Abstract
Importance
While computed tomography (CT) represents a tremendous advance in diagnostic imaging, it also creates the problem of incidental detection—the identification of tumors unrelated to the clinical symptoms that initiate the test.
Objective
To determine the geographic variation in the United States in CT imaging and the corresponding association with one of the most consequential sequelae of incidental detection: nephrectomy.
Design, Setting, and Participants
This study is a cross-sectional analysis of age-, sex-, and race-adjusted Medicare data (January 2010-December 2014) from 306 hospital referral regions (HRRs) in the United States and includes information from 15 million fee-for-service Medicare beneficiaries age 65 to 85 years.
Exposures
Regional CT risk (ie, the proportion of the population receiving either a chest or abdominal CT over 5 years).
Main Outcomes and Measures
Five-year risk of nephrectomy (partial or total).
Results
Data from 15 million fee-for-service Medicare beneficiaries age 65 to 85 years were gathered and illustrate that 43% of Medicare beneficiaries age 65 to 85 years received either a chest or abdominal CT from January 2010 to December 2014. This risk varied across the HRRs, ranging from 31% in Santa Cruz, California, to 52% in Sun City, Arizona. Increased regional CT risk was associated with a higher nephrectomy risk (r = 0.38; 95% CI, 0.28-0.47), particularly among HRRs with more than 50 000 beneficiaries (r = 0.47; 95% CI, 0.31-0.61). After controlling for HRR adult smoking rates, imaging an additional 1000 beneficiaries was associated with 4 additional nephrectomies (95% CI, 3-5). Case-fatality rates for those who underwent nephrectomy were 2.1% at 30 days and 4.3% at 90 days.
Conclusions and Relevance
Fee-for-service Medicare beneficiaries are commonly exposed to CT imaging. Those residing in high-scanning regions face a higher risk of nephrectomy, presumably reflecting the incidental detection of renal masses. Additional surgery should be considered one of the risks of excessive CT imaging.
This cross-sectional analysis of 15 million fee-for-service Medicare beneficiaries age 65 to 85 years examines the geographic variation in use of computed tomography imaging and its association with risk of nephrectomy.
Key Points
Question
How does the proportion of the population undergoing computed tomography (CT) relate to the risk of nephrectomy?
Findings
In this analysis of 306 hospital referral regions, regional CT risk was significantly correlated with nephrectomy, and scanning an additional 1000 Medicare beneficiaries was associated with 4 additional nephrectomies. Beneficiaries residing in high-scanning regions face a higher risk of nephrectomy, presumably reflecting the incidental detection of kidney masses.
Meaning
Additional surgery should be considered one of the risks of excessive CT scanning.
Introduction
The advent of computed tomography (CT) transformed medicine in the early 1970s. Prior to CT the ability to see internal anatomic structures was limited, as conventional radiographs superimpose overlying structures into a single 2-dimensional image. However, CT provides multiple 2-dimensional cross-sectional images that allow previously superimposed structures to be seen distinctly. The initial clinical application was imaging the head, where brain tumors and bleeding could be seen inside the cranium. When faster scanning eliminated the problems of respiratory motion and peristalsis, CT was extended to the chest and abdomen, where individual organs could now be seen in exquisite detail. The invention of CT was revolutionary, as recognized by a Nobel Prize in 1979.
This heightened ability to see inside the body, however, had an unintended side effect: doctors could see things that they had never been able to see before. Computed tomography identified abnormalities that were unrelated to the clinical symptoms that had initiated the diagnostic evaluation. These coincidently detected tumors led a new word to appear in the medical literature: incidentaloma. First applied to adrenal masses, the term was soon extended to incidentally detected tumors of the liver, pituitary gland, thyroid gland, ovary, pancreas, lung, and kidney.
In this study, we examine the breadth of population exposure to thoracoabdominal CT imaging; specifically, the risk of having either a chest or abdominal CT over 5 years (subsequently referred to as imaging risk). We describe the geographic variation of imaging risk across the United States and explore its relationship with one of the most consequential sequela of incidental detection: the risk of nephrectomy.
