Key Points
Question
Does the duration since initial use and new use of inhaled long-acting β2-agonists (LABAs) or antimuscarinic antagonists (LAMAs) for the treatment of chronic obstructive pulmonary disease (COPD) act as important determinants of the risk of cardiovascular disease?
Findings
In this nested case-control study of more than 280 000 patients with COPD, new use of LABAs or LAMAs is associated with an approximate 1.5-fold increased cardiovascular risk within 30 days of initiation therapy.
Meaning
Health care professionals need to be very vigilant with regard to any cardiovascular symptoms within 30 days of initiating LABA or LAMA treatment for COPD.
Abstract
Importance
The associations between cardiovascular disease (CVD) and inhaled long-acting β2-agonists (LABAs) or long-acting antimuscarinic antagonists (LAMAs) in chronic obstructive pulmonary disease (COPD) are greatly debated. Pivotal and relevant randomized clinical trials included prior LABA or LAMA users and excluded patients with baseline CVD; therefore, cardiovascular events arising from first-time LABA or LAMA use, if any, could not be observed. There is an urgent need to examine whether new use of and duration since initiating LABAs and LAMAs could act as important determinants of cardiovascular events.
Objective
To investigate risk of CVD associated with LABAs and LAMAs, focusing on the initiation and duration of LABA and LAMA therapies.
Design, Setting, and Participants
This nested case-control study included 284 220 LABA-LAMA–naïve patients with COPD at least 40 years old (mean age, 71.4 years; 68.9% men), retrieved from the Taiwan National Health Insurance Research Database for health care claims from 2007 to 2011.
Exposure
LABA or LAMA use was measured in the year preceding the event or index date, stratified by duration since initiation of LABA or LAMA treatment, new and prevalent users, concomitant COPD medications, and individual agents.
Main Outcomes and Measures
Cases with inpatient or emergency care visits for coronary artery disease, heart failure, ischemic stroke, or arrhythmia were identified and individually matched to 4 randomly selected controls. Conditional logistic regressions were performed to estimate odds ratios of CVD from LABA and LAMA treatment.
Results
During a mean follow-up of 2.0 years, 37 719 patients with CVD (mean age, 75.6 years; 71.6% men) and 146 139 matched controls (mean age, 75.2 years; 70.1% men) were identified. New LABA and LAMA use in COPD was associated with a 1.50-fold (95% CI, 1.35-1.67; P < .001) and a 1.52-fold (95% CI, 1.28-1.80; P < .001) increased cardiovascular risk within 30 days of initiation, respectively, whereas the risk was absent, or even reduced with prevalent use. Individual LABA agents, LAMA dosage forms, and concomitant COPD regimens did not differ in the CVD risks. The risk persisted in an alternative case-crossover study and remained across subgroups without CVD history or prior exacerbations.
Conclusions and Relevance
New initiation of LABAs or LAMAs in patients with COPD is associated with an approximate 1.5-fold increased severe cardiovascular risk, irrespective of prior CVD status and history of exacerbations.
This nested case-control study investigates the risk of cardiovascular disease associated with long-acting β2-agonists and long-acting antimuscarinic antagonists, focusing on the initiation and duration of both therapies.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic and irreversible inflammatory lung disease, presently posing a significant burden to health care systems across the world. Long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) are the mainstay therapies for COPD; however, these agents were found to increase the risk of cardiovascular disease (CVD), although the findings varied, concluding that there was no increased risk or that there was a 1.1- to 4.5-fold increased cardiovascular risk. These studies, even a large randomized clinical trial (RCT), generally observed few cardiovascular events, excluded patients with severe illness, and obtained incomplete medication records as well as dropped more than 50% of eligible patients. All of these limitations could potentially weaken the causality or generalizability of the association.
Notably, inclusion of patients with tolerability to the risk of CVD receiving LABA or LAMA therapy is probably a major drawback in previous large RCTs. The 4-year Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial and the 3-year Toward a Revolution in COPD Health (TORCH) trial, which observed a reduced or no increase in CVD risk, included more than 45% and 35% of participants who had received inhaled cholinergics and LABAs, respectively, and the 2 trials excluded patients with a history of recent CVD and life-threatening cardiovascular events. Accordingly, patients who had developed severe CVD with new use of LABAs or LAMAs could have been excluded, and only those LABA or LAMA prevalent users who had probably developed tolerance to the cardiovascular risk were included in the trials. Accordingly, we suspect that new use of and duration since initiating LABAs and LAMAs could act as important determinants of CVD risk, which to date have not been examined in details in previous studies.
