Collaborative Trial of Ovarian Cancer Screening clinical trial, this study examines the cost-effectiveness of multimodal screening for ovarian cancer in the United States and the association of screening with mortality.
Abstract
Importance
The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) is the largest randomized clinical trial to evaluate screening’s impact on ovarian cancer mortality, assigning women to multimodal screening (MMS) with serum cancer antigen 125 (CA-125) interpreted using a risk algorithm. If the MMS screening method is eventually shown to reduce mortality and be cost-effective, then it may be accepted by the medical community as a feasible screening tool.
Objective
To estimate the cost-effectiveness of an MMS screening program in the United States.
Design, Setting, and Participants
A Markov simulation model was constructed using data from UKCTOCS to compare MMS with no screening in the United States. Screening would begin at the age of 50 years for women in the general population. Published estimates of the long-term effect of MMS screening on ovarian cancer mortality and the trial’s published hazard ratios were used to simulate mortality estimates up to 40 years from start of screening. Base-case costs included CA-125, ultrasound, and false-positive work-up results, in addition to a risk algorithm cost estimate of $100. The utility and costs of ovarian cancer treatment were incorporated into the model.
Interventions
Screening strategies varied by costs of the algorithm and treatment for advanced ovarian cancer, rates of screening compliance, ovarian cancer incidence, and extrapolation of ovarian cancer mortality.
Main Outcomes and Measures
Costs, quality-adjusted life-years (QALYs), and mortality reduction of ovarian cancer screening.
Results
Multimodal screening is both more expensive and more effective in reducing ovarian cancer mortality over a lifetime than no screening. After accounting for uncertainty in the underlying parameters, screening women starting at age 50 years with MMS is cost-effective 70% of the time, when decision makers are willing to pay $150 000 per QALY. Screening reduced mortality by 15%, with an incremental cost-effectiveness ratio (ICER) ranging from $106 187 (95% CI, $97 496-$127 793) to $155 256 (95% CI, $150 369-$198 567).
Conclusions and Relevance
Ovarian cancer screening is potentially cost-effective in the United States depending on final significance of mortality reduction and cost of the CA-125 risk algorithm. These results are limited by uncertainty around the effect of screening on ovarian cancer mortality beyond the 11 years of UKCTOCS.
Introduction
Most women with ovarian cancer present with advanced-stage disease, with an estimated 5-year survival rate ranging from 17% to 29%. Because the 5-year relative survival rate for stage I disease is approximately 90%, screening to detect early-stage disease could theoretically reduce mortality. Ovarian cancer screening remains challenging owing to the low prevalence of disease and lack of reliable diagnostics to detect precursor lesions. Although several small studies have reported survival improvement in screen-detected cancers using cancer antigen 125 (CA-125) and transvaginal ultrasound, reduction in overall ovarian cancer mortality is the standard measure of screening success, and requires trials of much larger size.
The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) is the largest randomized clinical trial to evaluate screening’s impact on ovarian cancer mortality. The study randomly assigned 202 638 postmenopausal women to ultrasound-based screening (USS), multimodal screening (MMS) using CA-125, and reflex ultrasound based on the risk of ovarian cancer proprietary algorithm (ROCA), or no screening. After median follow-up of 11.1 years, there was a significant shift to earlier stages with MMS compared with no screening, and a nonsignificant reduction in ovarian cancer mortality in both screening arms (15% for MMS and 11% for USS). Posthoc analysis found significant mortality reduction in later years. The authors postulated that the difference in mortality between the no screening and screening groups would increase with time.
Despite enormous effort by clinicians and researchers, there is currently no evidence-proven screening method for ovarian cancer. If the MMS screening method is eventually shown to reduce mortality and be cost-effective, then it may be accepted by the medical community as a feasible screening tool. In the present analysis, we developed a Markov simulation model to estimate the potential cost-effectiveness of multimodal screening compared with no screening in the United States.
