Key Points
Question
What is the quality and output of different perimetric approaches in children with glaucoma?
Findings
In this cross-sectional study, test quality improved with increasing age for all approaches (65 children aged 5-15 years). Static perimetry appeared to be effective and appropriate in children younger than 10 years and for detecting mild field restriction, and far-peripheral kinetic perimetry was feasible and added value to assessment of older children, especially with severe visual field restriction, highlighting potential use in monitoring disease progression.
Meaning
These data suggest that monitoring visual fields in childhood glaucoma should consider age, disease severity, and different strengths of static vs kinetic tests.
Abstract
Importance
There is limited evidence to support the development of guidance for visual field testing in children with glaucoma.
Objective
To compare different static and combined static/kinetic perimetry approaches in children with glaucoma.
Design, Setting, and Participants
Cross-sectional, observational study recruiting children prospectively between May 2013 and June 2015 at 2 tertiary specialist pediatric ophthalmology centers in London, England (Moorfields Eye Hospital and Great Ormond Street Hospital). The study included 65 children aged 5 to 15 years with glaucoma (108 affected eyes).
Main Outcomes and Measures
A comparison of test quality and outcomes for static and combined static/kinetic techniques, with respect to ability to quantify glaucomatous loss. Children performed perimetric assessments using Humphrey static (Swedish Interactive Thresholding Algorithm 24-2 FAST) and Octopus combined static tendency-oriented perimetry/kinetic perimetry (isopter V4e, III4e, or I4e) in a single sitting, using standardized clinical protocols, administered by a single examiner. Information was collected about test duration, completion, and quality (using automated reliability indices and our qualitative Examiner-Based Assessment of Reliability score). Perimetry outputs were scored using the Aulhorn and Karmeyer classification. One affected eye in 19 participants was retested with Swedish Interactive Thresholding Algorithm 24-2 FAST and 24-2 standard algorithms.
Results
Sixty-five children (33 girls [50.8%]), with a median age of 12 years (interquartile range, 9-14 years), were tested. Test quality (Examiner-Based Assessment of Reliability score) improved with increasing age for both Humphrey and Octopus strategies and were equivalent in children older than 10 years (McNemar test, χ2 = 0.33; P = .56), but better-quality tests with Humphrey perimetry were achieved in younger children (McNemar test, χ2 = 4.0; P = .05). Octopus and Humphrey static MD values worse than or equal to −6 dB showed disagreement (Bland-Altman, mean difference, −0.70; limit of agreement, −7.74 to 6.35) but were comparable when greater than this threshold (mean difference, −0.03; limit of agreement, −2.33 to 2.27). Visual field classification scores for static perimetry tests showed substantial agreement (linearly weighted κ, 0.79; 95% CI, 0.65-0.93), although 25 of 80 (31%) were graded with a more severe defect for Octopus static perimetry. Of the 7 severe cases of visual field loss (grade 5), 5 had lower kinetic than static classification scores.
Conclusions and Relevance
A simple static perimetry approach potentially yields high-quality results in children younger than 10 years. For children older than 10 years, without penalizing quality, the addition of kinetic perimetry enabled measurement of far-peripheral sensitivity, which is particularly useful in children with severe visual field restriction.
This cross-sectional study compares different static and combined static/kinetic perimetry approaches in children with glaucoma.
Introduction
Childhood glaucoma is a relatively rare but potentially blinding eye disorder characterized by elevated intraocular pressure (IOP) and optic nerve damage, which can be caused by a diverse group of conditions. Five in 100 000 children born in Great Britain each year are diagnosed as having primary congenital glaucoma (PCG).
In adults, management of glaucoma relies on measuring IOP, assessing optic disc (OD) appearance, and monitoring visual field (VF) function. There is a focus on IOP as the key modifiable parameter of glaucoma progression, with strategies to control glaucoma and prevent irreversible loss of vision directed at lowering and maintaining an acceptable IOP, mostly through use of topical medication. In contrast, PCG, the most common glaucoma in infancy, is primarily treated surgically.
Monitoring IOP, OD appearance, and VF function in infants/young children is very challenging. Inhalational anesthesia, corneal opacities, and poor cooperation with testing (if unsedated) can all influence assessment. Thus, objective measures, such as increased axial length and increasing myopia, can be useful adjunct measures until children are old enough to cooperate with slitlamp biomicroscopy, assessment of IOP, and perimetry.