The present study investigated use of LABAs and LAMAs associated with the risk of CVD in a nationwide population of patients with COPD, focusing on new use and duration of therapy, individual agents, dosage forms, and concomitant COPD regimens.
Methods
Study Design and Data Source
We performed a nested case-control study of a nationwide COPD population 40 years or older with the data retrieved from the Taiwan National Health Insurance Research Database (NHIRD) from January 1, 2007, to December 31, 2011, as depicted in eFigure 1 in the Supplement. The NHIRD contains all medical and pharmacy claims records from all medical care settings for more than 99% of the 23 million Taiwanese inhabitants covered under a compulsory and universal national health insurance. The claims are audited quarterly by the National Health Insurance Administration, and multiple disease diagnosis codes in the database have been validated, including several CVD diagnoses. The study was exempt from a full review by the institutional review board of Tri-Service General Hospital, National Defense Medical Center.
Identification of Study Cohort
We identified patients with COPD 40 years or older who had made 2 outpatient visits or an inpatient visit for COPD (International Classification of Diseases, Ninth Revision [ICD-9] codes 491.xx, 492.xx, and 496.xx) in a single year from January 1, 2008, to June 30, 2011, accompanied by a record of filling at least 1 COPD medication at each visit. The cohort entry date was set as the date of the first COPD outpatient visit or the discharge date from a COPD hospitalization. We excluded patients who had received any LABA-LAMA therapy or who lacked continuous health insurance coverage for 1 year preceding cohort entry. We followed-up the remaining patients until the earliest of CVD outcome (defined in the outcome measurement), National Health Insurance program withdrawal, death, or the end of the study period (December 31, 2011). We determined mortality from the NHIRD based on a previously reported approach.
Case Identification
We identified CVD cases as patients who had made an inpatient or emergency department (ER) visit with a primary diagnosis of coronary heart disease (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM], codes 410–414), cardiac arrhythmia (code 427), heart failure (code 428), or ischemic stroke (codes 433-434). The adopted coding system for acute myocardial infarction, heart failure, and ischemic stroke has been validated with high accuracy. We set the first CVD event as the index date.
Each case was matched to 4 randomly selected controls from risk set samples by cohort entry date (±180 days) and disease risk score (DRS) (±0.01) of predicting the occurrence of a cardiovascular outcome during follow-up. Use of the DRS to match cases with controls yields better statistical precision than does exact matching on multiple discrete factors in a nested case-control setting. Estimation of the DRS is detailed in eMethods 1 in the Supplement.
Exposure Measurement
We examined all LABA and LAMA prescription records in the year before the index date for both cases and controls. Specifically, drug use was classified into current (≤30 days), recent (31-90 days), past (91-180 days), and remote (>180 days) use based on the most recent prescription date preceding the index date. Current users were further classified as new users if they had no other dispensing records in the 31 to 365 days before the index date and prevalent users otherwise. We also used restricted cubic spline models to analyze the duration of new LABA and new LAMA therapy (as detailed in eMethods 2 in the Supplement). New use of LABAs and LAMAs was also categorized according to various combinations with COPD medication agents, individual LABAs, and different drug-containing devices of LAMAs. Individual agents of LABAs and LAMAs comprised salmeterol, formoterol, and tiotropium. Nonusers of LABAs-LAMAs and new theophylline users were set as the reference group in the main analyses and sensitivity analyses, respectively.
Measurement of Covariates
Important determinants of CVD were considered, such as prior CVDs, hypertension, diabetes, hyperlipidemia, use of CVD medications, and agents related to cardiotoxicity. We also considered proxy indicators of COPD severity, including the number of COPD-related outpatient visits accompanied by oral corticosteroids or respiratory antibiotic prescription records (referred to as moderate exacerbation) and the number of severe exacerbation events (defined as any inpatient or ER admission for COPD), as well as the presence of incident or prevalent COPD. Other factors related to LABA-LAMA use or CVD were considered (as detailed in eTable 1 in the Supplement). We first measured these factors in the year preceding cohort entry, and included them in a logistic regression model to estimate the DRS of encountering a CVD outcome during follow-up (eMethods 1 in the Supplement). Second, we treated these confounders as time-varying effects by also assessing concomitant medications and the remaining factors during the 6 months and in the year prior to the index date, respectively.