Methods
Rationale for Nephrectomy
We chose nephrectomy because it is commonly performed for kidney cancer, which, in turn, is commonly diagnosed as an incidentally detected renal mass from the results of an abdominal CT or a chest CT (because of the curvature of the diaphragm, a complete image set of the chest typically includes the kidneys).
There is a growing recognition that not all kidney cancers have the same potential for insidious progression and metastasis. Some kidney tumors may meet the pathologic gold standard for “cancer” yet never progress to cause symptoms or death before the patient dies of other causes. The detection of such tumors has been labeled cancer overdiagnosis.
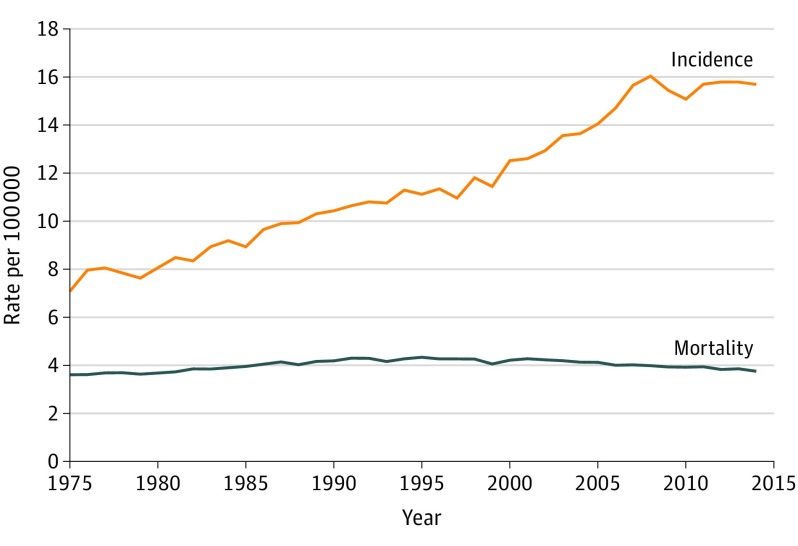
In the Surveillance, Epidemiology, and End Results (SEER) data (functionally, the cancer registry for the United States) the observed incidence of kidney cancer has roughly doubled since the advent of CT. However, as shown in Figure 1, there has been little change in the feared consequence of the disease: the mortality from kidney cancer. The combination of rising incidence and stable mortality is indicative of overdiagnosis. Kidney cancer thus joins melanoma, breast, prostate, and thyroid cancer as a cancer for which overdiagnosis appears to be occurring at the population level. As such many nephrectomies for incidentally detected small kidney tumors may represent overtreatment, causing more harm than benefit.
Figure 1. Kidney Cancer Incidence and Mortality in the United States From 1975 to 2014.
In the Surveillance, Epidemiology, and End Results data (functionally, the cancer registry for the United States), the observed incidence of kidney cancer has roughly doubled since the advent of computed tomography, while mortality has remained stable. The combination of rising incidence and stable mortality is indicative of overdiagnosis.
Unit of Analysis, Population, and Measures
We use Medicare claims to measure an individual’s 5-year risk of imaging and nephrectomy within each of the 306 hospital referral regions (HRRs) in the United States; HRRs are geographic areas representing regional health care markets in which most residents receive their medical care. The HRR is the primary unit of analysis in the Dartmouth Atlas of Health Care and is constructed using the method originally described by Wennberg et al in 1973. Hospital referral region data are population based: they reflect the experience of Medicare patients living in the region, regardless of where the care was actually delivered. We make use of the natural experiment provided by geographic variation to investigate the relationship between imaging risk and nephrectomy. This study was approved by the Dartmouth College institutional review board.
The study population included all Medicare beneficiaries age 65 through 85 years (inclusive) on January 1, 2010. Because of our interest in determining the risk of imaging over a 5-year period, our primary cohort included those who were continuously enrolled in fee-for-service Medicare (ie, excluding beneficiaries who transitioned to Medicare Advantage for whom claims data are unavailable) and who were still alive on December 31, 2014. The resulting study population was 15 513 426 beneficiaries. We also investigated a second, broader cohort that included those who died during the 5-year period while still covered under fee-for-service Medicare (n = 19 586 945).