Changes in VF sensitivity are useful in monitoring glaucoma progression, assuming perimetry is accurate. This is generally straightforward in adults using standard automated perimetry and automated reliability indices (such as false-positive/negative results and fixation losses). In children, the intense concentration and cooperation required to reliably perform perimetry often precludes its use, especially in early childhood. Moreover, the role of perimetry in the management of childhood glaucoma is unclear, with limited evidence about optimal approaches.
Perimetry in normal eyes is feasible beginning at age 4 years, but reliability of assessments improves with age. Children with glaucoma commonly commence VF testing from ages 7 to 8 years. While children are reported to have similar patterns of VF loss to adults, no guidance exists for interpreting visual field data longitudinally, accounting for both developing visual fields and changing procedures/algorithms (usually longer algorithms) with increasing age.
To address some of the evidence gaps, we investigated the feasibility and quality of perimetry in children with known glaucomatous disease, comparing static and combined static/kinetic techniques and testing both affected and unaffected eyes. We assessed the ability of each technique to detect glaucomatous defects and explored any potential benefit of the full-field examination capabilities of kinetic perimetry, with the aim of providing age-appropriate guidance on optimal perimetric approaches.
Methods
This cross-sectional study was undertaken as part of a wider program on perimetry in children (the Optimal Perimetric Testing In Children [OPTIC] study), drawing on our established, child-specific interpretation of test quality and outcomes. The objectives a priori were to compare different perimetric approaches to assess feasibility, quality, and relative strengths and weaknesses.
Children with glaucoma (all subtypes), aged 5 to 15 years with visual acuity better than 1.00 logMAR (6/60 Snellen equivalent) in at least 1 affected eye, were identified prospectively and sequentially from medical records of those attending specialist childhood glaucoma clinics at 2 specialist centers in London, England (Moorfields Eye Hospital and Great Ormond Street Hospital). Children with any significant impairment, such as severe learning disability, which would preclude cooperation with formal perimetry, were not eligible. Potential participants were approached during their routine clinical visit. They were given written study information sheets and opportunities (patients and parents) to ask questions about the study. Informed written consent was obtained from parents/guardians, while children gave verbal assent. The study was approved by the National Health Service Research Ethics Committee for London, Bloomsbury, and followed the tenets of the Declaration of Helsinki.
Assessments were performed using an Octopus 900 (Haag-Streit) and a Humphrey Visual Field Analyzer 740/750i (HFA; Carl Zeiss Meditec V). All tests were carried out by an experienced orthoptist (D. E. P.) in a darkened clinic room, using calibrated perimeters. The right eye was assessed first unless contraindicated clinically.
Participants were assessed in an age-appropriate manner, as described previously in detail, including our qualitative measure of test quality (Examiner-Based Assessment of Reliability [EBAR], shown in the eTable in the Supplement). Participants were given instructions regarding fixation and responding to stimuli and were given an opportunity to test their buzzer. One eye was occluded using a soft eye pad, and participants were aligned at a perimeter while sitting on a height-adjustable chair. Refractive errors were corrected for static perimetry only, using criteria modified from Henson: worse than +3.00 diopter spheres, worse than −1.00 diopter spheres, and more extreme than 1.00 diopter cylinder. Preparation time and any modifications required were noted. Encouragement and repetition of instructions were given throughout testing. Participants were offered a rest break during an assessment if required, and this was recorded.
The Humphrey Swedish Interactive Thresholding Algorithm (SITA) 24-2 FAST (with fixation monitoring and gaze-tracking) was performed first, followed by a combined static/kinetic approach using the Octopus perimeter. Automated fixation monitoring on the Octopus was switched off because it prolongs test duration in those with fixation losses/head movement. Participants were given a 1- to 2-minute rest break between assessments.
For Octopus perimetry, each eye was assessed with the tendency-oriented perimetry (G-TOP) algorithm followed by a kinetic isopter. The smallest visible target was chosen from V4e, III4e, or I4e. Twelve points were plotted initially (every 30°), followed by further points to a maximum of 24 to delineate isopter shape. Each point was manually defined, with an automated centripetal movement at 5°/s.
All participants were invited back for a second visit to test their eye with the most advanced glaucoma (except for those with visual acuity [VA] worse than 1.0 logMAR [6/60 Snellen equivalent] who had their fellow glaucomatous eye assessed). This comprised the HFA SITA 24-2 FAST and SITA 24-2 standard algorithms performed in an order assigned by a random number generator to enable a direct comparison of test quality, duration, and output.