Statistical Analysis
The covariate balance before and after DRS matching was assessed using the standardized difference, and meaningful imbalances between groups were determined when the standardized difference was greater than 0.1. We used conditional logistic regressions to estimate the odds ratio (OR) of CVD with LABA or LAMA use. We expressed the absolute risk of CVD arising from LABA and LAMA treatment as the number needed to harm (NNTH) according to a previously reported formula.
Data sets were constructed and analyzed by using SAS (version 9.3; SAS Institute Inc) and STATA (version 13; StataCorp) statistical software, respectively. We defined tests with 2-sided P < .05 as significant.
Sensitivity and Subgroup Analysis
Multiple additional analyses were performed. First, we adopted a case-crossover study to avoid selection bias and minimize time-invariant confounding (eMethods 3 and eFigure 2 in the Supplement). Second, to mitigate confounding by indication bias, we repeated the analyses by treating patients who began new treatment with theophyllines, another class of bronchodilators used for COPD therapy, in the 30 days before the index date as the reference group. Third, to address protopathic bias, we used a lag-time approach that disregarded any prescription records of LABAs and LAMAs in the 7 days before the index date and restricted new LABA and LAMA users to those who received spirometry testing within 30 days before or on the date of initiating these medications to justify respiratory medication use. We also excluded patients who started any cardiovascular medications in the 30 days before the index date and those with any chest pain (ICD-9 code 786.5) or dyspnea/breathing difficulty diagnoses (code 786.0) as possible presymptoms of CVD. Fourth, events of heart failure were excluded from the CVD outcome. Fifth, we also performed stratified analyses by CVD care type, baseline CVD status, CVD severity (fatal vs nonfatal), COPD severity, asthma comorbidity, use of theophyllines, and use of systemic short-acting β2-agonists. Sixth, we adjusted for all covariates that were measured preceding the index date in Table 1 and evaluated the impact of unmeasured confounding using a rule-out approach, as detailed in eFigure 3 in the Supplement.
Table 1. Clinical Characteristics Between Cases and Matched Controlsa.
| Characteristic | At Baseline, No. (%)a | During Follow-up, No. (%)b | ||||
|---|---|---|---|---|---|---|
| Cases (n = 37 719) |
Controls (n = 146 139) |
SDiffc | Cases (n = 37 719) |
Controls (n = 146 139) |
SDiffc | |
| Age, mean (SD), y | 75.6 (10.3) | 75.2 (10.2) | 0.042 | 75.6 (10.3) | 75.2 (10.2) | 0.042 |
| Sex, male No. (%) | 27 019 (71.6) | 102 404 (70.1) | 0.034 | 27 019 (71.6) | 102 404 (70.1) | 0.034 |
| Prior CVDd | ||||||
| CAD | ||||||
| None | 21 304 (56.5) | 86 351 (59.1) | 0.008 | 22 565 (59.8) | 92 072 (63.0) | 0.065 |
| Hospitalization or ER visits | 3687 (9.8) | 7795 (5.3) | 0 | 0 | ||