Our exposure was the cumulative risk of an individual having either a chest or abdominal CT scan over a 5-year period. It is important to emphasize the distinction between this risk measure and a typical utilization rate. A patient who has multiple CTs over 5 years contributes only once to cumulative risk, while the same patient contributes multiple observations to a utilization rate. We believe this cumulative risk measure best addresses the question most relevant to the amount of incidental detection within a region’s population: What proportion of the population has had a CT?
Our outcome measures were the 5-year risk for partial and total nephrectomy. In addition, we also determined the risk of an alternative intervention for kidney cancer: radiofrequency or cryoablation (Current Procedural Terminology codes used for all procedures are provided in eTable 1 in the Supplement). We constructed 2 combined outcomes: any nephrectomy (partial or total nephrectomy) and any renal procedure (any nephrectomy or renal ablation).
Analyses
Our primary analysis is the correlation between imaging risk and renal procedures using the HRR as the unit of analysis. All HRR-level measures were age-, sex-, and race-adjusted using the indirect method (which used 20 age-sex-race cells); Pearson correlation coefficients were weighted by the number of beneficiaries in the HRR.
To determine whether our findings were sensitive to the restriction to those who survived 5 years, we repeated the analysis on the second, broader cohort that included beneficiaries who died. Because the findings from this sensitivity analysis were not materially different (details of this analysis are provided in eMethods in the Supplement), we report on the primary cohort. The second cohort, however, allowed us to determine 30-day and 90-day case-fatality rates for those who underwent nephrectomy.
Finally, because smoking appears to moderately increase the risk of kidney cancer and is also likely associated with imaging, we considered the potential confounding effect of smoking. We derived HRR-level data on the proportion of adults who smoked from the 2010 Behavioral Risk Factor Surveillance Survey. Using the HRR as the unit of analysis (n = 306), we used multiple linear regression to estimate the number of additional nephrectomies and renal procedures associated with an additional 1000 people undergoing thoracoabdominal CT imaging, while controlling for smoking.
Results
Geographic Variation in Imaging Risk
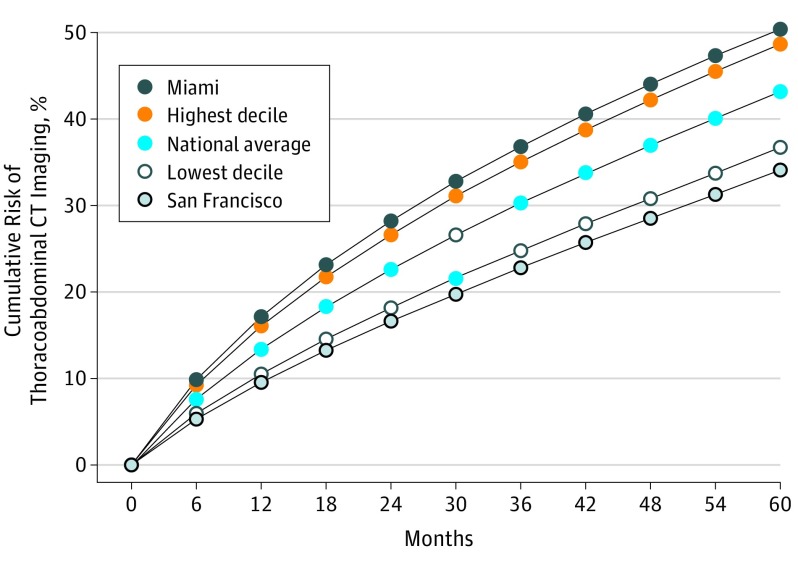
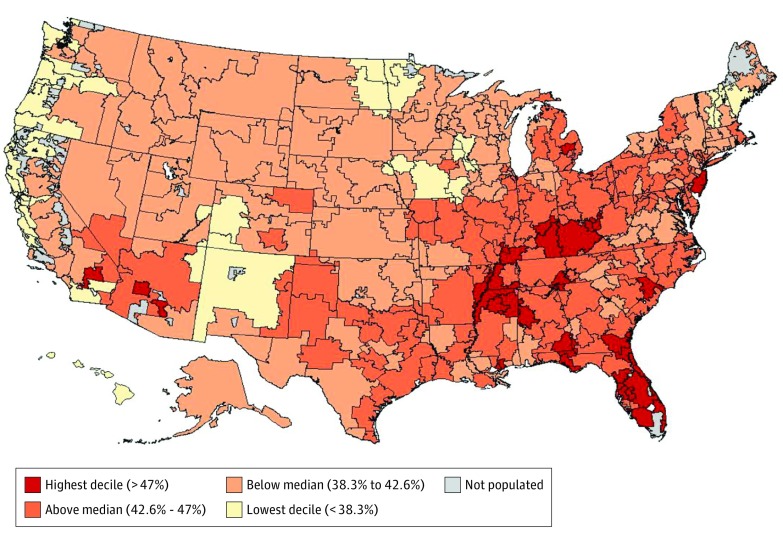
