Key Points
Question
Is high-risk plaque, as detected by coronary computed tomographic angiography, associated with incident major adverse cardiovascular events?
Findings
In this substudy of the PROMISE trial, we evaluated the presence of high-risk plaque in 4415 patients. High-risk plaque was present in 676 patients (15%) and carried a 70% increased risk of major adverse cardiovascular events independent of cardiovascular risk factors and obstructive coronary artery disease; detection was most useful in younger patients, women, and those with nonobstructive coronary artery disease.
Meaning
The findings suggest the potential significance of detecting high-risk plaques in patients with stable ischemic heart disease; however, the low absolute rates of major adverse cardiovascular events and low positive predictive value of high-risk plaque detection might limit its clinical applicability.
This nested observational cohort study seeks to determine whether high-risk plaque detected by coronary computed tomographic angiography was independently associated with major adverse cardiovascular events.
Abstract
Importance
Coronary computed tomographic angiography (coronary CTA) can characterize coronary artery disease, including high-risk plaque. A noninvasive method of identifying high-risk plaque before major adverse cardiovascular events (MACE) could provide practice-changing optimizations in coronary artery disease care.
Objective
To determine whether high-risk plaque detected by coronary CTA was associated with incident MACE independently of significant stenosis (SS) and cardiovascular risk factors.
Design, Setting, and Participants
This prespecified nested observational cohort study was part of the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial. All stable, symptomatic outpatients in this trial who required noninvasive cardiovascular testing and received coronary CTA were included and followed up for a median of 25 months.
Exposures
Core laboratory assessment of coronary CTA for SS and high-risk plaque (eg, positive remodeling, low computed tomographic attenuation, or napkin-ring sign).
Main Outcomes and Measures
The primary end point was an adjudicated composite of MACE (defined as death, myocardial infarction, or unstable angina).
Results
The study included 4415 patients, of whom 2296 (52%) were women, with a mean age of 60.5 years, a median atherosclerotic cardiovascular disease (ASCVD) risk score of 11, and a MACE rate of 3% (131 events). A total of 676 patients (15.3%) had high-risk plaques, and 276 (6.3%) had SS. The presence of high-risk plaque was associated with a higher MACE rate (6.4% vs 2.4%; hazard ratio, 2.73; 95% CI, 1.89-3.93). This association persisted after adjustment for ASCVD risk score and SS (adjusted hazard ratio [aHR], 1.72; 95% CI, 1.13-2.62). Adding high-risk plaque to the ASCVD risk score and SS assessment led to a significant continuous net reclassification improvement (0.34; 95% CI, 0.02-0.51). Presence of high-risk plaque increased MACE risk among patients with nonobstructive coronary artery disease relative to patients without high-risk plaque (aHR, 4.31 vs 2.64; 95% CI, 2.25-8.26 vs 1.49-4.69). There were no significant differences in MACE in patients with SS and high-risk plaque as opposed to those with SS but not high-risk plaque (aHR, 8.68 vs. 9.31; 95% CI, 4.25-17.73 vs 4.21-20.61). High-risk plaque was a stronger predictor of MACE in women (aHR, 2.41; 95% CI, 1.25-4.64) vs men (aHR, 1.40; 95% CI, 0.81-2.39) and younger patients (aHR, 2.33; 95% CI, 1.20-4.51) vs older ones (aHR, 1.36; 95% CI, 0.77-2.39).
Conclusions and Relevance
High-risk plaque found by coronary CTA was associated with a future MACE in a large US population of outpatients with stable chest pain. High-risk plaque may be an additional risk stratification tool, especially in patients with nonobstructive coronary artery disease, younger patients, and women. The importance of findings is limited by low absolute MACE rates and low positive predictive value of high-risk plaque.
Trial Registration
clinicaltrials.gov Indentifier: NCT01174550
Introduction
Evaluation of stable chest pain often requires diagnostic testing with the goals of detecting obstructive coronary artery disease (CAD) and assessing the risk of a future major adverse cardiovascular event (MACE), defined as death, myocardial infarction, or unstable angina. Coronary computed tomography angiography (CTA) has developed into a reliable, noninvasive technique and a viable alternative to functional testing in outpatients with new onset of symptoms suggestive of obstructive CAD. The predictive value of coronary CTA for MACE is similar to that of conventional functional testing. The traditional assessment of coronary CTA for obstructive CAD provides prognostic information for MACE. However, more than half of MACEs occur in patients with nonobstructive CAD; coronary plaque detected by coronary CTA provides additional prognostic value in this group. The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) trial, which studied coronary plaques using intravascular ultrasound in survivors of acute coronary syndrome (ACS), found that plaque characteristics (eg, a thin-cap fibroatheroma and a plaque burden >70%) were associated with 2.5-fold to 5-fold increased risk of repeat events.
Previous research using tissue samples and intravascular imaging has defined characteristics of coronary plaques (eg, a large necrotic lipid pool, a thin fibrous cap, and a large plaque burden) that are associated with sudden cardiac death and ACS. Coronary CTA can characterize coronary plaques, including the detection of high-risk features such as positive remodeling, low computed tomography (CT) attenuation, or napkin-ring sign. High-risk plaques were associated with advanced atherosclerotic plaque, thin-cap fibroatheroma, and large lipid pools, as well as with culprit lesions of ACS. Recent studies in Japanese patients with stable chest pain suggested that the presence of high-risk plaque on coronary CTA was associated with 5-fold to 8-fold increased risk of MACE. We performed standardized core laboratory assessment of coronary CTA in a large contemporary North American population of outpatients undergoing evaluation for new-onset stable chest pain in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial (clinicaltrials.gov No. NCT01174550). We tested the hypothesis that the presence of high-risk plaque was associated with incident MACE independent of clinically significant stenosis (SS) and cardiovascular risk factors.
Methods
Study Design and Population
The PROMISE trial was a pragmatic comparative effectiveness trial that enrolled 10 003 patients at 193 sites in North America between July 2010 and September 2013. Details regarding the PROMISE study have been described elsewhere. The study enrolled stable symptomatic outpatients without known CAD who required noninvasive cardiovascular testing. Participants were randomly assigned to either the coronary CTA or functional testing group. Patient demographics and cardiovascular risk factors were documented at the time of enrollment. Atherosclerotic cardiovascular disease (ASCVD) and Framingham Heart Study risk scores were calculated. After randomization, follow-up visits were performed at 60 days at the study sites and centrally at 6-month intervals for a minimum of 1 year. Local or central institutional review boards approved the study, and all participants provided written informed consent.