| History of CAD | 12 728 (33.7) | 51 993 (35.6) | 15 154 (40.2) | 54 067 (37.0) | ||
| Heart failure | ||||||
| None | 27 958 (74.1) | 115 949 (79.3) | 0.078 | 28 674 (76.0) | 117 107 (80.1) | 0.100 |
| Hospitalization or ER visits | 2912 (7.7) | 4877 (3.3) | 0 | 0 | ||
| History of heart failure | 6849 (18.2) | 25 313 (17.3) | 9045 (24.0) | 29 032 (19.9) | ||
| Ischemic stroke | ||||||
| None | 32 072 (85.0) | 126 408 (86.5) | 0.028 | 33 282 (88.2) | 129 718 (88.8) | 0.017 |
| Hospitalization or ER visits | 1903 (5.1) | 5610 (3.8) | 0 | 0 | ||
| History of ischemic stroke | 3744 (9.9) | 14 121 (9.7) | 4437 (11.8) | 16 421 (11.2) | ||
| Cardiac arrhythmia | ||||||
| None | 29 143 (77.3) | 116 390 (79.6) | 0.046 | 30 034 (79.6) | 118 225 (80.9) | 0.032 |
| Hospitalization or ER visits | 902 (2.4) | 1917 (1.3) | 0 | 0 | ||
| History of arrhythmia | 7674 (20.4) | 27 832 (19.0) | 7685 (20.4) | 27 914 (19.1) | ||
| Hypertension | 26 501 (70.3) | 103 676 (70.9) | 0.015 | 24 136 (64.0) | 92 373 (63.2) | 0.016 |
| Diabetes | 11 482 (30.4) | 43 119 (29.5) | 0.020 | 10 977 (29.1) | 39 884 (27.3) | 0.040 |
| Asthma | 7979 (21.2) | 29 743 (20.4) | 0.020 | 8087 (21.4) | 27 889 (19.8) | 0.059 |
| Newly diagnosed COPD | 16 030 (42.5) | 58 272 (39.9) | 0.053 | 16 030 (42.5) | 58 272 (39.9) | 0.053 |
| Severe COPD exacerbations. No.e | ||||||
| 0 | 33 081 (87.7) | 131 099 (89.7) | 0.066 | 33 592 (89.1) | 136 272 (93.3) | 0.148 |
| 1 | 3778 (10.0) | 12 563 (8.6) | 3404 (9.0) | 8452 (5.8) | ||
| ≥2 | 860 (2.3) | 2477 (1.7) | 723 (1.9) | 1415 (1.0) | ||
| COPD Severity Indicators, No. (%) | ||||||
| Moderate COPD exacerbations, No.f | ||||||
| 0 | 33 281 (88.2) | 128 393 (87.9) | 0.013 | 33 418 (88.6) | 132 875 (90.9) | 0.071 |
| 1 | 2938 (7.8) | 11 627 (8.0) | 3353 (8.9) | 10 295 (7.0) | ||
| ≥2 | 1500 (4.0) | 6119 (4.2) | 948 (2.5) | 2969 (2.0) | ||
| Type of COPD medications, No. | ||||||
| 0 | 16 003 (42.4) | 63 413 (43.4) | 0.033 | 16 137 (42.8) | 65 959 (45.1) | 0.106 |
| 1 | 14 755 (39.1) | 57 933 (39.6) | 15 221 (40.4) | 62 961 (43.1) | ||
| ≥2 | 6961 (18.5) | 24 793 (17.0) | 6361 (16.9) | 17 219 (11.8) | ||
| Site of the initial diagnosis of COPD | ||||||
| Outpatient | 35 618 (94.4) | 139 686 (95.6) | 0.053 | 35 618 (94.4) | 139 686 (95.6) | 0.053 |
| Inpatient | 2101 (5.6) | 6453 (4.4) | 2101 (5.6) | 6453 (4.4) | ||
| Health Care Use, No. (%) | ||||||
| Outpatient visits, No. | ||||||
| ≤16 | 8717 (23.1) | 31 972 (21.9) | 0.022 | 16 789 (44.5) | 67 789 (46.4) | 0.045 |
| 12-31 | 11 915 (31.6) | 47 272 (32.4) | 8582 (22.8) | 33 452 (22.9) | ||
| ≥32 | 17 087 (45.3) | 66 895 (45.8) | 12 348 (32.7) | 44 898 (30.7) | ||
| Comorbidities, No. (%) | ||||||
| Pulmonary disease | ||||||
| Acute bronchitis | 14 137 (37.5) | 55 088 (37.7) | 0.004 | 10 155 (26.9) | 38 019 (26.0) | 0.021 |
| Pneumonia | 9379 (24.9) | 30 843 (21.1) | 0.089 | 8782 (23.3) | 29 493 (20.2) | 0.075 |