Statistical Analysis
Data were hosted securely in a Research Electronic Data Capture database at University College London Great Ormond Street Institute of Child Health and exported to Stata, version 12 (StataCorp) for analysis. Visual field defects were graded using the Aulhorn and Karmeyer classification, allowing categorization of static and kinetic fields on the same scale and using a grading of 0 (no VF loss) to 5 (greatest VF loss).
Agreement between VF loss classification scores was measured with linearly weighted κ statistics (perfect agreement weighted as 1, with an incremental decrease of 0.2 per level increase in disagreement) and EBAR quality ratings between perimeters/algorithms with McNemar test. Quantitative analysis of agreement between mean deviation (MD) values between perimeters/algorithms was measured using Spearman correlation and the Bland-Altman method. Comparisons of test duration between perimeters were made using paired t tests.
Multivariable linear and logistic regression models were fitted to investigate the relationship between static and kinetic sensitivity outputs as well as associations between test duration and VA, MD, IOP, age (continuous variable), sex, and race/ethnicity including only factors significant at a 10% level (2-sided P < .10) in univariable analyses. Robust variance estimates were obtained to allow for the within-patient correlation owing to paired data (2 eyes). Analysis of feasibility and quality data for the HFA and Octopus combined static/kinetic perimetry drew only on initial visit data (65 patients).
Results
Between June 2013 and May 2015, 65 children (33 girls [50.8%]) were recruited, with a median age of 12 years (interquartile range [IQR], 9-14 years) (Table 1). Most were white (n = 51; 78.5%), 6 were black (9.2%), 5 were Asian (7.7%), and 3 were mixed race/ethnicity (4.6%). Overall participation rate was 79.3% (65 of 82 eligible participants identified over a 2-year period), with similar age distribution for nonparticipants (median age, 11 years; IQR, 10-14 years) but different ethnicity distribution (6 were white [35.2%], 6 were black [35.2%], 4 were Asian [23.5%], and 1 was other [5.8%]).
Table 1. Test Feasibility and Quality for Humphrey SITA 24-2 FAST and Octopus Combined Static/Kinetic Perimetry.
| Age Group, y | Sex, No. | Completed Assessments, No. (%) | Test Duration,a Mean (SD), min | Good-Quality Tests, No. (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Humphrey (n = 65) |
Octopus (n = 64) |
Humphrey (n = 60) |
Octopus (n = 59) |
Humphrey (n = 65) |
Octopus (n = 64) |
|
| 5-7 | 2 | 4 | 6 (100) | 6 (100) | 18.0 (3.1) | 20.2 (4.8) | 2 (33.3) | 1 (16.7) |
| 8-11 | 15 | 11 | 26 (100) | 24 (96) | 13.9 (2.6) | 17.3 (2.7) | 20 (76.9) | 16 (64) |
| 12-15 | 15 | 18 | 33 (100) | 33 (100) | 13.3 (2.2) | 17.0 (2.0) | 33 (100) | 32 (97.0) |
Abbreviation: SITA, Swedish Interactive Thresholding Algorithm.
Test duration values include preparation and assessment tasks as well as any rest breaks required for participants performing testing with both eyes only.
Twenty-nine of 65 participants had PCG (45%), and 13 of 65 (20%) had aphakic glaucoma; all diagnoses are shown in Table 2. Twenty-one participants (32.3%) had never undertaken perimetry. Of these, 14 (66.7%) were younger than 10 years. Forty-four participants had prior experience using the HFA, ranging from 1 to 8 years (median, 2 years’ experience; IQR, 1-4 years), with a median of 1 test (IQR, 1-1.5) per year. One participant withdrew after 1 perimetric test, citing time constraints. Humphrey data for this participant are included where appropriate.
Table 2. Classification of Diagnoses for 65 Participants.