Methods
Model
A Markov simulation model was constructed from a health care sector perspective using data from UKCTOCS to compare MMS with no screening. The primary outcome of the model was cost-effectiveness, calculated using discounted costs and discounted life expectancy. Incremental cost-effectiveness ratios (ICERs) were calculated in 2016 US dollars per quality-adjusted life-year (QALY) saved. In the base-case analysis, we modeled a lifetime horizon and assumed, based on age criteria for UKCTOCS, that women would undergo annual screening between ages 50 to 75 years. Screening would not be available to women with a history of ovarian cancer, increased risk of developing ovarian cancer based on family history/genetic susceptibility, or prior bilateral oophorectomy.
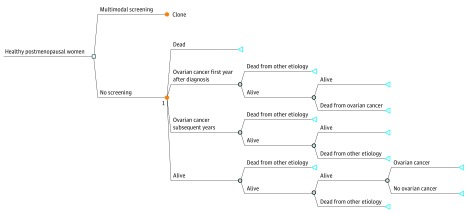
The model was constructed using TreeAge Pro software (version R2, TreeAge Software Inc) and included the following mutually exclusive Markov states including: alive, ovarian cancer first year after diagnosis, ovarian cancer subsequent years, and dead (Figure 1).
Figure 1. Decision Tree With Markov States.
Triangles indicate terminal nodes; blue circles, chance nodes; and orange circles, Marhov nodes.
Clinical Estimates
The model incorporated incidence and mortality from the UKCTOCS trial, which had a median follow up of 11.1 years. To evaluate the long-term cost-effectiveness of screening, it was necessary to extrapolate estimates of ovarian cancer mortality beyond the follow-up period. Ovarian cancer mortality beyond the study period was previously modeled in a cost-effectiveness analysis from a United Kingdom health system perspective, by Kearns et al. To reproduce the mortality rates reported in that analysis, we digitized mortality curves using Engauge software (version 4.1, Engauge Digitizer) and incorporated the resulting annual mortality estimates into our model. In our base-case scenario, we applied the log-normal model of Kearns et al to estimate ovarian cancer mortality in the no screening arm for up to 40 years. While the UKCTOCS statisticians applied a Royston-Parmer model to their analysis, long-term projections by Kearns et al using this model resulted in over 3% of all patients dying from ovarian cancer without screening by 40 years. Based on SEER data indicating 1% lifetime mortality from ovarian cancer in the United States, we believe that Kearns’ log-normal model (1.4% cumulative mortality) provides a more realistic estimate of long-term cancer mortality in a control. As validation, our mortality estimates in the no screening arm over a 40-year period (1.47%) closely approximated those of Kearns et al (1.41%).
To model the uncertainty surrounding the screening effectiveness estimate of the UKCTOCS study, we parameterized the hazard ratio for mortality attributable to screening as a lognormal distribution with a mean of 0.85 (95% CI, 0.7-1.03). The model was run as a probabilistic analysis using 100 000 samples from each modeled distribution. All-cause mortality in both arms was modeled using US Life Tables.
Costs
We incorporated direct medical costs into our model, reported in 2016 US dollars using medical inflation and discounted at 3% annually. To incorporate the costs of treating ovarian cancer, incidence was modeled as 57 per 100 000 woman years using study data. Stage-specific costs of initial, ongoing, and end-of-life care for ovarian cancer were estimated using previously published retrospective analyses of Medicare claims data. The overall cost of a diagnosis in the MMS screening compared with no screening arms incorporated the stage distribution in each arm and the stage-specific lifetime costs; overall costs in each arm were modeled as triangular distributions and sampled over reasonable ranges for base-case Monte Carlo analysis (eTable in the Supplement). We assumed that all patients diagnosed with ovarian cancer would receive treatment.
The estimated annual weighted cost of MMS screening was $39.46, which excluded the cost of the ROCA propriety algorithm. We calculated MMS cost as a weighted average using the probability that an individual in the screening arm would undergo a transvaginal ultrasound, clinical assessment, imaging, consultation with a gynecologic oncologist, or oophorectomy for benign disease based on published data for the initial year of screening (eTable in the Supplement). The number of surgeries based on false-positive results was modeled per numbers reported in UKCTOCS. Costs of CA-125 and benign oophorectomy were estimated from previously published Medicare claims data. Costs of procedures were incorporated using 2016 Medicare reimbursement data: level II ultrasound, outpatient assessment by an internist, consultation fee by a gynecologic oncologist, imaging (computed tomography of the thorax, abdomen, and pelvis), and oophorectomy for benign disease.