| Influenza | 1978 (5.2) | 7782 (5.3) | 0.004 | 1412 (3.7) | 5256 (3.6) | 0.008 |
| Pulmonary embolism | 136 (0.4) | 479 (0.3) | 0.006 | 153 (0.4) | 389 (0.3) | 0.024 |
| CVD | ||||||
| Peripheral vascular disease | 2585 (6.9) | 9357 (6.4) | 0.018 | 2141 (5.7) | 6908 (4.7) | 0.043 |
| Rheumatic heart disease | 1432 (3.8) | 3512 (2.4) | 0.080 | 1008 (2.7) | 2192 (1.5) | 0.082 |
| Hemorrhagic stroke | 860 (2.3) | 3146 (2.2) | 0.009 | 672 (1.8) | 2333 (1.6) | 0.014 |
| Hyperlipidemia | 8245 (21.9) | 32 994 (22.6) | 0.017 | 6483 (17.2) | 25 480 (17.4) | 0.007 |
| Cancer | 5394 (14.3) | 18 644 (12.8) | 0.045 | 4987 (13.2) | 18 843 (12.9) | 0.010 |
| Renal failure | 3971 (10.5) | 12 882 (8.8) | 0.058 | 4063 (10.8) | 11 536 (7.9) | 0.099 |
| Dementia | 3472 (9.2) | 11 904 (8.2) | 0.038 | 3594 (9.5) | 11 892 (8.1) | 0.049 |
| Chronic liver disease | 3498 (9.3) | 13 466 (9.2) | 0.002 | 2707 (7.2) | 10 187 (7.0) | 0.008 |
| Parkinsonism | 1806 (4.8) | 6668 (4.6) | 0.011 | 1688 (4.5) | 5971 (4.1) | 0.019 |
| Comedication, No. (%) | ||||||
| CV medication | ||||||
| Antiplatelets | 23 272 (61.7) | 89 672 (61.4) | 0.007 | 18 967 (50.3) | 65 648 (44.9) | 0.108 |
| Calcium channel blockers | 23 836 (63.2) | 92 158 (63.1) | 0.003 | 18 595 (49.3) | 69 021 (47.2) | 0.041 |
| Diuretics | 22 495 (59.6) | 84 070 (57.5) | 0.043 | 18 420 (48.8) | 63 820 (43.7) | 0.104 |
| Angiotensin receptor blockers | 14 032 (37.2) | 52 040 (35.6) | 0.033 | 11 716 (31.1) | 41 621 (28.5) | 0.056 |
| Angiotensin-converting enzyme inhibitor | 11 149 (29.6) | 41 967 (28.7) | 0.019 | 6241 (16.6) | 19 453 (13.3) | 0.091 |
| β-Blockers | ||||||
| CV-selective | 7762 (20.6) | 29 760 (20.4) | 0.005 | 4998 (13.3) | 16 740 (11.5) | 0.055 |
| Non–CV-selective | 10 520 (27.9) | 39 172 (26.8) | 0.024 | 5945 (15.8) | 19 300 (13.2) | 0.073 |
| Digoxin | 5346 (14.2) | 17 067 (11.7) | 0.074 | 4261 (11.3) | 12 811 (8.8) | 0.084 |
| Antiarrhythmic agents | 4972 (13.2) | 15 769 (10.8) | 0.074 | 3525 (9.4) | 10 835 (7.4) | 0.070 |
| Nitrates | 5459 (14.5) | 17 746 (12.1) | 0.069 | 3032 (8.0) | 9129 (6.3) | 0.070 |
| Anticoagulants | 4701 (12.5) | 15 263 (10.4) | 0.063 | 2280 (6.0) | 6338 (4.3) | 0.077 |
| Lipid-lowering agents | ||||||
| Statins | 7323 (19.4) | 27 768 (19.0) | 0.010 | 5114 (13.6) | 18 485 (12.7) | 0.027 |
| Others | 152 (0.4) | 546 (0.4) | 0.005 | 70 (0.2) | 254 (0.2) | 0.003 |
| COPD medications | ||||||
| Methylxanthines | 14 037 (37.2) | 59 209 (40.5) | 0.068 | 16 726 (44.3) | 65 386 (44.7) | 0.008 |
| Short-acting β2-agonists | ||||||
| Oral/injection | 17 027 (45.1) | 64 531 (44.2) | 0.020 | 17 027 (45.1) | 64 531 (44.2) | 0.020 |
| Nebulized | 3749 (9.9) | 12 077 (8.3) | 0.058 | 2398 (6.4) | 6246 (4.3) | 0.093 |
| Inhaled | ||||||
| 0 Canister | 25 096 (66.5) | 104 088 (71.2) | 0.100 | 28 153 (74.6) | 119 237 (81.6) | 0.163 |
| ≤6 Canisters | 10 717 (28.4) | 36 068 (24.7) | 8441 (22.4) | 23 890 (16.4) | ||
| >6 Canisters | 1906 (5.1) | 5983 (4.1) | 1125 (3.0) | 3012 (2.1) | ||