| Ophthalmic Diagnosis | Participants, No. |
|---|---|
| Primary childhood glaucoma | |
| Primary congenital glaucoma | |
| Bilateral | 20 |
| Unilateral | 9 |
| Juvenile open-angle glaucoma | 6 |
| Secondary childhood glaucoma | |
| Glaucoma associated with nonacquired ocular anomalies | |
| Aniridia | 1 |
| Axenfeld-Rieger anomaly | 2 |
| Posterior polymorphous dystrophy | 1 |
| Glaucoma associated with nonacquired systemic disease or syndrome | |
| Sturge-Weber syndrome | 5 |
| Neurofibromatosis | 1 |
| Glaucoma associated with an acquired condition | |
| Uveitis | 4 |
| Trauma | 1 |
| Glaucoma following cataract surgery | 13 |
| Glaucoma suspect | 2 |
| Total | 65 |
Five participants with bilateral glaucoma had severely reduced VA (>1.3 logMAR [6/120 Snellen equivalent]) in 1 eye and therefore contributed data from 2 right and 3 left eyes. Seventeen participants had unilateral glaucoma. Thus, in total, 125 eyes were tested in the main protocol (ie, Humphrey vs Octopus perimetry), of which, 108 had glaucoma (eFigure 1 in the Supplement). Median logMAR acuity of tested eyes with glaucoma was 0.22 (IQR, 0.04-0.4; approximately [6/10 Snellen equivalent], with median spherical equivalent of 0.0 diopters (IQR, −2.0 to 1.0).
Nineteen eyes with glaucoma were tested in the subsidiary protocol (SITA standard vs SITA FAST).
Feasibility of Perimetry
Sixty-five of 65 and 63 of 64 participants (98.4%) completed assessments with the HFA and Octopus perimeters, respectively (Table 1). The single child who was unable to complete Octopus perimetry could not see the central fixation target with 1 eye (VA, 1.0 logMAR [6/60 Snellen equivalent]) but was able to complete a Humphrey assessment with this eye.
Table 1 summarizes total test duration (ie, from test start to test end as a clinically meaningful metric) for both perimeters by age. Humphrey visual field analyzer test duration decreased with decreasing severity of visual field loss (such that tests took 0.16 minutes longer per 1 dB loss of MD; 95% CI, 0.10-0.21) and increasing EBAR ratings (3.43 minutes shorter from poor to good quality; 95% CI, 1.02-5.84; P = .006) but did not vary by VA, IOP, age, sex, or race/ethnicity. Octopus perimetry test duration did not vary by VA, IOP, and test quality (EBAR) but reduced with increasing age (0.37 minutes per year increase in age; 95% CI, 0.07-0.67; P = .02) and MD, such that tests took 0.13 minutes longer per 1 dB loss of MD (95% CI, 0.04-0.21). Octopus perimetry took a mean of 3.3 minutes longer than HFA perimetry (t test; 95% CI, 2.7-4.0; P < .001).
Nine of 65 children (13.9%) required a break in testing to complete a Humphrey assessment, while only 3 of 64 children (4.7%) did so during Octopus perimetry: 11 of 12 children (92%) were younger than 8 years.
Quality of Perimetry
The proportion of good-quality tests, as measured with EBAR, increased with increasing age for both Humphrey and Octopus perimeters (Table 1). In children older than 10 years, no significant difference was found between the proportion of good-quality ratings for Humphrey and Octopus assessments (McNemar χ2 = 0.33; P = .56). In children younger than 10 years, Humphrey perimetry had better EBAR ratings (McNemar χ2 = 4.0, P = .05).
In eyes with glaucoma, the number of false-positive results (Humphrey, 1.4%; 95% CI, 0.4-2.3 and Octopus, 4.8%; 95% CI, 3.0-6.7) and fixation losses (Humphrey, 3.1%; 95% CI, 0.6-5.5) decreased per year increase in age. No change with age was noted for false-negative metrics with Humphrey (−0.43%; 95% CI, −1.90 to 1.04; P = .56) or Octopus (−0.61%; 95% CI, −1.88 to 0.67; P = .34) perimetry.
Traditional reliability indices, a composite measure including false positive results and fixation losses together, disagreed with EBAR ratings in 55 of 125 cases (44%). False-positive results alone showed better agreement with EBAR ratings (n = 104 of 125; 83%) (Table 3).
Table 3. Comparison EBAR With Automated Reliability Indices for Humphrey Perimetry in 65 Patients.
| EBAR Rating | No. | |||||
|---|---|---|---|---|---|---|
| False-Positive Results | Fixation Losses | Traditional Reliability Indicesa | ||||
| <15% | ≥15% | <25% | ≥25% | Reliable | Unreliable | |
| Good | 99 | 8 | 56 | 51 | 53 | 54 |
| Fair | 7 | 3 | 2 | 8 | 2 | 8 |
| Poor | 3 | 5 | 1 | 7 | 1 | 7 |
| Total, No. (%) | 109 | 16 (13) | 59 | 66 (53) | 56 | 69 (57) |
Abbreviation: EBAR, Examiner-Based Assessment of Reliability.
Traditional reliability indices are defined here as fixation losses of at least 25% or false positives of at least 15%.