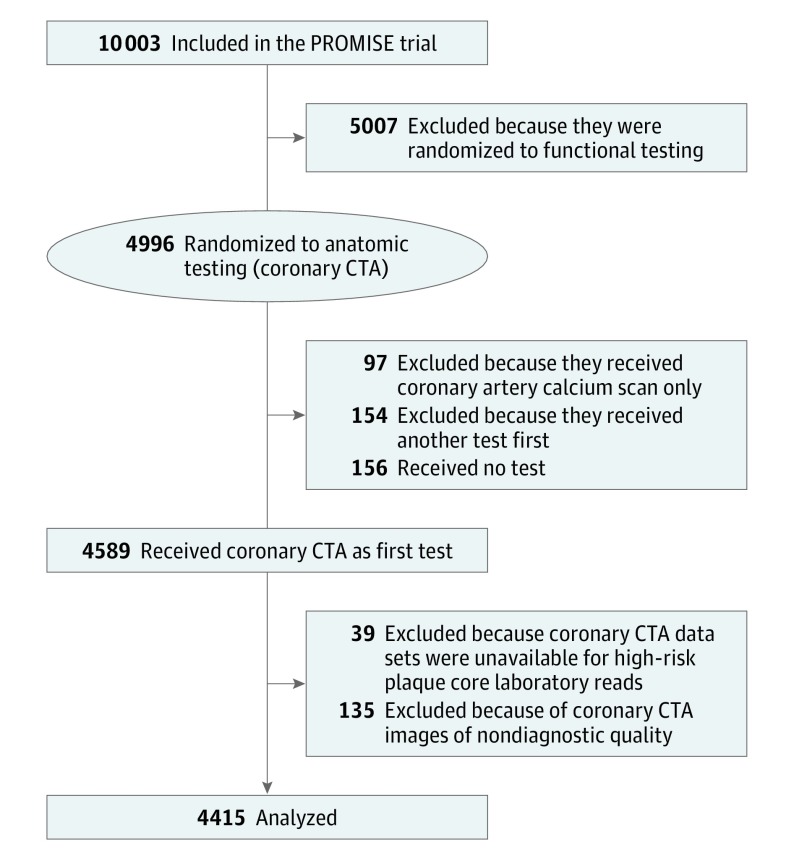
In our cohort study nested in the original PROMISE trial, we included all patients who were randomized to the coronary CTA arm of the trial and who received the initial diagnostic test as randomized. (Eligible participants were randomly assigned in a ratio of 1:1 to either the anatomical or functional diagnostic testing arm of the trial. A computer-generated permuted block randomization scheme with stratification by clinical site was used.) We excluded patients who received other tests as their first test, did not undergo any diagnostic test, or received noncontrast CT testing only, as well as those for whom coronary CTA data sets were unavailable or were of nondiagnostic quality (Figure 1).
Figure 1. Patient Inclusion and Exclusion.
This flowchart outlines patient selection. CTA indicates computed tomography angiography; PROMISE, the Prospective Multicenter Imaging Study for Evaluation of Chest Pain.
Coronary CTA Analysis
Coronary CTA images were acquired using either retrospectively electrocardiogram-gated or prospectively electrocardiogram-triggered protocols according to guidelines and local protocols using scanners from 4 vendors (Siemens, General Electric, Toshiba, and Philips) and different generations (64-row, 128-row, 256-row, 320-row, and dual source). The images were transferred to the core laboratory for the analysis at a cardiac image-viewing workstation (TeraRecon). Six readers with level 3 training in coronary CTA were randomly assigned data sets for analysis. A further 50 randomly selected coronary CTA data sets were analyzed by all 6 readers to determine interobserver agreement (high-risk plaque: κ = 0.56; ≥70% stenosis or left main ≥50% stenosis: κ = 0.69). The coronary CTA analysis was performed per coronary segment. Coronary segments with nondiagnostic image quality (1602 of 79 470; 2.0%) were treated as noninformative for the analysis.
Each evaluable coronary segment was assessed for the presence of stenosis. The severity of stenosis was quantified by visual estimation into 5 categories (0%, 1%-29%, 30%-49%, 50%-69%, or ≥70% stenosis). We defined SS as the presence of 70% stenosis or greater in any vessel or 50% stenosis or greater in the left main coronary artery. We performed a sensitivity analysis using the definition of 50% stenosis or greater in any coronary artery as SS. Nonobstructive CAD was defined as the presence of coronary plaque with no SS.
For each evaluable coronary segment, we noted the presence of plaque (calcified, noncalcified, or partially calcified; eFigure 1 in the Supplement). Each coronary segment with plaque was evaluated for the presence of high-risk plaque. High-risk plaque features were defined as positive remodeling (remodeling index, >1.1), low CT attenuation (mean CT number <30 HU), or napkin-ring sign (a ringlike peripheral higher attenuation with central low CT attenuation; eFigure 1 in the Supplement). Each patient was classified as having high-risk plaque if at least 1 high-risk plaque feature was present. We also performed sensitivity analyses using the definition of high-risk plaque as the presence of both positive remodeling and low CT attenuation, the presence of positive remodeling or low CT attenuation, and the presence of any 2 or 3 high-risk plaque features.
Study Outcomes
The primary end point was a composite of time to MACE, the definition of which included death from any cause, myocardial infarction, and hospitalization for unstable angina. We performed a sensitivity analysis with MACE defined as the secondary composite end point of cardiovascular death, myocardial infarction, or hospitalization for unstable angina, and as the tertiary composite end point of death or myocardial infarction. An independent clinical events committee adjudicated all end point events in a blinded fashion on the basis of standard, prospectively determined definitions, as described previously.