| Short-acting muscarinic antagonists | ||||||
| Nebulized | 8788 (23.3) | 28 177 (19.3) | 0.098 | 5320 (14.1) | 12 953 (8.9) | 0.165 |
| Inhaled | ||||||
| 0 Canister | 31 267 (82.9) | 125 117 (85.6) | 0.071 | 32 428 (86.0) | 131 247 (89.8) | 0.114 |
| ≤6 Canisters | 5652 (15.0) | 18 413 (12.6) | 4891 (13.0) | 13 776 (9.4) | ||
| >6 Canisters | 800 (2.1) | 2609 (1.8) | 400 (1.1) | 1116 (0.8) | ||
| Inhaled corticosteroids | 1662 (4.4) | 5734 (3.9) | 0.024 | 3972 (10.5) | 13 785 (9.4) | 0.037 |
| Oral long-acting β2-agonists | 2 (0.01) | 13 (0.01) | 0.004 | 546 (1.5) | 1766 (1.2) | 0.021 |
| Systemic anticholinergic | ||||||
| Antihistamine | 24 062 (63.8) | 92 993 (63.6) | 0.003 | 13 218 (35.0) | 47 868 (32.8) | 0.048 |
| Gastrointestinal antispasmodics | 8299 (22.0) | 31 814 (21.8) | 0.006 | 3460 (9.2) | 12 304 (8.4) | 0.027 |
| Bladder antimuscarinics | 3036 (8.1) | 11 780 (8.1) | <0.001 | 1521 (4.0) | 5614 (3.8) | 0.010 |
| Others | 4622 (12.3) | 18 105 (12.4) | 0.004 | 2086 (5.5) | 7439 (5.1) | 0.020 |
| NSAIDs | 31 105 (82.5) | 120 663 (82.6) | 0.003 | 19 788 (52.5) | 75 109 (51.4) | 0.021 |
| Systematic corticosteroids | 20 149 (53.4) | 73 517 (50.3) | 0.062 | 13 606 (36.1) | 44 309 (30.3) | 0.122 |
| Antipsychotic | 9396 (24.9) | 34 394 (23.5) | 0.032 | 5432 (14.4) | 17 237 (11.8) | 0.077 |
| Antidepressant | 7373 (19.6) | 28 311 (19.4) | 0.004 | 4326 (11.5) | 15 345 (10.5) | 0.031 |
| Vaccine | 13 330 (35.3) | 54 564 (37.3) | 0.042 | 12 997 (34.5) | 54 170 (37.1) | 0.054 |
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; CVD, cardiovascular disease; ER, emergency department; DRS, disease risk score; NSAIDs, nonsteroidal anti-inflammatory drugs; SDiff, standardized difference.
All comorbidities and comedications were measured in the year preceding entry date.
All comorbidities were measured in the year, and comedications in the 6 months before index date.
Covariates with SDiff> 0.1 represent meaningful differences between groups.
Prior CVDs were measured in 3 severity levels.
Severe COPD exacerbation was defined as patients requiring hospital or ER visits for COPD.
Moderate COPD exacerbation included patients who were prescribed with either an antibiotics or oral corticosteroid in an outpatient COPD visit.
Results
The study cohort consisted of 284 220 LABA-LAMA-naive patients with COPD with a mean age of 71.4 years, 68.9% of whom were male. During a mean follow-up of 2.0 years, we identified 37 719 patients with severe CVD requiring hospitalization or emergency care, at a rate of 6.6 per 100 person-years, and included 146 139 matched controls (eFigure 4 in the Supplement).
All characteristics were balanced prior to cohort entry between cases and controls (Table 1). During follow-up, most of the covariates remained balanced between the 2 groups, and only a few factors differed significantly higher between cases and controls, for which statistical adjustment was performed.