Detection of VF Defects
Isopter I4e increased by 382.0°2 (95% CI, 248.5-515.5), and isopter III4e (used in those with poor VA) increased by 154.7°2 (95% CI, 21.0-288.3) per 1 dB increase in Octopus MD (glaucoma eyes only).
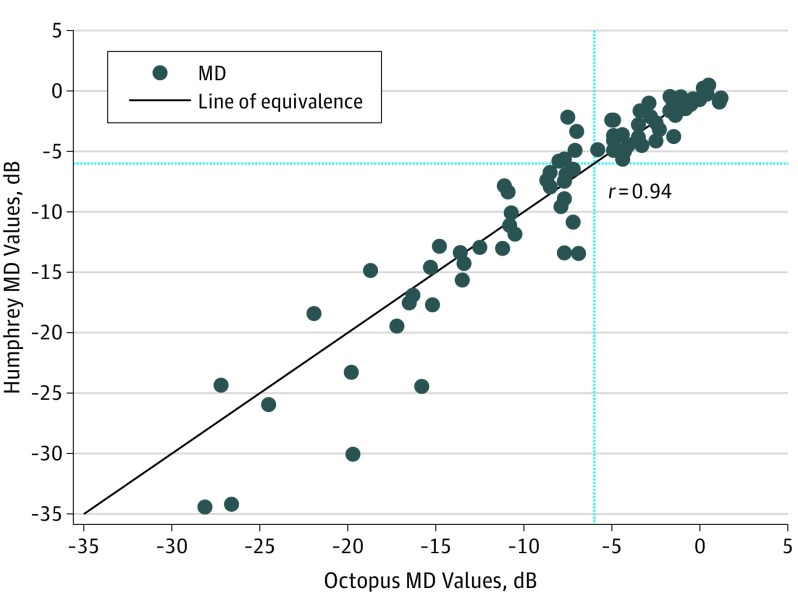
The Figure shows Humphrey compared with Octopus MD (decibels) for eyes with glaucoma and good EBAR ratings for both tests (n = 80, correlation coefficient r = 0.94). Bland-Altman analysis showed wide limits of agreement (LoA) (n = 80; mean difference, −0.38; 95% CI, −0.97 to 0.22; LoA, −5.73; 95% CI, −5.85 to −5.62; 4.98; 95% CI, 4.86-5.09), with increasing variation for more severe defects. There was good agreement for Octopus MD values better than −6 dB (eFigure 2 in the Supplement; n = 38; mean difference, −0.03; LoA, −2.33 to 2.27). Values worse than or equal to −6 dB (n = 42), ie, severe defects, were not comparable (mean difference, −0.70; LoA, −7.74 to 6.35).
Figure. Humphrey vs Octopus Static MD Values With Dashed Lines at −6 dB.
There was evidence of strong agreement between Humphrey and Octopus static classification scores (κ, 0.79; 95% CI, 0.65-0.93; good EBAR results only [Table 4]). Three of 80 participants (3.8%) had a higher classification score based on Humphrey perimetry, with 25 of 80 (31.3%) having higher scores on Octopus perimetry. Of the 7 severe cases of VF loss (grade 5), 5 had lower kinetic than static classification scores.
Table 4. Comparison of Humphrey Static and Octopus Static and Kinetic Classification Scores in Glaucomatous Eyes With Good EBAR Ratings.
| Humphrey Classification Scorea | Eyes, No. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Octopus Static Classification Score | Total | Octopus Kinetic Classification Scorea | Total | |||||||||||
| 0 | I | II | III | IV | V | 0 | I | II | III | IV | V | |||
| 0 | 11a | 7 | 0 | 0 | 0 | 0 | 18 | 14a | 3 | 1 | 0 | 0 | 0 | 18 |
| I | 0 | 8a | 5 | 2 | 0 | 0 | 15 | 3 | 9a | 3 | 0 | 0 | 0 | 15 |
| II | 0 | 1 | 4a | 5 | 0 | 0 | 10 | 0 | 1 | 7a | 2a | 0 | 0 | 10 |
| III | 0 | 0 | 0 | 16a | 6 | 0 | 22 | 2 | 5 | 5a | 6a | 4 | 0 | 22 |
| IV | 0 | 0 | 0 | 2 | 6a | 0 | 8 | 0 | 1 | 0 | 3 | 4a | 0 | 8 |
| V | 0 | 0 | 0 | 0 | 0 | 7a | 7 | 0 | 0 | 1 | 2 | 2 | 2a | 7 |
| Total | 11 | 16 | 9 | 25 | 12 | 7 | 80 | 19 | 19 | 17 | 13 | 10 | 2 | 80 |
Equivalent scores.