Statistical Analysis
Continuous variables are presented as mean (SD) or median (interquartile range [IQR]). Categorical variables are presented as frequencies and percentages. Comparisons between groups were performed with the use of a 2-sample t test or a Wilcoxon rank-sum test for continuous variables and with a Fisher exact test for categorical variables. Interobserver agreement was calculated using the κ statistic. The Cox proportional hazards model was used to assess the relationship of the presence of high-risk plaque and time to the first clinical event (or censoring) for the composite end point. To appropriately account for heterogeneity among the participants, analyses were adjusted for a prespecified set of baseline covariates, including SS and ASCVD risk score or Framingham Heart Study risk score. Adjusted hazard ratios (aHRs) and 95% confidence intervals were computed using Cox models to characterize the relative risks of patients with high-risk plaque vs those without high-risk plaque. Cumulative event rates based on test results were computed using the Kaplan-Meier method. The C index (also known as the concordance statistic) and continuous net reclassification improvement was calculated using the risk prediction (incrisk) package in Stata (version 14.2, StataCorp). Bootstrap standard errors and 95% CIs were calculated using 10 000 bootstrap samples. The calibration of the model containing high-risk plaque, SS, and ASCVD risk score was tested using Grønnesby and Borgan test for goodness of fit. All P values were 2-sided, and were considered significant at the nominal .05 level. All statistical analyses were performed using Stata 14.2 (StataCorp).
Results
Study Population
The baseline characteristics of 4415 patients included in our study are summarized in Table 1, stratified by the presence of high-risk plaque. Patients with high-risk plaque, compared with those without the condition, were more often men (428 of 676, or 63.3%, vs 1704 of 3739, or 45.6%, P < .001), more often smokers (420 of 676, or 62.1%, vs 1836 of 3738, or 49.1%; P < .001), and had mean (SD) body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 29.4 (5.1) vs 30.6 (6.1) (P < .001). No other individual risk factors were associated with the presence of high-risk plaque. The mean (SD) number of risk factors was higher in patients with high-risk plaque (2.49 [1.10]) compared with patients without high-risk plaque (2.33 [1.07]). Patients with high-risk plaque compared with those without high-risk plaque had elevated median (IQR) ASCVD risk scores (13.9 [8.3-23.2] vs 10.5 [5.8-18.4]), and elevated median (IQR) Framingham Heart Study risk scores (21.6 [13.8-34.0] vs 16.2 [10.0-26.3]).
Table 1. Baseline Characteristics, Stratified by the Presence of High-Risk Plaque (HRP).
| Variables | No. (%) | P Value | ||
|---|---|---|---|---|
| All Patients (N = 4415) |
No HRP (n = 3739) |
Any HRP (n = 676) |
||
| Age, mean (SD), y | 60.5 (8.2) | 60.4 (8.2) | 61.0 (8.2) | .07 |
| Male | 2132 (48.3) | 1704 (45.6) | 428 (63.3) | <.001 |
| Race/ethnicity | ||||
| Non-Hispanic white | 3401/4370 (77.8) | 2874/3704 (77.6) | 527/666 (79.1) | .39 |
| Asian | 131/4370 (3.0) | 110/3704 (3.0) | 21/666 (3.2) | .81 |
| Non-Hispanic black | 444/4370 (10.2) | 393/3704 (10.6) | 51/666 (7.7) | .02 |
| Hispanic | 316/4370 (7.2) | 268/3704 (7.2) | 48/666 (7.2) | >.99 |
| Cardiovascular risk factors | ||||
| Body mass index, mean (SD) | 30.4 (5.9) | 30.6 (6.1) | 29.4 (5.1) | <.001 |
| Hypertension | 2829 (64.1) | 2406 (64.4) | 423 (62.6) | .38 |
| Diabetes | 908 (20.6) | 762 (20.4) | 146 (21.6) | .47 |
| Dyslipidemia | 2965 (67.2) | 2514 (67.2) | 451 (66.7) | .79 |
| Family history of premature CAD, No./total No. (%) | 1441/4401 (32.7) | 1201/3730 (32.2) | 240/671 (35.8) | .07 |
| Peripheral arterial or cerebrovascular disease, No./total No. (%) | 221/4414 (5.0) | 177/3739 (4.7) | 44/675 (6.5) | .06 |
| CAD risk equivalent | 1066 (24.1) | 888 (23.8) | 178 (26.3) | .16 |
| Metabolic syndrome | 1630 (36.9) | 1384 (37.0) | 246 (36.4) | .80 |
| Current or past tobacco use, No./total No. (%) | 2256/4414 (51.2) | 1836/3738 (49.1) | 420/676 (62.1) | <.001 |
| Sedentary lifestyle, No./total No. (%) | 2126/4406 (48.3) | 1925/3730 (51.6) | 355/676 (52.5) | .68 |
| History of depression | 868 (19.7) | 726 (19.4) | 142 (21.0) | .34 |
| Risk burden | ||||
| No risk factors | 114 (2.6) | 99 (2.7) | 15 (2.2) | .60 |
| No. of risk factors per patient, mean (SD) | 2.36 (1.08) | 2.33 (1.07) | 2.49 (1.10) | <.001 |
| Combined Diamond and Forrester and Coronary Artery Surgery Study risk score, mean (SD) | 53.2 (21.3) | 52.3 (21.2) | 57.9 (20.9) | <.001 |
| Framingham risk score categories, No./total No. (%) | <.001 | |||
| Low risk (<6%) | 289/4408 (6.6) | 270/3736 (7.2) | 19/672 (2.8) | NA |
| Intermediate risk (6%-20%) | 2325/4408 (52.8) | 2028/3736 (54.3) | 297/672 (44.2) | NA |
| High risk (>20%) | 1794/4408 (40.7) | 1438/3736 (38.5) | 356/672 (53.0) | NA |
| Framingham risk score, median (IQR) | 16.9 (10.5-27.8) | 16.2 (10.0-26.3) | 21.6 (13.8-34.0) | <.001 |
| ASCVD risk, No./total No. (%) | <.001 | |||
| Low risk (<7.5%) | 1450/4368 (33.2) | 1317/3706 (35.5) | 133/662 (20.1) | NA |
| Elevated risk (≥7.5%) | 2918/4368 (66.8) | 2389/3706 (64.5) | 529/662 (79.9) | NA |
| ASCVD risk, median (IQR) | 11.0 (6.1-19.1) | 10.5 (5.8-18.4) | 13.9 (8.3-23.2) | <.001 |
| Baseline medications, No./total No. (%) | ||||
| β-Blocker | 1041/4223 (24.7) | 898/3573 (25.1) | 143/650 (22.0) | .09 |
| ACE inhibitor or ARB | 1811/4223 (42.9) | 1554/3573 (43.5) | 257/650 (39.5) | .06 |
| Statin | 1926/4223 (45.6) | 1615/3573 (45.2) | 311/650 (47.9) | .22 |
| Aspirin | 1905/4223 (45.1) | 1589/3573 (44.5) | 316/650 (48.6) | .05 |
| Primary presenting symptom, No./total No. (%) | ||||
| Chest pain | 3247/4412 (73.6) | 2734/3736 (73.2) | 513/676 (75.9) | .16 |
| Dyspnea on exertion | 625/4412 (14.2) | 544/3736 (14.6) | 81/676 (12.0) | .08 |
| Other | 540/4412 (12.2) | 458/3736 (12.3) | 82/676 (12.1) | >.99 |
| Type of angina | .57 | |||
| Typical | 503 (11.4) | 418 (11.2) | 85 (12.6) | NA |
| Atypical | 3440 (77.9) | 2920 (78.1) | 520 (76.9) | NA |
| Nonanginal pain | 472 (10.7) | 401 (10.7) | 71 (10.5) | NA |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; HRP, high-risk plaque; IQR, interquartile range; NA, not applicable.