As indicated in Table 2, overall use of inhaled long-acting bronchodilators was not associated with an increased risk of CVD across different recency of therapy, although a 10% decrease in cardiovascular risk was observed with past LABA use. Among current users, new initiation of LABA and LAMA treatment was associated with a 1.50-fold (95% CI, 1.35-1.67; P < .001) and a 1.52-fold (95% CI, 1.28-1.80; P < .001) increased cardiovascular risk, respectively, while prevalent LABA or LAMA use yielded a 9% to 12% reduction in risk. In addition, new LABA use vs new LAMA use yielded no difference in the risk of CVDs (P = .93) (eTable 2 in the Supplement).
Table 2. Risk of CVD With Prior Use of LABA and LAMA Compared With Nonuse, Stratified by Recencya.
| Bronchodilator | Casesb (n = 37 719) |
Controlsb (n = 146 139) |
Crude OR (95% CI) | P Value | Adjusted ORc (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Nonuse of LABA or LAMA, No. (%) | 31 732 (84.1) | 124 943 (85.5) | 1 [Reference] | 1 [Reference] | ||
| Current bronchodilator use (≤30 d) | ||||||
| LABA | 1482 (3.9) | 4981 (3.4) | 1.18 (1.12-1.26) | <.001 | 1.06 (0.99-1.12) | .08 |
| New use | 520 (1.4) | 1186 (0.8) | 1.74 (1.57-1.93) | <.001 | 1.50 (1.35-1.67) | <.001 |
| Prevalent use | 962 (2.6) | 3795 (2.6) | 1.01 (0.94-1.08) | .81 | 0.91 (0.85-0.98) | .02 |
| LAMA | 648 (1.7) | 2440 (1.7) | 1.05 (0.96-1.15) | .26 | 1.00 (0.92-1.10) | .97 |
| New use | 190 (0.5) | 463 (0.3) | 1.62 (1.37-1.92) | <.001 | 1.52 (1.28-1.80) | <.001 |
| Prevalent use | 458 (1.2) | 1977 (1.4) | 0.92 (0.83-1.02) | .11 | 0.88 (0.79-0.98) | .02 |
| LABA and LAMA | 581 (1.5) | 1706 (1.2) | 1.37 (1.24-1.50) | <.001 | 1.16 (1.05-1.28) | .003 |
| New use | 50 (0.1) | 84 (0.1) | 2.38 (1.68-3.38) | <.001 | 2.03 (1.42-2.91) | <.001 |
| Prevalent use | 531 (1.4) | 1622 (1.1) | 1.31 (1.19-1.45) | <.001 | 1.11 (1.00-1.23) | .04 |
| Recent bronchodilator use (31-90 d) | ||||||
| LABA | 787 (2.1) | 2770 (1.9) | 1.13 (1.04-1.22) | .004 | 0.97 (0.89-1.05) | .47 |
| LAMA | 304 (0.8) | 1129 (0.8) | 1.07 (0.94-1.22) | .28 | 1.00 (0.88-1.14) | .98 |
| LABA and LAMA | 192 (0.5) | 604 (0.4) | 1.28 (1.09-1.51) | .003 | 1.09 (0.92-1.29) | .31 |
| Past bronchodilator use (91-180 d) | ||||||
| LABA | 621 (1.7) | 2384 (1.6) | 1.03 (0.95-1.13) | .46 | 0.90 (0.83-0.99) | .03 |
| LAMA | 205 (0.5) | 875 (0.6) | 0.93 (0.80-1.08) | .36 | 0.86 (0.74-1.00) | .06 |
| LABA and LAMA | 98 (0.3) | 338 (0.2) | 1.14 (0.91-1.43) | .26 | 0.95 (0.76-1.20) | .69 |
| Remote bronchodilator use (>180 d) | ||||||
| LABA | 738 (2.0) | 2643 (1.7) | 1.11 (1.02-1.21) | .01 | 1.04 (0.96-1.13) | .33 |
| LAMA | 259 (0.7) | 1046 (0.7) | 0.98 (0.86-1.13) | .82 | 0.96 (0.84-1.10) | .55 |
| LABA and LAMA | 72 (0.2) | 280 (0.2) | 1.03 (0.80-1.34) | .82 | 0.95 (0.73-1.24) | .71 |
Abbreviations: CVD, cardiovascular disease; LABA, inhaled long-acting β2-agonist; LAMA, inhaled antimuscarinic antagonist; OR, odds ratio.