In those with unilateral glaucomatous disease (n = 17), median MD in unaffected eyes was −0.4 dB (IQR, −1.6 to 0.3) with Humphrey perimetry and −0.4 dB (IQR, −1.4 to 0.1) with Octopus perimetry.
Comparison of SITA FAST and Standard Algorithms
Nineteen participants (19 eyes) underwent assessment with both the SITA 24-2 FAST and SITA 24-2 standard algorithms. Mean (SD) overall test duration, including preparation tasks, was 6 (1.5) minutes with the FAST algorithm and 8.6 (2.1) minutes with the standard algorithm. There was no significant difference between the proportions of good EBAR score tests (18 of 19 [94.7%] for the SITA FAST vs 16 of 19 [84.2%] using the standard algorithm; McNemar P = .16), but the small sample size may have precluded detection of true differences.
Bland-Altman analysis showed comparable outputs for MD (n = 19, mean difference, 0.2 dB; LoA: −2.6 to 3.0), and pattern standard deviation (−0.25 dB; LoA, −2.3 to 1.8).
Discussion
Our cross-sectional study, examining a representative group of children aged 5 to 15 years with glaucoma, shows that perimetry can be undertaken from age 5 years with an expectation that quality will improve with increasing age, such that by age 8 years, more than 50% of children can perform a high-quality/reliable static assessment with a good EBAR rating and less than 15% rate of false-positive responses. Static perimetry (either using Humphrey or Octopus) is most likely to detect mild glaucomatous VF loss and is most likely to be feasible in younger children. The Octopus G-TOP algorithm potentially identifies milder VF loss than the SITA 24-2 FAST algorithm, but longitudinal studies of VF progression are required to confirm whether this translates to earlier detection of VF loss per se.
Our sample included experienced and nonexperienced participants, a high proportion of myopic participants and a small proportion of aphakic children, all with varying levels of visual loss, reflecting the population served by specialist childhood glaucoma centers, permitting generalization of findings.
Examiner-Based Assessment of Reliability ratings add value to interpretation by capturing behaviors associated with test performance that are not captured by automated perimeters. False-positive measures provide complementary data to EBAR ratings, and the use of both could allow greater accuracy in the interpretation of findings. The sole use of fixation loss measures to assess reliability in children may lead to potentially useful assessments being disregarded inappropriately.
Our data suggest that mild glaucomatous defects can be detected using kinetic perimetry, although sometimes this is less sensitive than static perimetry. However, detection could be improved by use of isopter I2e. Specifically, our findings (Table 4) show that the value of assessing the far-peripheral field using kinetic perimetry lies in the assessment of children with moderate/severe VF loss in whom assessment of the full extent of the field, ie, sensitivity outside 30° eccentricity, may enable detection of residual islands of sensitivity, which is not possible with conventional static perimetry alone.
We found that combined static/kinetic perimetry was feasible, and owing to multiple short components, children required fewer breaks to perform this than static perimetry alone. In children older than 10 years, combined static/kinetic perimetry can be used effectively for full field testing. However, the quality of combined tests was poorer in younger participants, although this may reflect partly the longer overall test duration or fatigue induced through systematic bias (test order). In children younger than 10 years, a short static algorithm, with additional kinetic assessment at a separate sitting in those with moderate/severe VF loss, may be the best approach.
Although our sample of children with unilateral glaucoma was small, unaffected eyes showed normal VF sensitivity, highlighting the potential value of these as control eyes, providing information on underlying longitudinal developmental changes that are also occurring in the glaucomatous eye.
We found no meaningful differences in quality or output between SITA FAST and SITA standard algorithms, which have equivalent precision for detecting progressive loss in adults. Thus, if testing is started at a very young age, the shorter algorithm may be used, with the caveat that the optimal approach to alignment of baseline measures with subsequent standard algorithm tests is not known.
Humphrey and Octopus MD values showed good agreement for values greater than −6 dB, but in those with severe loss, Humphrey MD values were on average larger. Thus, care should be taken when interpreting and comparing values from different perimeters, and, where possible, the same perimetric approach should be used to monitor for change over time.