Figure 2 shows how the risk of thoracoabdominal CT imaging accumulates over time. Over the 5-year period we examined, 43% of Medicare beneficiaries had either a chest or abdominal CT. The risk varied across the 306 HRRs, ranging from 31% in Santa Cruz, California, to 52% in Sun City, Arizona, and among HRRs with more than 50 000 beneficiaries, from 34% in San Francisco, California, to 50% in Miami, Florida. Figure 3 illustrates the geographic variation in imaging risk across the United States.
Figure 2. Cumulative Risk of Thoracoabdominal CT Imaging Among Medicare Beneficiaries Age 65 to 85 Years.
Data show how the risk of thoracoabdominal computed tomography (CT) imaging accumulates over time. The risk varied across the 306 hospital referral regions, from 34% in San Francisco, California, to 50% in Miami, Florida.
Figure 3. Five-Year Risk of Thoracoabdominal CT Imaging for Medicare Beneficiaries Age 65 to 85 Years.
This heatmap shows the geographic variation in thoracoabdominal computed tomography (CT) imaging risk across the United States.
Association With Nephrectomy
As shown in the Table, imaging risk was positively correlated with the risk of both total nephrectomy and partial nephrectomy (Pearson r = 0.28; P < .001 for both). The association was strengthened when combining the 2 procedures to consider any nephrectomy (r = 0.38; 95% CI, 0.28-0.47) and further strengthened when restricted to HRRs with more than 50 000 beneficiaries (r = 0.47; 95% CI, 0.31-0.61). After controlling for HRR adult smoking rates, imaging an additional 1000 beneficiaries was associated with 4 additional nephrectomies (95% CI, 3-5). Case-fatality rates for those who underwent nephrectomy were 2.1% at 30 days and 4.3% at 90 days (additional detail on operative case-fatality is provided in eTable 2 in the Supplement).
Table. Correlations Between Imaging Risk and Renal Procedures With Sensitivity to Population Sizea.
| Correlation | Level of Restriction (95% CI) | ||
|---|---|---|---|
| No Restriction (All HRRs) [n = 306] | HRRs >20 000 Beneficiaries [n = 229] | HRRs >50 000 Beneficiaries [n = 104] | |
| Total nephrectomy | 0.28 (0.18-0.38) | 0.31 (0.19-0.42) | 0.34 (0.16-0.50) |
| Partial nephrectomyb | 0.28 (0.17-0.39) | 0.28 (0.16-0.40) | 0.30 (0.11-0.47) |
| Any nephrectomy | 0.38 (0.28-0.47) | 0.41 (0.30-0.51) | 0.47 (0.31-0.61) |
| Renal ablationc | 0.18 (0.05-0.30) | 0.20 (0.06-0.33) | 0.25 (0.06-0.42) |
| Any renal procedure | 0.46 (0.37-0.54) | 0.49 (0.39-0.58) | 0.56 (0.41-0.68) |
Abbreviation: HRR, hospital referral region.
All coefficients are weighted for the HRR fee-for-service Medicare population and have P values less than .01.