We estimated that in addition to the cost of screening, ROCA would cost payers $100. While this estimate is somewhat arbitrary, this would make it comparable in cost to US Food and Drug Administration (FDA)-approved screening tests, such as mammogram and colonoscopy. The current Medicare reimbursement for a mammogram ranges from $82 to $135, compared with approximately $1035 for a colonoscopy, which is recommended every 10 years.
The modeled overall cost of screening and the cost of ROCA were reduced annually to reflect reductions in screening compliance in UKCTOCS. At last follow-up, 47% of patients were compliant with screening. In the base case, we assumed that after 11 years of screening, there would be no further decrease in compliance. These assumptions were tested in sensitivity analyses.
Quality of Life
We modeled previously published quality of life-related utilities to estimate the average utility of ovarian cancer treatment. A literature search was performed to estimate the overall rates of chemotherapy administration at each stage. We assumed that patients who received chemotherapy would have an additive disutility of 0.21. Stage-specific weighted utilities incorporated the disutility of chemotherapy, stage-specific chemotherapy rates and stage distributions observed in each study arm. The utility associated with ovarian cancer treatment was applied to year of diagnosis and returned to the general population baseline (0.84) in subsequent years. The utilities associated with no screening and MMS were 0.54 and 0.51, respectively. In the absence of reported distributions, utilities were parameterized as triangular distributions using clinically reasonable ranges and varied in the base-case Monte Carlo analysis (eTable in the Supplement).
Sensitivity Analysis
We performed 2 major alternative analyses to test assumptions and data surrounding the possible mortality benefit of screening. In the first analysis, to account for the lower ovarian cancer incidence and mortality observed in the United States compared with the UKCTOCS trial population, we imposed a maximum cumulative ovarian cancer mortality of 1% at 40 years on the no screening arm. This constituted a 31% reduction in baseline ovarian cancer mortality compared with that observed in the UKCTOCS trial. In the second alternative analysis, we duplicated the lognormal MMS mortality curve of Kearns et al instead of using the published HR to model ovarian cancer mortality that is associated with screening. This analysis effectively increased the expected mortality benefit of screening.
The following parameters were varied as distributions in the base-case probabilistic analysis: cost of ovarian cancer treatment, utility of ovarian cancer treatment, and degree of mortality reduction afforded by screening. To further account for uncertainty around estimates, 1-way sensitivity analyses were performed using the base-case model with clinical and cost estimates varied over reasonable ranges. Specifically, we varied the cost of the algorithm, cost of treatment for advanced ovarian cancer, quality of life-related utilities, and rates of screening compliance. Finally, value of information methodology previously described was used to estimate the value to society of further research on ovarian cancer screening.
Results
In the base case, MMS screening beginning at age 50 years with an algorithm costing $100 reduced ovarian cancer mortality by 15%. The MMS had an average cost of $3008 and no screening had an average cost of $1633. The discounted difference in life expectancy between strategies was 0.01 years. The MMS was therefore more costly and more effective than no screening, with an ICER of $106 187 per QALY (95% CI, $97 496-$127 793). In acceptability curve analysis, screening was cost-effective in 47% of simulations at a willingness-to-pay threshold of $100 000 per QALY and in 70% of simulations at a willingness to pay of $150 000 per QALY (eFigure in the Supplement).
In the first alternative analysis, the expected mortality from ovarian cancer after 40 years in the absence of screening was adjusted from 1.4% in the base case to 1%, as observed after age 50 years in the US SEER database. Multimodal screening in this scenario resulted in an ICER of $155 256 per QALY (95% CI, $150 369-$198 567).
In the second alternative analysis using the modeled data of Kearns et al to simulate the mortality reduction associated with screening, MMS screening reduced ovarian cancer mortality by 24% and had an ICER of $65 205 per QALY compared with no screening.