Very little literature exists against which we can compare our findings directly. Much prior literature predates the use of short static algorithms and thus is not comparable with this study or is limited by sample size or inability to describe changes in test feasibility or quality with age. Other strategies, such as frequency doubling technology, microperimetry, and continuous light increment perimetry, have been described in pediatric populations, with similar success to those used here, but to our knowledge, there are no available data on their use in children with glaucoma. The value of game-based and other suprathreshold approaches lies in offering a snapshot of VF sensitivity in children who cannot otherwise be assessed rather than detailed longitudinal evaluation.
Our study shows that the choice of perimetric test will depend on the child’s clinical features, age, and capability. For example, children with limited ability to cooperate should be assessed initially with the least demanding test, commonly a short static test. In those who cannot perform static perimetry, there is merit in attempting kinetic perimetry alone, although results should be interpreted with awareness that early/mild defects may not be captured using far-peripheral stimuli alone. In children with reduced visual acuity and severely impaired central visual fields (measured by static perimetry), we suggest that the addition of kinetic perimetry to map the full visual field extent may hold value for monitoring a child with the potential for a lifetime of further progressive visual loss. Combined static/kinetic perimetry gives detailed information about central and far-peripheral sensitivity and can be used successfully in children older than 10 years.
Limitations
The small sample of children returning for repeated testing precludes detailed investigation of test repeatability. As discussed earlier, test order effects may have affected test quality, though this appears to be limited to children younger than 10 years.
Conclusions
We believe a gap remains in our understanding of the role of perimetry in identifying progressive visual field loss in young children with glaucoma, and our cross-sectional study may inform such future research. Our findings may offer guidance to clinicians about optimal perimetric approaches and interpretation of findings to improve clinical management now.
eFigure 1. Flowchart of participants at initial and follow-up visit
eFigure 2. Bland-Altman plot of Octopus and Humphrey MD values for Octopus MD better than -6, in glaucomatous eyes with good EBAR ratings in both tests
eTable. Examiner-Based Assessment of Reliability (EBAR) scoring system
References
- 1.Beck A, Chang TCP, Freedman S. Definition, classification and differential diagnosis In: Weinreb RN, Grajewski A, Papadopoulos M, Grigg J, Freedman S, eds. Childhood Glaucoma. WGA Consensus Series - 9. Amsterdam, the Netherlands: Kugler Publications; 2013:3-10. [Google Scholar]
- 2.Papadopoulos M, Cable N, Rahi J, Khaw PT; BIG Eye Study Investigators . The British Infantile and Childhood Glaucoma (BIG) eye study. Invest Ophthalmol Vis Sci. 2007;48(9):4100-4106. [DOI] [PubMed] [Google Scholar]
- 3.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295-1304. [DOI] [PubMed] [Google Scholar]
- 4.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S; Low-Pressure Glaucoma Study Group . A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151(4):671-681. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367-1377. [DOI] [PubMed] [Google Scholar]
- 6.Garway-Heath DF. Early diagnosis in glaucoma. Prog Brain Res. 2008;173:47-57. [DOI] [PubMed] [Google Scholar]
- 7.de Souza EC, Berezovsky A, Morales PH, de Arruda Mello PA, de Oliveira Bonomo PP, Salomão SR. Visual field defects in children with congenital glaucoma. J Pediatr Ophthalmol Strabismus. 2000;37(5):266-272. [PubMed] [Google Scholar]
- 8.Walters BC, Rahi JS, Cumberland PM. Perimetry in children: survey of current practices in the United Kingdom and Ireland. Ophthalmic Epidemiol. 2012;19(6):358-363. [DOI] [PubMed] [Google Scholar]
- 9.Quinn GE, Fea AM, Minguini N. Visual fields in 4- to 10-year-old children using Goldmann and double-arc perimeters. J Pediatr Ophthalmol Strabismus. 1991;28(6):314-319. [DOI] [PubMed] [Google Scholar]