Partial nephrectomies were performed in 270 of the 306 HRRs and in 222 of the 229 HRRs with over 20 000 beneficiaries. Partial nephrectomies were performed in all HRRs with over 50 000 beneficiaries.
Renal ablations were performed in 221 of the 306 HRRs, in 197 of 229 HRRs with over 20 000 beneficiaries, and in 103 of the 104 HRRs with over 50 000 beneficiaries.
Association With Any Renal Procedure
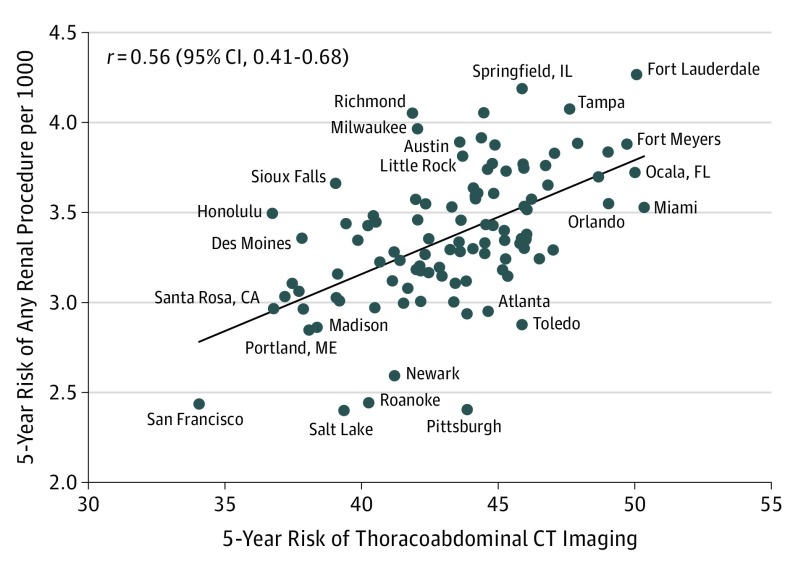
The Table also shows the correlation between CT risk and nephrectomy increases further as renal ablation is combined with nephrectomies, suggesting the 3 individual procedures may be substituting for one another across HRRs. Imaging risk was strongly correlated with any renal procedure (r = 0.46; 95% CI, 0.37-0.54), particularly among HRRs with more than 50 000 beneficiaries (r = 0.56; 95% CI, 0.41-0.68). Figure 4 shows a scatterplot of this relationship. After controlling for HRR adult smoking rates, imaging an additional 1000 beneficiaries was associated with 5.5 additional renal procedures (95% CI, 4-7).
Figure 4. Imaging Risk and the Risk of Any Renal Procedure in the 104 HRRs With More Than 50 000 Beneficiaries.
Imaging risk was strongly correlated with any renal procedure (r = 0.46; 95% CI, 0.37-0.54), particularly among HRRs with more than 50 000 beneficiaries (r = 0.56; 95% CI, 0.41-0.68). HRR indicates hospital referral region.
Discussion
Thoracoabdominal CT is one of the most common procedures in medical care. We found that more than 40% of Medicare beneficiaries undergo the procedure within a 5-year period. A conservative extrapolation would suggest that the majority of the US population ultimately have either a chest or abdominal CT at some point during their life.
We also found that beneficiaries residing in high-scanning regions faced higher risks of total nephrectomy, partial nephrectomy, and renal ablation. The association was apparent for each of the 3 procedures and was strengthened as they were combined. The associations were further strengthened as the sample was restricted to larger HRRs, as the statistical noise of smaller HRRs was eliminated. We believe these findings reflect the incidental detection of small renal masses by CT.
Limitations
Because our findings are based on observational data, our ability to make causal inferences is limited. While claims data are a reliable data source for use of medical care (billing Medicare for services not provided constitutes fraud), they are less reliable indicators of the reason services are being used. Thus, we cannot make any judgments about the appropriateness of CT imaging. Although inappropriate imaging is difficult to define (much less, quantify), there is a widespread consensus that it is common—a view shared by policy makers, payers, and health care providers, including radiologists. This realization has led to the creation of national guidelines for the appropriate use of imaging: the American College of Radiology Appropriateness Criteria and as part of the Choosing Widely Campaign. Starting in January 2018, Medicare providers will be required to consult appropriate use criteria prior to ordering CT and other advanced imaging examinations.