High-Risk Plaque Prevalence
The presence of high-risk plaque was detected in 676 patients (15.3%). Of these patients, 628 (92.9%) had positive remodeling; 222 (32.8%) had low CT attenuation; 169 (25.0%) had napkin-ring sign; 179 (26.5%) had both positive remodeling and low CT attenuation; and 671 (99.3%) had at least 1 of the high-risk plaque features of positive remodeling and low CT attenuation plaque. The prevalence of high-risk plaque in subgroups was 10.9% for women vs 20.1% for men (P < .001), 13.7% for those under median age vs 16.9% for those at or above median age (P = .004); 17.1% for those with a BMI below the median value of 30.4 vs 13.6% for those with a BMI equal to or greater than the median (P = .001); 15.1% for those without diabetes vs 16.1% for those with diabetes (P = .47); 11.9% for patients reporting no tobacco use vs 18.6% for those reporting current or past tobacco use (P < .001); and 14.4% for non-Hispanic white people vs 15.5% for people of other races (P = .39).
Association of High-Risk Plaque With Nonobstructive and Obstructive CAD
The prevalence of high-risk plaque gradually increased with the increasing degree of stenosis (Table 2): no stenosis (0 of 1525 patients; 0%), 1% to 29% stenosis (186 of 1391 patients; 13.4%), 30% to 49% stenosis (196 of 884 patients; 22.2%), 50% to 69% (125 of 345 patients; 36.2%), and stenosis equal to or greater than 70% (169 of 270 patients; 62.6%). The presence of both high-risk plaque and SS was observed in 171 of the 4415 patients (3.9%). High-risk plaque without SS was present in 505 patients (11.4%), and SS without high-risk plaque in 105 patients (2.4%).
Table 2. Coronary Atherosclerosis Characteristics, Stratified by the Presence of High-Risk Plaque (HRP).
| Variables | No. (%) | P Value | ||
|---|---|---|---|---|
| All Patients (N = 4415) |
No HRP (n = 3739) |
Any HRP (n = 676) |
||
| Plaque observed by coronary CTA | ||||
| None | 1525 (34.5) | 1525 (40.8) | 0 (0.0) | NA |
| Calcified | 361 (8.2) | 357 (9.6) | 4 (0.6) | <.001 |
| Noncalcified | 162 (3.7) | 127 (3.4) | 35 (5.2) | .03 |
| Partially calcified | 2367 (53.6) | 1730 (46.3) | 637 (94.2) | <.001 |
| Stenosis observed by coronary CTA | ||||
| No stenosis | 1525 (34.5) | 1525 (40.8) | 0 (0.0) | NA |
| 1%-29% | 1391 (31.5) | 1205 (32.2) | 186 (27.5) | .02 |
| 30%-49% | 884 (20.0) | 688 (18.4) | 196 (29.0) | <.001 |
| 50%-69% | 345 (7.8) | 220 (5.9) | 125 (18.5) | <.001 |
| 70%-100% | 270 (6.1) | 101 (2.7) | 169 (25.0) | <.001 |
| Clinical CAD categories | ||||
| Stenosis ≥50% | 615 (13.9) | 321 (8.6) | 294 (43.5) | <.001 |
| Stenosis ≥70% or LM ≥50% | 276 (6.3) | 105 (2.8) | 171 (25.3) | <.001 |
Abbreviations: CAD, coronary artery disease; CTA, computed tomographic angiography; LM, left main coronary artery; NA, not applicable.
Association of High-Risk Plaque With MACE
The primary outcome of composite MACE occurred in 131 of 4415 patients (total incidence, 3.0%). This included death from all causes (60 patients; 1.4%), cardiovascular death (31 patients; 0.7%), nonfatal myocardial infarction (24 patients; 0.5%), and hospitalization for unstable angina (47 patients; 1.1%) during a median (IQR) follow-up of 25 (18-34) months. Patients with high-risk plaque had an increased risk of MACE (43 of 676 patients, 6.4%) compared with patients without high-risk plaque (88 of 3739 patients, 2.4%; unadjusted HR, 2.73; 95% CI, 1.89-3.93) (eTable 1 in the Supplement). The sensitivity analysis using alternative definitions of high-risk plaque (eg, the presence of both positive remodeling and low CT attenuation, the presence of positive remodeling or low CT attenuation, or the presence of any 2 or 3 high-risk plaque features) rendered similar results (eTable 1 in the Supplement).