Extensive 1-way sensitivity analyses of the base-case model were performed, with the cost of ROCA the only significant variable driving a change in the ICER. In varying the cost of ROCA, the ICER was below $50 000 per QALY if the algorithm costed $25 or less, below $100 000 per QALY if $95 or less, and below $150 000 per QALY if $150 or less. The ICER remained below $100 000 per QALY if the algorithm cost was less than $55 in the first alternative analysis or less than $170 in the second alternative analysis (Figure 2). When varying lifetime costs of treating advanced ovarian cancer between $120 000 and $220 000, there was no impact on overall ICER. Quality of life-related utilities were examined in sensitivity analyses, with minimal effect on cost-effectiveness results. Assuming no quality of life decrement with an ovarian cancer diagnosis, or alternatively, a permanent decrement, the ICER comparing screening with no screening remains near $100 000 per QALY.
Figure 2. One-Way Sensitivity Analysis Adjusting the Cost of Screening Algorithm.
QALY indicates quality-adjusted life-years. Incremental cost-effectiveness ratio comparing the base-case scenario with the 2 alternative scenarios (1% maximum ovarian cancer mortality; extrapolated screening mortality). Willingness-to-pay (WTP) threshold: $100 000/QALY (blue solid line); $150 000 (blue dashed line).
We examined our assumptions regarding compliance with screening. In the base case, we modeled decrements in compliance as observed in UKCTOCS. Assuming that all patients were 100% compliant with annual screening, screening had an ICER of $164 191 per QALY compared with no screening. When alternatively assuming that compliance continued to drop indefinitely by 2% annually, the ICER of screening dropped to $98 859 per QALY compared with no screening.
In a value of information analysis of the base-case model, at a societal willingness to pay (WTP) threshold of $100 000 per QALY, the per-patient expected value of perfect information (EVPI) was $259 and the expected value of partial perfect information (EVPPI) for the mortality hazard ratio was $244 per patient. Estimating that 2.2 million women aged 50 years in the United States are potentially eligible to begin screening annually, the upper limit of value of information for the hazard ratio alone was $536 million annually and greater than $2.5 billion over a 5-year time horizon using a 3% annual discount rate. This means a clinical trial more clearly defining the ovarian cancer mortality effect of screening in a population of postmenopausal women and costing less than $2.5 billion would be of value to society.
Discussion
Our model suggests that multimodal screening using the ROCA test may be a potentially cost-effective screening option for ovarian cancer in the United States. However, our analysis demonstrates that the cost-effectiveness of screening is highly dependent on the overall cost of ROCA, modeled as $100 in our base case.
The ROCA test was briefly marketed in the United States at $295 but is not currently available. The manufacturer stopped sales of the test following an FDA safety alert reporting that “available data do not demonstrate that currently available ovarian cancer screening tests are accurate and reliable in screening asymptomatic women for early ovarian cancer.” The US Preventive Services Task Force does not currently recommend screening for ovarian cancer. Should UKCTOCS follow-up reveal a significant mortality reduction with MMS, US screening guidelines may ultimately change.
Since the 1960s, $50 000 per QALY has been the medical community’s value threshold to define cost-effective care. Recently, experts in the field of cost-effectiveness analyses agree that using a cutoff of $100 000 or $150 000 per QALY is more reflective of the current per-capita income and reasonable in the context of our current state of resources. Based on our model’s assumptions, an algorithm cost of $295 would result in an MMS ICER of $258 609 per QALY and would be cost-effective in 0.06% of Monte-Carlo simulations at a willingness-to-pay threshold of $100 000 per QALY. Therefore, if the previously marketed price of the algorithm were borne by third party payers, screening could not be considered cost-effective.