We know more about the indications for surgery. In our data, 67% of the nephrectomies were accompanied with a primary diagnosis code indicating neoplasm, while another 20% were coded as “Unspecified Renal Disease,” a broad category that includes unspecified renal masses. While we lack details regarding the renal masses—for example, whether a biopsy-confirmed cancer prior to nephrectomy or how suspicious the masses appeared on imaging—published series indicate that 70% to 80% of small renal masses are kidney cancer. We are thus confident that most of the nephrectomies are, in fact, for the removal of renal masses, most of which represent kidney cancer.
An alternative explanation for our findings might be that imaging risk is not specifically related to the treatment of small renal masses but is instead simply a reflection of the regional intensity of medical care. In other words, the 5-year risk of thoracoabdominal CT might be associated with many procedures that have nothing to do with the specific CT findings.
To consider this possible limitation, we explored the relationship between imaging risk and radical prostatectomy, which is a procedure that should have nothing to do with incidental CT detection. Here the correlation was insignificant and, in fact, negative (eFigure 1 in the Supplement). This lack of correlation suggests that our findings do not simply reflect regional medical intensity in general, and, further, they do not reflect the supply of urologists in particular.
Clinical Context
Instead, there is a strong theoretical basis for why more imaging would lead to more nephrectomies and renal ablations. There is an autopsy reservoir of undetected kidney cancer: a series of 412 forensic autopsies in North Dakota revealed a prevalence of unsuspected renal cell carcinoma of 1.2% to 1.7%. Because CT is likely more sensitive than autopsy (as its typical ≤5 mm slices are much thinner than those at autopsy), CT is likely capable of uncovering an even larger reservoir. Advanced imaging has increasingly tapped this reservoir, and while most renal cell carcinomas presented as symptomatic disease in the past, most are now the result of incidental detection. The natural history of this newly detected subset has been found to be generally favorable. In a cohort of patients undergoing active surveillance, small (<4 cm diameter) biopsy-proven renal cell carcinomas had an average growth rate of less than 0.2 cm per year, and in fact approximately 25% regressed. These observations juxtaposed with the epidemiologic time trends in Figure 1 suggest that a large proportion incidentally detected kidney cancer represents overdiagnosis. Furthermore, the roughly 40% increase in the rate of nephrectomy over the past 20 years in the face of stable mortality suggests that more nephrectomies have had little effect on the risk of death from kidney cancer. This association is suggestive of overtreatment.
Whether it entails radical or partial nephrectomy or renal ablation, treatment for an incidentally detected renal mass is associated with a significant risk of harm. In addition to the perioperative case-fatality rates reported here, all these interventions require general anesthesia, elevating the risk of cardiovascular events. Furthermore, each is associated with periprocedural complications such as hemorrhage, infection, urine leak, and injury to surrounding organs. Recent studies using the SEER-Medicare data report overall complication rates in the range of 30% to 40% for partial and radical nephrectomy and about 20% for image-guided ablation. Interventions to treat small renal masses also lead to a loss of functioning nephrons and consequent decline in renal function. The resulting renal insufficiency has been associated with lower long-term survival, although this is the subject of some debate. There is less debate, however, that these procedures are also accompanied by human costs: pain, a convalescence period interfering with normal activities, and the distress of being diagnosed with cancer.
Policy Implications
In surgical decision making for small renal masses, the frequency of overdiagnosis combined with the harms of immediate intervention make active surveillance an important option, particularly for elderly patients. In a prospective cohort of 497 patients with small renal masses, the 223 who chose active surveillance had the same 5-year cancer-specific survival as those choosing immediate intervention. Furthermore, only 21 (9%) progressed to receive delayed intervention and none developed distant metastases. Given these data, we believe surgeons should routinely offer active surveillance for small renal masses, and that patients, after being informed about the small risk of developing metastatic disease, should give the option serious consideration.