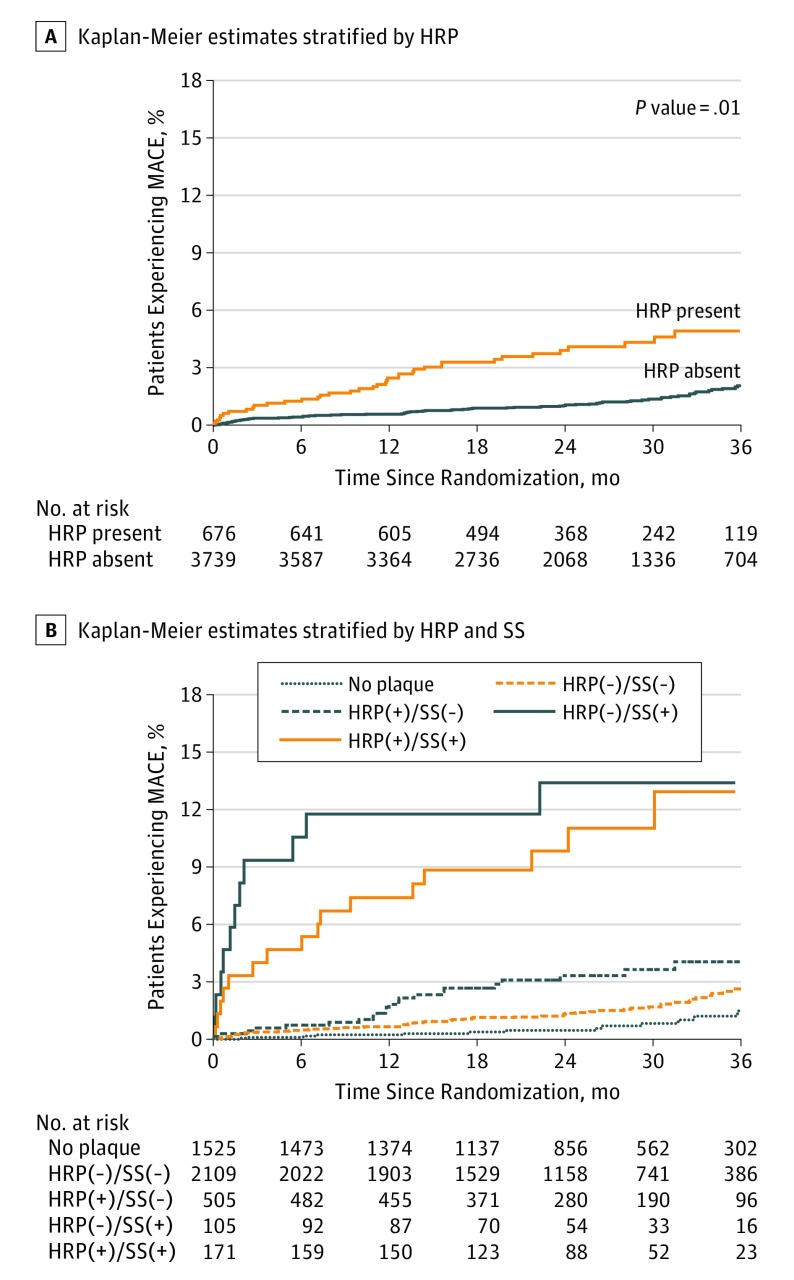
The association of high-risk plaque with MACE remained significant after adjustment for SS (defined as ≥70% stenosis in any coronary artery or ≥50% stenosis in the left main coronary artery) and ASCVD risk score (aHR, 1.72; 95% CI, 1.13-2.62) (Figure 2).
Figure 2. Kaplan-Meier Estimates of the Composite Primary End Point as a Function of Time After Randomization.
A, Kaplan-Meier estimates stratified by the presence of high-risk plaque (HRP), adjusted for significant stenosis [SS], defined as 70% or greater stenosis in any coronary artery or 50% or greater stenosis in the left main coronary artery and for atherosclerotic cardiovascular disease risk score. B, Kaplan-Meier estimates stratified by both the presence of HRP and SS with adjustment for atherosclerotic cardiovascular disease risk score. The absolute number of patients with high relative risk of major adverse cardiovascular events (MACE) was low. Only 105 patients (2.3%) had SS without high-risk plaque, and 171 patients (3.9%) had both SS and HRP.
Furthermore, adding high-risk plaque to a baseline model that included SS and ASCVD risk score led to a significant continuous net reclassification improvement (0.34; 95% CI, 0.02-0.51). The C index did not increase significantly when a model including only ASCVD score and SS had high-risk plaque added to it (0.69 [95% CI, 0.63-0.74] vs 0.71 [95% CI, 0.66-0.76]; P = .12). A Grønnesby and Borgan test for goodness of fit of the model including ASCVD score, SS, and high-risk plaque was also nonsignificant, suggesting there was no gross model violation. The sensitivity, specificity, positive predictive value, and negative predictive value of high-risk plaque for MACE were 32.8%, 85.2%, 6.4%, and 97.6%, respectively.
Similar results were observed when SS was defined as stenosis equal to or greater than 50% in any coronary artery (HR, 1.58; 95% CI, 1.04-2.40); when Framingham Heart Study risk score was used for adjustment (aHR, 1.68; 95% CI, 1.10-2.56); when the secondary composite end point of cardiovascular death, myocardial infarction, or hospitalization for unstable angina was used (aHR, 1.63; 95% CI, 1.01-2.62) (eFigure 2A in the Supplement); and when the tertiary composite end point of death or myocardial infarction (aHR, 1.85; 95% CI, 1.10-3.09; eFigure 2B in the Supplement) was used as the outcome.
In patients with nonobstructive CAD, in whom most incidents of MACE occurred (86 of 131; 65.6%), the presence of high-risk plaque identified a subgroup with increased risk of MACE. Of 505 patients with high-risk plaque, 24 experienced MACE (4.8%; aHR, 4.31; 95% CI, 2.25-8.26) compared with just 62 of the 2109 patients with no high-risk plaque (2.9%; aHR, 2.64; 95% CI, 1.49-4.69; Figure 2; eTable 2 in the Supplement). In contrast, the increase of MACE risk was similar whether high-risk plaque was present or absent among patients with SS; 19 of 171 patients with high-risk plaque (11.1%; aHR, 8.68; 95% CI, 4.25-17.73) experienced these events, compared with 11 of 105 patients with no high-risk plaque (10.5%; HR, 9.31; 95% CI, 4.21-20.61; eFigure 3 in the Supplement).