Our analysis has inconsistencies with 2 previously published studies evaluating the cost-effectiveness of MMS in the United Kingdom. In the first study, Kearns et al reported that MMS cost £8864 per QALY (approximately $11 099 per QALY) compared with no screening. This lower ICER is explained by differences in costs and in assumptions about the mortality benefit of screening. Kearns et al did not incorporate any algorithm cost, as this was assumed to be supported by both “public and charitable funds.” In addition, their base-case model incorporated Royston-Parmar mortality estimates, which amplify the mortality benefit of screening compared with the log-normal mortality curves. We used the most conservative ovarian cancer mortality estimates of Kearns et al, used the hazard ratio reported in UKCTOCS and its confidence intervals to model base-case screening-associated mortality, and performed an additional analysis in which we lowered expected baseline mortality in the no screening arm to 1%. When alternatively using the extrapolated mortality estimates of Kearns for the screening arm, the apparent mortality benefit and cost-effectiveness of screening are higher, with ICERs consistently less than $100 000 per QALY.
A second, newer cost-effectiveness analysis by the primary UKCTOCS team extrapolates posttrial mortality over a 25-year period and reports that MMS compared with no screening has an ICER of £35 544 ($46 000) per life-year gained, from a National Health Service (NHS) perspective. This favorable ICER is likely owing to the authors’ primary screening cost estimate of £20 for CA125 plus ROCA combined, estimated using an observed 8-fold difference between private charges and NHS payments in the UK. In contrast, we estimated the US cost of CA125 ($28) plus ROCA ($100) to be $128, more conservatively assuming that US payers will bear considerable additional cost for the screening algorithm itself. If we reduce our estimate of CA125 plus ROCA cost to $65 (approximately £50), the ICER for MMS is around $30 000 per QALY. The Menon model is otherwise similar to ours in estimating effects; our model estimates a 0.01-year difference in discounted life expectancy over a lifetime, compared with 0.01 years difference over a 25-year horizon in the Menon model.
We estimate that ovarian cancer screening beginning at age 50 years could potentially reduce ovarian cancer mortality by approximately 15%. This is a conservative estimate compared with the 28% mortality reduction seen in the MMS screening arm after 7 years. In ovarian cancer screening trials, mortality reduction is often difficult to evaluate owing to the low disease prevalence and necessary length of follow-up. The UKCTOCS authors noted that mortality reduction was 8% in years 0 to 7 and 28% in years 7 to 14, reflecting a potential delayed effect of screening. In contrast, clinical trials of screening tests for colon cancer showed statistically significant mortality reduction within 11 years. There is inherent uncertainty in modeling that is based on extrapolated mortality expectations; only prolonged follow-up will determine whether the mortality benefit observed in the later years of UKCTOCS persists.
Limitations
Our model has a number of limitations, most notably the uncertainty inherent in modeling long-term mortality. Second, our target population includes women who are insured by either a commercial payer or Medicare. Private insurers may have higher reimbursement rates, resulting in a higher ICER than calculated in our model. Third, although we incorporated utility estimates for ovarian cancer outcomes, we did not include the potential utility decrements associated with screening itself and false-positive results—these might increase the estimated ICER for screening. Finally, a more robust cost-effectiveness model schematic would also incorporate the natural history of ovarian, fallopian tube, and primary peritoneal cancer. This would also allow us to incorporate the effects on screening of other ovarian cancer mortality reduction interventions, such as the impact of rising rates of opportunistic risk-reducing salpingectomy on the future effectiveness and cost-effectiveness of postmenopausal screening.
Conclusions
Our model suggests that ovarian cancer screening is potentially cost-effective in the United States using the multimodality screening method. Screening has the potential to reduce ovarian cancer mortality by approximately 15% if begun at age 50 years. Cost-effectiveness estimates are less favorable when lower baseline mortality similar to that observed in the US population is assumed. These results and conclusions are entirely dependent on the results of UKCTOCS follow up analysis. If screening is shown to significantly and persistently reduce mortality on longer-term follow-up, and the algorithm’s cost is similar to that of other screening tools, such as the mammogram, ovarian cancer screening may be cost-effective compared with no screening. We eagerly await the results of further follow-up to more fully evaluate the impact of screening on mortality reduction. Additional data will allow for a more robust model that will enable researchers to better quantify the harms and benefits of ovarian cancer screening.
eFigure. Cost-effectiveness acceptability curves, multi-modal screening
eTable. Model parameters
References
- 1.Are W. The Key Statistics About Ovarian Cancer? Cancer A-Z 2017; https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed April 5, 2017.