As our diagnostic technologies are increasingly able to detect small internal abnormalities, incidental detection has become an increasingly common problem for medicine. The US Presidential Commission on Bioethics recently addressed the issue, advising that incidental findings should be anticipated and communicated. We believe that, at a minimum, physicians ordering CT and other scans ought to prepare patients for incidental findings. Doing so could make their occurrence less worrisome and conservative management more acceptable. The same commission also recommended that federal agencies and others fund research on the nature and frequency of incidental findings. Based on our experience, a high priority for research funding is for tumor registries (such as SEER) to collect data on the mode of cancer detection. One 3-level variable—clinically detected (based on symptoms and signs), screen detected, or incidentally detected—would add important information on cancer prognosis and epidemiology.
The decision to perform a diagnostic test requires consideration of its potential benefits and harms. Computed tomography scanning potentially provides tremendous benefits in revealing diagnoses in those who are acutely sick and injured. Perhaps its most widely understood harm is the radiation risk, which is estimated to result in 0.2 cancer deaths per 1000 abdominal CTs in adults. Our findings suggest that the risk of nephrectomy is more than an order of magnitude higher. Given that there are other surgical procedures triggered by incidental detection in other organs, it is likely that unnecessary surgery for overdiagnosed tumors is, in fact, the more important harm.
Conclusions
We should emphasize that surgeons are not purposely performing unnecessary surgery any more than radiologists are purposely engaged in overdiagnosis. Many clinicians understand the downsides of incidental detection. Nevertheless, they are caught in a complex web of forces promoting earlier cancer diagnosis and earlier intervention: strong financial interests, fear of litigation, and unquestioning beliefs among the public about the value of early diagnosis and treatment. More balanced medical care will require addressing these forces.
eTable 1. Current Procedural Terminology (CPT) codes
eMethods. Sensitivity of findings to the choice of population
eTable 2. 30- and 90-day operative case-fatality
eFigure 1. Negative Control: Imaging Prevalence and Radical Prostatectomy
References
- 1.Cormack AM, Hounsfield. Perspectives: With a Little Help From My Friends. https://www.nobelprize.org/nobel_prizes/medicine/laureates/1979/perspectives.html. Accessed October 31, 2017.
- 2.Geelhoed GW, Druy EM. Management of the adrenal “incidentaloma”. Surgery. 1982;92(5):866-874. [PubMed] [Google Scholar]
- 3.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913-924. [DOI] [PubMed] [Google Scholar]
- 4.Daskivich TJ, Tan H-J, Litwin MS, Hu JC. Life expectancy and variation in treatment for early stage kidney cancer. J Urol. 2016;196(3):672-677. [DOI] [PubMed] [Google Scholar]
- 5.Gill IS, Aron M, Gervais DA, Jewett MAS. Clinical practice: small renal mass. N Engl J Med. 2010;362(7):624-634. [DOI] [PubMed] [Google Scholar]
- 6.Williamson SR, Cheng L. Clear cell renal cell tumors: not all that is “clear” is cancer. Urol Oncol. 2016;34(7):292.e17-292.e22. [DOI] [PubMed] [Google Scholar]
- 7.The Surveillance, Epidemiology, and End Results SEER Program (http://www.seer.cancer.gov) SEER*Stat Database: (1975-2014). DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016 submission. https://seer.cancer.gov/faststats/selections.php?run=runit&output=1&statistic=1&cancer=72&year=201701&race=1&sex=1&age=1&series=data&data=1;2. Accessed November 3, 2017.
- 8.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. [DOI] [PubMed] [Google Scholar]
- 9.Wennberg J, Gittelsohn AM. Small area variations in health care delivery. Science. 1973;182(4117):1102-1108. [DOI] [PubMed] [Google Scholar]
- 10.Naing NN. Easy way to learn standardization: direct and indirect methods. Malays J Med Sci. 2000;7(1):10-15. [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114(1):101-108. [DOI] [PubMed] [Google Scholar]
- 12.Hendee WR, Becker GJ, Borgstede JP, et al. Addressing overutilization in medical imaging. Radiology. 2010;257(1):240-245. [DOI] [PubMed] [Google Scholar]
- 13.https://www.acr.org/Quality-Safety/Appropriateness-Criteria/About-AC.