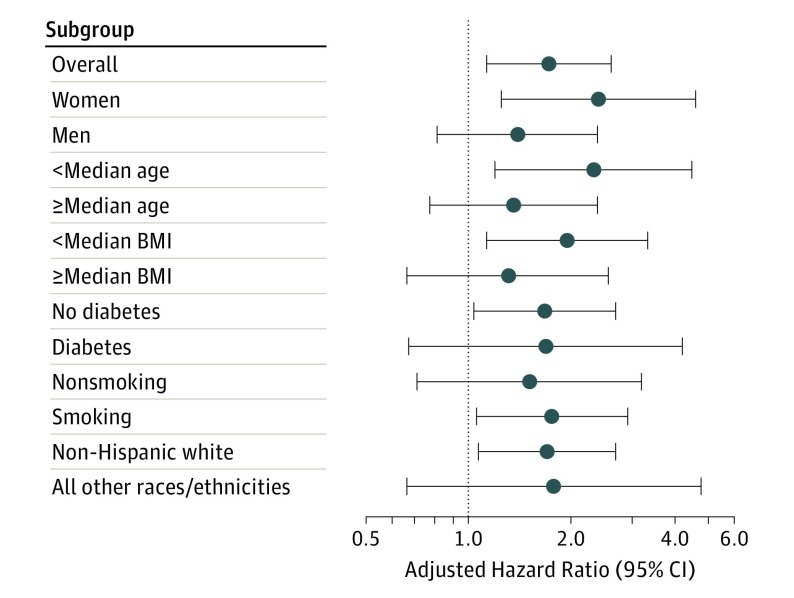
Association of High-Risk Plaque With MACE in Subgroups
We also performed multivariable Cox proportional hazards analyses adjusted for SS and ASCVD score in patient subgroups (Figure 3; eTable 3 in the Supplement). There was a stronger association of the presence of high-risk plaque with MACE in women (aHR, 2.41; 95% CI, 1.25-4.64) than in men (aHR, 1.40; 95% CI, 0.81-2.39). The association of high-risk plaque and MACE was also stronger in younger patients (aHR, 2.33; 95% CI, 1.20-4.51) than in older patients (aHR, 1.36; 95% CI, 0.77-2.39). In a subgroup of women younger than the median age of 59.6 years (n = 952), we found that 4 of 84 women with high-risk plaque (4.8%) vs 7 of the 868 with no high-risk plaque (0.8%) experienced MACE (aHR, 4.16; 95% CI, 1.00-17.28), suggesting a strong predictive value of high-risk plaque in this subgroup.
Figure 3. Multivariable Cox Proportional Analyses in Patient Subgroups.
The analyses demonstrate the predictive value of high-risk plaque for the outcome of major adverse cardiovascular events (death, nonfatal myocardial infarction, or hospitalization for unstable angina) adjusted for significant stenosis (≥70% stenosis in any coronary artery or ≥50% in the left main coronary artery) and atherosclerotic cardiovascular disease risk score. Error bars show adjusted hazard ratios with 95% CIs, adjusted for atherosclerotic cardiovascular disease risk score and significant stenosis. Wide and overlapping 95% CIs were present. The median age of participants was 59.6 years; median body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), 29.5.
Discussion
Our study demonstrated that the presence of high-risk plaque (as observed by signs such as positive remodeling, low CT attenuation, and napkin-ring sign) carried a 70% increased risk of future MACE in a large contemporary North American population of outpatients with stable chest pain, and that this risk was independent of cardiovascular risk factor burden and obstructive CAD. Detection of high-risk plaque added the most value for patients with nonobstructive CAD and those with a lower atherosclerosis burden such as younger patients and women. Our results demonstrate the clinical significance of detecting individual high-risk plaques relative to standard clinical risk factors and obstructive CAD. This suggests the potential to use this information to optimize management of this large group of patients.
High-Risk Plaque and Future MACE
Our observation of the independent predictive value of high-risk plaque for future MACE in a North American population with stable chest pain expands studies performed in Japanese populations that reported 5-fold to 8-fold increased risk of MACE in patients with high-risk plaque. Although our study concurred with observations that high-risk plaque was independently associated with MACE after adjusting for cardiovascular risk factors and SS, we could not confirm that high-risk plaque features might identify vulnerable lesions with a high positive predictive value for future MACE (aHR, 1.72). In part, this was because 43 of 676 patients with high-risk plaque (6.4%) experienced MACE.
Some of the differences could be explained by differences in risk profiles and clinical presentations in Japanese and North American populations (eg, lower BMIs and lower prevalence of statin treatment in the Japanese study cohorts, as well as the inclusion of some patients with CAD history in the Japanese studies). The lower predictive value of high-risk plaque also reflects temporal changes in cardiovascular disease during the last 2 decades, which includes a shift in acute coronary syndome presentations from myocardial infarctions with ST-segment elevation to those without ST-segment elevation, decrease in plaque ruptures in culprit lesions of acute coronary syndromes, and more stable characteristics of atherosclerotic plaques (eg, smaller lipid cores and less intraplaque inflammation). These changes can be explained by increased use of lipid-lowering therapies (statins), better blood pressure control, and decreased prevalence of smoking; they are responsible for low MACE rates in contemporary cardiovascular clinical trials.
However, the most striking difference, which might have significantly affected the large difference in the aHRs between our study and those completed in Japan, is the weak association of SS with MACE in the Japanese studies (aHR, 1.6-1.7). This is in contrast with our data (which found an aHR of approximately 9 for SS) and other studies from North America and Europe (which reported aHR values ranging from 3 to 9). As a result of the strong association of stenosis with MACE, there was no incremental value in high-risk plaque detection for the prediction of MACE in patients with SS. We speculate that in patients with SS the presence of stenosis plays an important role in MACE in the short term. Indeed, we observed that a significant portion of MACE occurred in the first 6 months after coronary CTA (Figure 2). In contrast, in patients with nonobstructive CAD and high-risk plaque, we found an increase in MACE more than 12 months after coronary CTA, suggesting that plaque progression and rupture may have played a role in the progression to these events.
Because the PROMISE trial had a fairly low prevalence of obstructive CAD, the present cohort included a large number of patients who had nonobstructive CAD. This meant the study identified a large additional group of at-risk patients, in whom over half of MACEs occurred. Our results are in agreement with extensive research in interventional cardiology that has found that a significant number of MACEs occur at locations in the coronary artery tree where previously no obstructive CAD was present. Indeed, the presence of high-risk plaque doubles the risk of MACE in the group of patients with nonobstructive CAD, and this provides considerably more information. Since the presence of high-risk plaque can be easily determined during routine evaluation of coronary CTA and because it permits risk reclassification especially in younger patients and women, information on the presence of high-risk plaque should be included in standardized reports in accordance with the current guideline recommendations.
High-Risk Plaque in Patient Subgroups
The large size of our population permitted subgroup analyses that provided additional insights into the role of high-risk plaque for MACE prediction. We observed that the predictive value of high-risk plaque was stronger in younger patients and women. Women with CAD tend to have lower coronary plaque burden but also smaller size of coronary arteries, which may lead to symptomatic disease with lower plaque burden or thrombus load. Previous studies of differences in coronary plaque development in men and women have suggested that lower prevalence of plaque ruptures and thin-cap fibroatheroma but higher prevalence of plaque erosions exist in women; however, these results have not been consistent across studies. Sex-based differences in plaque size and composition have been more pronounced in younger women and more attenuated in women older than 65 years. We speculate that when high-risk plaques are present in younger women, they portend a higher risk of future MACE. Furthermore, younger patients and women have a lower prevalence of obstructive CAD, and thus, consistent with our findings, high-risk plaque may be most helpful for risk stratification in patients without SS.