- 2.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol. 2015;126(3):491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih IeM. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198(4):351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Nagell JR Jr, DePriest PD, Ueland FR, et al. Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer. 2007;109(9):1887-1896. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs IJ, Skates SJ, MacDonald N, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353(9160):1207-1210. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327-340. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs IJ, Parmar M, Skates SJ, Menon U. Ovarian cancer screening: UKCTOCS trial-authors’ reply. Lancet. 2016;387(10038):2603-2604. [DOI] [PubMed] [Google Scholar]
- 9.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093-1103. [DOI] [PubMed] [Google Scholar]
- 10.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. [DOI] [PubMed] [Google Scholar]
- 11.Kearns B, Chilcott J, Whyte S, Preston L, Sadler S. Cost-effectiveness of screening for ovarian cancer amongst postmenopausal women: a model-based economic evaluation. BMC Med. 2016;14(1):200-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchel M. Engauge Digitizer: Extracts data points from images of graphs. http://markummitchell.github.io/engauge-digitizer. Accessed May 9, 2017.
- 13.Overview of the SEER Program https://seer.cancer.gov/about/overview.html. Accessed May 1, 2017.
- 14.Havrilesky LJ, Sanders GD, Kulasingam S, et al. Development of an ovarian cancer screening decision model that incorporates disease heterogeneity: implications for potential mortality reduction. Cancer. 2011;117(3):545-553. [DOI] [PubMed] [Google Scholar]
- 15.Havrilesky LJ, Sanders GD, Kulasingam S, Myers ER. Reducing ovarian cancer mortality through screening: Is it possible, and can we afford it? Gynecol Oncol. 2008;111(2):179-187. [DOI] [PubMed] [Google Scholar]
- 16.Arias E, Heron M, Xu J. United States life tables, 2013. Natl Vital Stat Rep. 2017;66(3):1-64. [PubMed] [Google Scholar]
- 17.Urban RR, He H, Alfonso-Cristancho R, Hardesty MM, Goff BA. The cost of initial care for medicare patients with advanced ovarian cancer. J Natl Compr Canc Netw. 2016;14(4):429-437. [DOI] [PubMed] [Google Scholar]
- 18.Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2006-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esselen KM, Cronin AM, Bixel K, et al. Use of CA-125 tests and computed tomographic scans for surveillance in ovarian cancer. JAMA Oncol. 2016;2(11):1427-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. http://www.cms.hhs.gov/. Accessed April 5, 2017.
- 21.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015;220(5):940-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee S, Chen L, Tergas AI, et al. Utilization and outcomes of chemotherapy in women with intermediate-risk, early-stage ovarian cancer. Obstet Gynecol. 2016;127(6):992-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havrilesky LJ, Chino JP, Myers ER. How much is another randomized trial of lymph node dissection in endometrial cancer worth? A value of information analysis. Gynecol Oncol. 2013;131(1):140-146. [DOI] [PubMed] [Google Scholar]
- 26.Administration USFaD Ovarian Cancer Screening Tests: Safety Communication. 2016; https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm519540.htm. Accessed May 8, 2017.
- 27.Moyer VA; U.S. Preventive Services Task Force . Screening for ovarian cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900-904. [DOI] [PubMed] [Google Scholar]
- 28.Menon U, McGuire AJ, Raikou M, et al. The cost-effectiveness of screening for ovarian cancer: results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Br J Cancer. 2017;117(5):619-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkin WS, Edwards R, Kralj-Hans I, et al. ; UK Flexible Sigmoidoscopy Trial Investigators . Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624-1633. [DOI] [PubMed] [Google Scholar]
- 30.Robinson J. Hospitals respond to Medicare payment shortfalls by both shifting costs and cutting them, based on market concentration. Health Aff (Millwood). 2011;30(7):1265-1271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Cost-effectiveness acceptability curves, multi-modal screening
eTable. Model parameters