- 14.Rao VM, Levin DC. The overuse of diagnostic imaging and the Choosing Wisely initiative. Ann Intern Med. 2012;157(8):574-576. [DOI] [PubMed] [Google Scholar]
- 15.American College of Radiology. About the ACR Appropriateness Criteria. https://www.acr.org/Advocacy/Economics-Health-Policy/Clinical-Decision-Support. Accessed October 31, 2017.
- 16.Akdogan B, Gudeloglu A, Inci K, Gunay LM, Koni A, Ozen H. Prevalence and predictors of benign lesions in renal masses smaller than 7 cm presumed to be renal cell carcinoma. Clin Genitourin Cancer. 2012;10(2):121-125. [DOI] [PubMed] [Google Scholar]
- 17.Richard PO, Jewett MAS, Bhatt JR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol. 2015;68(6):1007-1013. [DOI] [PubMed] [Google Scholar]
- 18.Sens MA, Zhou X, Weiland T, Cooley AM. Unexpected neoplasia in autopsies: potential implications for tissue and organ safety. Arch Pathol Lab Med. 2009;133(12):1923-1931. [DOI] [PubMed] [Google Scholar]
- 19.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203-205. [DOI] [PubMed] [Google Scholar]
- 20.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982-1997). Urology. 2000;56(1):58-62. [DOI] [PubMed] [Google Scholar]
- 21.Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60(1):39-44. [DOI] [PubMed] [Google Scholar]
- 22.HCUPnet. Healthcare Cost and Utilization Project (HCUP). 1993-2014. Agency for Healthcare Research and Quality, Rockville, MD. http://hcupnet.ahrq.gov/. Accessed August 30, 2017. [PubMed]
- 23.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):2215-2245. [DOI] [PubMed] [Google Scholar]
- 24.Tan H-J, Wolf JS Jr, Ye Z, Wei JT, Miller DC. Complications and failure to rescue after laparoscopic versus open radical nephrectomy. J Urol. 2011;186(4):1254-1260. [DOI] [PubMed] [Google Scholar]
- 25.Larcher A, Fossati N, Tian Z, et al. Prediction of complications following partial nephrectomy: implications for ablative techniques candidates. Eur Urol. 2016;69(4):676-682. [DOI] [PubMed] [Google Scholar]
- 26.Tan H-J, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65(2):372-377. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RH. Partial versus radical nephrectomy: the debate regarding renal function ends while the survival controversy continues. Eur Urol. 2014;65(2):378-379. [DOI] [PubMed] [Google Scholar]
- 29.Vetterlein MW, Jindal T, Becker A, et al. Small renal masses in the elderly: Contemporary treatment approaches and comparative oncological outcomes of nonsurgical and surgical strategies. Investig Clin Urol. 2016;57(4):231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abouassaly R, Lane BR, Novick AC. Active surveillance of renal masses in elderly patients. J Urol. 2008;180(2):505-508. [DOI] [PubMed] [Google Scholar]
- 31.Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7(10):754-773. [DOI] [PubMed] [Google Scholar]
- 32.Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. 2015;68(3):408-415. [DOI] [PubMed] [Google Scholar]
- 33.Gutmann A. Ethics. The bioethics commission on incidental findings. Science. 2013;342(6164):1321-1323. [DOI] [PubMed] [Google Scholar]
- 34.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. [DOI] [PubMed] [Google Scholar]
- 35.Welch HG, Schwartz LM, Woloshin S. Overdiagnosed: Making People Sick in the Pursuit of Health. Boston, Massachusetts: Beacon Press; 2011:151-166. [Google Scholar]
- 36.Schwartz LM, Woloshin S, Welch HG. Not so silver lining. Arch Intern Med. 2011:171(6):489-490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Current Procedural Terminology (CPT) codes
eMethods. Sensitivity of findings to the choice of population
eTable 2. 30- and 90-day operative case-fatality
eFigure 1. Negative Control: Imaging Prevalence and Radical Prostatectomy