Local Interventional Treatment of High-Risk Plaque
The results of our study challenge the feasibility of local therapies for high-risk plaque. We found that only 6.4% of patients with high-risk plaque developed MACE and that, among 131 patients with MACEs, 88 patients (67.2%) had no high-risk plaque. These results are similar to observations from the PROSPECT trial, in which only 26 of 595 patients (4.9%) with thin-cap fibroatheromata experienced the development of culprit lesions of acute coronary syndrome. Therefore, local interventional therapies for high-risk plaque may be of limited value. Overall, the presence of high-risk plaque, especially in those with nonobstructive CAD, may constitute an additional risk stratification tool guiding management, including lifestyle modification (eg, diet and exercise) and pharmacologic treatments (eg, treatment with lipid-lowering and antiplatelet therapies).
Limitations
In the setting of low MACE rates observed in the PROMISE trial, HRs may inflate the importance of our observations. This is especially true given the low positive predictive value of high-risk plaque for MACE. The options for changes in preventive therapies (eg, aspirin or statin) based on the detection of high-risk plaque by coronary CTA are limited, and no data on whether such interventions would change long-term outcomes are available from our study. The PROMISE trial was not a study of natural history, and patients were treated based on coronary CTA results, which might have affected MACE. We performed a qualitative analysis of high-risk plaque with simple manual measurements that can be easily incorporated into clinical practice. An identical approach was used by other investigators studying the predictive value of high-risk plaque. Quantitative plaque assessment is feasible but remains time-consuming, not widely used in clinical practice, and of unproven value.
Conclusions
In a large contemporary North American population of outpatients with stable chest pain, noninvasive detection of high-risk plaque improved risk assessment for MACE. The detection of high-risk plaque added the most value in patients with nonobstructive CAD, younger patients, and women. High-risk plaque may constitute an additional risk stratification tool for clinical management. However, because absolute event rates were low and the positive predictive value of high-risk plaque was diminished, the findings may be of limited applicability to clinical management strategies.
eFigure 1. Evaluation of Coronary CTA Datasets for the Detection of Coronary Plaque, Stenosis, and High-risk Plaque Features
eFigure 2. Kaplan–Meier Estimates of the Composite Secondary End Point of Cardiovascular Death, Myocardial Infarction, or Hospitalization for Unstable Angina and Tertiary End Point of Death, or Myocardial Infarction as a Function of Time After Randomization.
eFigure 3. Major Adverse Cardiovascular Event Rate (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina) Stratified by the Degree of Stenosis and Presence of High-Risk Plaque
eTable 1. Results of Univariable and Multivariable Cox Proportional Hazard Analysis Demonstrating the Predictive Value of High-Risk Plaque for the Outcome of Major Adverse Cardiovascular Events (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina Pectoris)
eTable 2. Results of Univariable and Multivariable Cox Proportional Hazard Analysis Demonstrating the Predictive Value of the Presence of any Coronary Plaque, High-Risk Plaque, and Significant Coronary Stenosis for the Outcome of Major Adverse Cardiovascular Events (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina Pectoris)
eTable 3. Results of Univariable and Multivariable Cox Proportional Hazard Analysis Demonstrating the Predictive Value of High-Risk Plaque for the Outcome of Major Adverse Cardiovascular Events (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina Pectoris) in Patient Subgroups
References
- 1.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724-1732. [DOI] [PubMed] [Google Scholar]
- 2.Meijboom WB, Meijs MFL, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135-2144. [DOI] [PubMed] [Google Scholar]
- 3.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324-2336. [DOI] [PubMed] [Google Scholar]
- 4.Douglas PS, Hoffmann U, Patel MR, et al. ; PROMISE Investigators . Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SCOT-HEART investigators CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385(9985):2383-2391. [DOI] [PubMed] [Google Scholar]
- 6.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282-291. [DOI] [PubMed] [Google Scholar]
- 7.Hadamitzky M, Täubert S, Deseive S, et al. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J. 2013;34(42):3277-3285. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann U, Ferencik M, Udelson JE, et al. ; PROMISE Investigators . Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (prospective multicenter imaging study for evaluation of chest pain). Circulation. 2017;135(24):2320-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone GW, Maehara A, Lansky AJ, et al. ; PROSPECT Investigators . A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226-235. [DOI] [PubMed] [Google Scholar]
- 10.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8)(suppl):C13-C18. [DOI] [PubMed] [Google Scholar]
- 11.Bom MJ, van der Heijden DJ, Kedhi E, et al. Early detection and treatment of the vulnerable coronary plaque: can we prevent acute coronary syndromes? Circ Cardiovasc Imaging. 2017;10(5):e005973. [DOI] [PubMed] [Google Scholar]
- 12.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66(4):337-346. [DOI] [PubMed] [Google Scholar]
- 13.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64(7):684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurovich-Horvat P, Schlett CL, Alkadhi H, et al. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging. 2012;5(12):1243-1252. [DOI] [PubMed] [Google Scholar]
- 15.Maurovich-Horvat P, Schlett CL, Alkadhi H, et al. Differentiation of early from advanced coronary atherosclerotic lesions: systematic comparison of CT, intravascular US, and optical frequency domain imaging with histopathologic examination in ex vivo human hearts. Radiology. 2012;265(2):393-401. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Terashima M, Kaneda H, et al. Comparison of in vivo assessment of vulnerable plaque by 64-slice multislice computed tomography versus optical coherence tomography. Am J Cardiol. 2011;107(9):1270-1277. [DOI] [PubMed] [Google Scholar]
- 17.Kröner ESJ, van Velzen JE, Boogers MJ, et al. Positive remodeling on coronary computed tomography as a marker for plaque vulnerability on virtual histology intravascular ultrasound. Am J Cardiol. 2011;107(12):1725-1729. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann U, Moselewski F, Nieman K, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47(8):1655-1662. [DOI] [PubMed] [Google Scholar]