- 10.Patel DE, Cumberland PM, Walters BC, Russell-Eggitt I, Rahi JS; OPTIC study group . Study of Optimal Perimetric Testing in Children (OPTIC): feasibility, reliability and repeatability of perimetry in children. PLoS One. 2015;10(6):e0130895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wabbels BK, Wilscher S. Feasibility and outcome of automated static perimetry in children using continuous light increment perimetry (CLIP) and fast threshold strategy. Acta Ophthalmol Scand. 2005;83(6):664-669. [DOI] [PubMed] [Google Scholar]
- 12.Wilscher S, Wabbels B, Lorenz B. Feasibility and outcome of automated kinetic perimetry in children. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1493-1500. [DOI] [PubMed] [Google Scholar]
- 13.Bjerre A, Codina C, Griffiths H. Peripheral visual fields in children and young adults using semi-automated kinetic perimetry: feasibility of testing, normative data, and repeatability. Neuroophthalmology. 2014;38(4):189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulos M, Brandt JD, Sugiyama K, et al. Establishing the diagnosis and determining glaucoma progression In: Weinreb RN, Grajewski A, Papadopoulos M, Grigg J, Freedman S, eds. Childhood Glaucoma: WGA Consensus Series - 9. Amsterdam, the Netherlands: Kugler Publications; 2013:15-41. [Google Scholar]
- 15.Lopes Filho JG, Betinjane AJ, Carvalho CA. Automated perimetry in patients with primary congenital glaucoma [in Portuguese]. Arq Bras Oftalmol. 2007;70(1):37-40. [DOI] [PubMed] [Google Scholar]
- 16.Sinha G, Patil B, Sihota R, et al. Visual field loss in primary congenital glaucoma. J AAPOS. 2015;19(2):124-129. [DOI] [PubMed] [Google Scholar]
- 17.Patel DE, Cumberland PM, Walters BC, Russell-Eggitt I, Cortina-Borja M, Rahi JS; OPTIC Study Group . Study of Optimal Perimetric Testing In Children (OPTIC): normative visual field values in children. Ophthalmology. 2015;122(8):1711-1717. [DOI] [PubMed] [Google Scholar]
- 18.Patel DE, Viswanathan AC, Garway-Heath D, et al. ; OPTIC Study Group . Study of Optimal Perimetric Testing In Children (OPTIC): development and feasibility of the kinetic perimetry reliability measure (KPRM). Br J Ophthalmol. 2017;101(2):94-96. [DOI] [PubMed] [Google Scholar]
- 19.Henson D. Visual Fields. 2nd ed Oxford, England: Butterworth-Heinemann; 2000. [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aulhorn E, Karmeyer H. Frequency distribution in early glaucomatous defects. Doc Ophthalmol. 1977;14:75-83. [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. [PubMed] [Google Scholar]
- 23.Mantel N, Fleiss JL. The equivalence of the generalized McNemar tests for marginal homogeneity in 2(3) and 3(2) tables. Biometrics. 1975;31(3):727-729. [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. [PubMed] [Google Scholar]
- 25.Saunders LJ, Russell RA, Crabb DP. Measurement precision in a series of visual fields acquired by the standard and fast versions of the Swedish interactive thresholding algorithm: analysis of large-scale data from clinics. JAMA Ophthalmol. 2015;133(1):74-80. [DOI] [PubMed] [Google Scholar]
- 26.Sampaolesi R, Casiraghi JF. Computerized visual fields in pediatric glaucoma In: Heijl A, Mills RP, eds. Perimetry Update 1990/1991. Amsterdam, the Netherlands: Kugler Publications; 1991. [Google Scholar]
- 27.Marraffa M, Pucci V, Marchini G, Morselli S, Bellucci R, Bonomi L. HPR perimetry and Humphrey perimetry in glaucomatous children. Doc Ophthalmol. 1995;89(4):383-386. [DOI] [PubMed] [Google Scholar]
- 28.Becker K, Semes L. The reliability of frequency-doubling technology (FDT) perimetry in a pediatric population. Optometry. 2003;74(3):173-179. [PubMed] [Google Scholar]
- 29.Jones PR, Yasoubi N, Nardini M, Rubin GS. Feasibility of Macular Integrity Assessment (MAIA) microperimetry in children: sensitivity, reliability, and fixation stability in healthy observers. Invest Ophthalmol Vis Sci. 2016;57(14):6349-6359. [DOI] [PubMed] [Google Scholar]
- 30.Aslam TM, Rahman W, Henson D, Khaw PT. A novel paediatric game-based visual-fields assessor. Br J Ophthalmol. 2011;95(7):921-924. [DOI] [PubMed] [Google Scholar]
- 31.Murray IC, Fleck BW, Brash HM, Macrae ME, Tan LL, Minns RA. Feasibility of saccadic vector optokinetic perimetry: a method of automated static perimetry for children using eye tracking. Ophthalmology. 2009;116(10):2017-2026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of participants at initial and follow-up visit
eFigure 2. Bland-Altman plot of Octopus and Humphrey MD values for Octopus MD better than -6, in glaucomatous eyes with good EBAR ratings in both tests
eTable. Examiner-Based Assessment of Reliability (EBAR) scoring system