Abstract
This cohort study uses 2002-2015 Medical Expenditure Panel Survey data to characterize the use of gabapentinoids among the US adult population.
Gabapentin and pregabalin are the 2 clinically used medications in the gabapentinoid drug class. Pregabalin and gabapentin have both been approved by the US Food and Drug Administration for treatment of partial seizures and postherpetic neuralgia. Gabapentin enacarbil has also been approved for restless legs syndrome, while pregabalin has additional approvals for diabetic peripheral neuropathy, neuropathic pain associated with spinal cord injury, and fibromyalgia. Gabapentin, in particular, is frequently used for off-label indications. There has been controversy around the use of these drugs due to off-label marketing, the short-term follow-up of studies, potential for addiction, possible increased risk of overdose among individuals concomitantly taking opiates, and ineffectiveness for common indications. Given the safety concerns and the implications of widespread use, I set out to characterize the use of gabapentinoids among the adult population.
Methods
The 2002-2015 Medical Expenditure Panel Survey (MEPS) was used for the analysis. The MEPS is cosponsored by the Agency for Healthcare, Research, and Quality and the Centers for Disease Control and Prevention and is nationally representative of the noninstitutionalized population of the United States. It consists of 2 overlapping cohorts who are included in the survey for 2 years through 5 interviews and includes sociodemographic characteristics, self-reported medical conditions, health indicators, and prescribed medication use.
All adults (>17 years) in the MEPS were included. Gabapentinoid, benzodiazepine (including zolpidem tartrate and eszopiclone), and opioid prescriptions were identified. Medical conditions were identified by self-report, and an Elixhauser comorbidity index was calculated. Simple logistic regression was used to determine trends over time.
The OhioHealth Institutional Review Board considered the study exempt from review. All analyses were conducted using survey weighting in Stata, version 13 (StataCorp).
Results
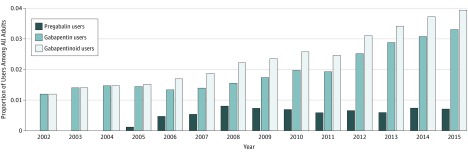
The study included 346 177 adults between 2002 and 2015. The mean age of gabapentinoid users was 59.0 years (95% CI, 58.4-59.6 years) and 62.1% (95% CI, 60.3%-63.9%) of users were women. The percentage of individuals who used gabapentin and/or pregabalin increased from 1.2% (95% CI, 1.1%-1.4%) during a year in 2002 to 3.9% (95% CI, 3.6%-4.3%) in 2015 (trend, 1.10 odds ratio [OR] per year; 95% CI, 1.09-1.11; P < .001) (Figure 1). Gabapentin was the predominantly used medication in the class, with 82.6% (95% CI, 81.0%-84.2%) of individuals. Prior to 2008, gabapentin use did not increase (OR per year, 1.02; 95% CI, 0.98-1.05; P = .39 for trend), but it increased after 2008 (OR, 1.12; 95% CI, 1.10-1.15; P < .001 for trend). Pregabalin use plateaued in 2008 and did not increase after that time (OR per year, 0.99; 95% CI, 0.95-1.03; P = .47 for trend).
Figure 1. Gabapentinoid Use in the United States, 2002 Through 2015.
The figure identifies the proportion of adults (>17 years) who reported a filled prescription for gabapentin, pregabalin, or a gabapentinoid during a calendar year between 2002 and 2015.
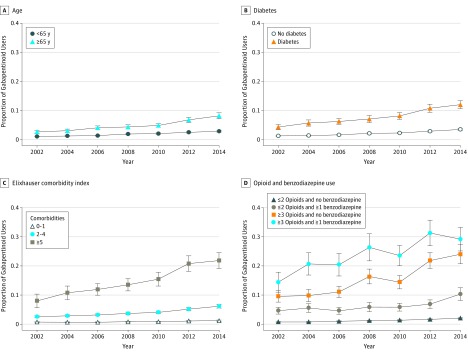
There were increases in use among individuals older than 64 years (Figure 2A) and individuals with diabetes (Figure 2B). Additionally, there was increasing use of gabapentinoids in conjunction with increasing comorbidities (Figure 2C), along with individuals who reported more than 2 opioid prescriptions and/or a benzodiazepine prescription (Figure 2D). In 2014 through 2015, 11.0% (95% CI, 10.5%-11.6%) of the population reported more than 2 opioid prescriptions or a benzodiazepine prescription and accounted for 52.6% (95% CI, 49.2%-56.0%) of gabapentinoid users.
Figure 2. Gabapentinoid Use by Subgroups, 2002 Through 2015.
The figure identifies the proportion of gabapentinoid users among adults stratified by age (<65 vs ≥65 years), presence of diabetes, Elixhauser comorbidity index, and concomitant use of opioids (0-2 and ≥3 prescriptions) and benzodiazepines (any benzodiazepine prescription).
Discussion
The use of gabapentinoids more than tripled between 2002 and 2015. A proportion of the increase was from the introduction of pregabalin, but more recently the increase has been from gabapentin. Importantly, the use of medications from the class was not evenly distributed through the population but concentrated among individuals who were older with numerous comorbidities and/or had numerous opioid prescriptions and/or had a benzodiazepine prescription.
The study had many limitations. Notably, medication use is self-reported and short-term medications, such as opioids, are likely underreported. Gabapentinoid expenditures could not be accurately reported due to high rebate levels for types of branded gabapentin.
The combination of a dearth of long-term safety data, small effect sizes, concern for increased risk of overdose in combination with opioid use, and high rates of off-label prescribing, which are associated with high rates of adverse effects, raises concern about the levels of gabapentinoid use. While individual clinical scenarios can be challenging, caution should be advised in the use of gabapentinoids, particularly for those individuals who are long-term opioid users, given the lack of proven long-term efficacy and the known and unknown risks of gabapentinoid use.
References
- 1.Kesselheim AS, Darby D, Studdert DM, Glynn R, Levin R, Avorn J. False Claims Act prosecution did not deter off-label drug use in the case of neurontin. Health Aff (Millwood). 2011;30(12):2318-2327. [DOI] [PubMed] [Google Scholar]
- 2.Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs. 2014;28(6):491-496. [DOI] [PubMed] [Google Scholar]
- 4.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieson S, Maher CG, McLachlan AJ, et al. Trial of pregabalin for acute and chronic sciatica. N Engl J Med. 2017;376(12):1111-1120. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality (AHRQ), Center for Financing, Access, and Cost Trends MEPS HC-181: 2015 Full Year Consolidated Data File. https://meps.ahrq.gov/data_stats/download_data/pufs/h181/h181doc.pdf. Published August 2017. Accessed September 18, 2017.