- 19.Pflederer T, Marwan M, Schepis T, et al. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis. 2010;211(2):437-444. [DOI] [PubMed] [Google Scholar]
- 20.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49-57. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging. 2013;6(4):448-457. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H, Kitagawa T, Ohashi N, et al. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J Cardiovasc Comput Tomogr. 2013;7(3):192-199. [DOI] [PubMed] [Google Scholar]
- 23.Douglas PS, Hoffmann U, Lee KL, et al. ; PROMISE investigators . PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J. 2014;167(6):796-803.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. [DOI] [PubMed] [Google Scholar]
- 25.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S49-S73. [DOI] [PubMed] [Google Scholar]
- 26.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10(6):435-449. [DOI] [PubMed] [Google Scholar]
- 27.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342-358. [DOI] [PubMed] [Google Scholar]
- 28.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109(1):14-17. [DOI] [PubMed] [Google Scholar]
- 29.Gauss S, Achenbach S, Pflederer T, Schuhbäck A, Daniel WG, Marwan M. Assessment of coronary artery remodelling by dual-source CT: a head-to-head comparison with intravascular ultrasound. Heart. 2011;97(12):991-997. [DOI] [PubMed] [Google Scholar]
- 30.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50(4):319-326. [DOI] [PubMed] [Google Scholar]
- 31.Maurovich-Horvat P, Hoffmann U, Vorpahl M, Nakano M, Virmani R, Alkadhi H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging. 2010;3(4):440-444. [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasterkamp G, den Ruijter HM, Libby P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat Rev Cardiol. 2017;14(1):21-29. [DOI] [PubMed] [Google Scholar]
- 34.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57(10):1237-1247. [DOI] [PubMed] [Google Scholar]
- 35.Chow BJW, Small G, Yam Y, et al. ; CONFIRM Investigators . Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging. 2011;4(5):463-472. [DOI] [PubMed] [Google Scholar]
- 36.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78(5 Pt 1):1157-1166. [DOI] [PubMed] [Google Scholar]
- 37.Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12(1):56-62. [DOI] [PubMed] [Google Scholar]
- 38.Mancini GBJ, Hartigan PM, Bates ER, et al. ; COURAGE Investigators and Coordinators . Angiographic disease progression and residual risk of cardiovascular events while on optimal medical therapy: observations from the COURAGE Trial. Circ Cardiovasc Interv. 2011;4(6):545-552. [DOI] [PubMed] [Google Scholar]
- 39.Cury RC, Abbara S, Achenbach S, et al. ; Endorsed by the American College of Cardiology . CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10(4):269-281. [DOI] [PubMed] [Google Scholar]
- 40.Wykrzykowska JJ, Mintz GS, Garcia-Garcia HM, et al. Longitudinal distribution of plaque burden and necrotic core-rich plaques in nonculprit lesions of patients presenting with acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3)(suppl):S10-S18. [DOI] [PubMed] [Google Scholar]
- 41.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3)(suppl):S62-S72. [DOI] [PubMed] [Google Scholar]
- 42.Chandrasekhar J, Mehran R. Sex-based differences in acute coronary syndromes: insights from invasive and noninvasive coronary technologies. JACC Cardiovasc Imaging. 2016;9(4):451-464. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-García J, Lerman A, Weisz G, et al. Age- and gender-related changes in plaque composition in patients with acute coronary syndrome: the PROSPECT study. EuroIntervention. 2012;8(8):929-938. [DOI] [PubMed] [Google Scholar]
- 44.Nicholls SJ, Wolski K, Sipahi I, et al. Rate of progression of coronary atherosclerotic plaque in women. J Am Coll Cardiol. 2007;49(14):1546-1551. [DOI] [PubMed] [Google Scholar]
- 45.Pundziute G, Schuijf JD, van Velzen JE, et al. Assessment with multi-slice computed tomography and gray-scale and virtual histology intravascular ultrasound of gender-specific differences in extent and composition of coronary atherosclerotic plaques in relation to age. Am J Cardiol. 2010;105(4):480-486. [DOI] [PubMed] [Google Scholar]
- 46.Bharadwaj AS, Vengrenyuk Y, Yoshimura T, et al. Multimodality intravascular imaging to evaluate sex differences in plaque morphology in stable CAD. JACC Cardiovasc Imaging. 2016;9(4):400-407. [DOI] [PubMed] [Google Scholar]
- 47.Guagliumi G, Capodanno D, Saia F, et al. ; OCTAVIA Trial Investigators . Mechanisms of atherothrombosis and vascular response to primary percutaneous coronary intervention in women versus men with acute myocardial infarction: results of the OCTAVIA study. JACC Cardiovasc Interv. 2014;7(9):958-968. [DOI] [PubMed] [Google Scholar]
- 48.Chia S, Christopher Raffel O, Takano M, Tearney GJ, Bouma BE, Jang I-K. In-vivo comparison of coronary plaque characteristics using optical coherence tomography in women vs. men with acute coronary syndrome. Coron Artery Dis. 2007;18(6):423-427. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Mintz GS, Witzenbichler B, et al. Differences in underlying culprit lesion morphology between men and women: an IVUS analysis from the ADAPT-DES study. JACC Cardiovasc Imaging. 2016;9(4):498-499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Evaluation of Coronary CTA Datasets for the Detection of Coronary Plaque, Stenosis, and High-risk Plaque Features
eFigure 2. Kaplan–Meier Estimates of the Composite Secondary End Point of Cardiovascular Death, Myocardial Infarction, or Hospitalization for Unstable Angina and Tertiary End Point of Death, or Myocardial Infarction as a Function of Time After Randomization.
eFigure 3. Major Adverse Cardiovascular Event Rate (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina) Stratified by the Degree of Stenosis and Presence of High-Risk Plaque
eTable 1. Results of Univariable and Multivariable Cox Proportional Hazard Analysis Demonstrating the Predictive Value of High-Risk Plaque for the Outcome of Major Adverse Cardiovascular Events (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina Pectoris)
eTable 2. Results of Univariable and Multivariable Cox Proportional Hazard Analysis Demonstrating the Predictive Value of the Presence of any Coronary Plaque, High-Risk Plaque, and Significant Coronary Stenosis for the Outcome of Major Adverse Cardiovascular Events (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina Pectoris)
eTable 3. Results of Univariable and Multivariable Cox Proportional Hazard Analysis Demonstrating the Predictive Value of High-Risk Plaque for the Outcome of Major Adverse Cardiovascular Events (Death, Non-Fatal Myocardial Infarction, or Hospitalization for Unstable Angina Pectoris) in Patient Subgroups