Recency was defined from the first supply date of the most recent prescription until the index date.
Data are given as number (percentage).
Adjusted for all covariates with standardized difference >0.1 in Table 1.
Various clinical usages of LABAs and LAMAs within 30 days of therapy initiation were scrutinized (Table 3). The adjusted ORs ranged minimally from 1.42 to 1.51 across the various LABA medication regimens. Salmeterol and formoterol were found to present similar CVD risks. Different LAMA regimens, including use of tiotropium only, carried similar risks, ranging from 1.39-fold to 1.58-fold increases. Analyses of individual CVD outcomes revealed increased risks of coronary artery disease and heart failure with LABA and LAMA treatment, and an increased risk for cardiac arrhythmias with LAMA therapy (eTable 3 in the Supplement).
Table 3. Risk of CVD With New Use of LABAs and LAMAs, Stratified by Characteristics of Therapy.
| Bronchodilator | Cases (n = 37 719)a |
Controls (n = 146 139)a |
Crude OR (95% CI) | P Value | Adjusted OR (95% CI)b | P Value |
|---|---|---|---|---|---|---|
| Nonuse of LABA or LAMA, No. (%) | 31 732 (84.1) | 124 943 (85.5) | 1 [Reference] | 1 [Reference] | ||
| New LABA Use, No. (%) | ||||||
| Regimenc | ||||||
| LABA + ICS | 517 (1.4) | 1173 (0.8) | 1.75 (1.57-1.94) | <.001 | 1.51 (1.36-1.68) | <.001 |
| LABA + SABA | 230 (0.6) | 448 (0.3) | 2.02 (1.72-2.37) | <.001 | 1.42 (1.21-1.68) | <.001 |
| LABA + ipratropium | 199 (0.5) | 371 (0.3) | 2.11 (1.78-2.51) | <.001 | 1.42 (1.19-1.70) | <.001 |
| LABA + methylxanthines | 231 (0.6) | 514 (0.4) | 1.80 (1.54-2.10) | <.001 | 1.50 (1.28-1.76) | <.001 |
| Individual drugsc | ||||||
| Salmeterol | 368 (1.0) | 828 (0.6) | 1.76 (1.56-1.99) | <.001 | 1.49 (1.31-1.69) | <.001 |
| Formoterol | 149 (0.4) | 353 (0.2) | 1.67 (1.38-2.02) | <.001 | 1.52 (1.25-1.85) | <.001 |
| New LAMA Use, No. (%) | ||||||
| Regimend | ||||||
| LAMA only | 66 (0.2) | 170 (0.1) | 1.52 (1.14-2.03) | .004 | 1.58 (1.19-2.11) | .002 |
| LAMA + SABA | 68 (0.2) | 150 (0.1) | 1.78 (1.33-2.37) | <.001 | 1.39 (1.04-1.87) | .03 |
| LAMA + ipratropium | 52 (0.1) | 94 (0.1) | 2.17 (1.54-3.05) | <.001 | 1.47 (1.04-2.08) | .03 |
| LAMA + methylxanthines | 101 (0.3) | 247 (0.2) | 1.62 (1.29-2.05) | <.001 | 1.47 (1.16-1.86) | .001 |
| Route | ||||||
| DPI only | 176 (0.5) | 425 (0.3) | 1.63 (1.37-1.95) | <.001 | 1.53 (1.28-1.83) | <.001 |
| Mist | 14 (0.04) | 38 (0.03) | 1.48 (0.80-2.73) | .21 | 1.37 (0.74-2.55) | .32 |
Abbreviations: CVD, cardiovascular disease; DPI, dry powder inhaler; ICS, inhaled corticosteroid; LABA, inhaled long-acting β2-agonist; LAMA, inhaled antimuscarinic antagonist; OR, odds ratio; SABA, short-acting β2-agonist.
Data are given as number (percentage).
Adjusted for all covariates with standardized difference > 0.1 in Table 1.
Patients using LABA only and LAMA plus ICS regimens were not shown owing to small sample sizes.
Excluding analysis for combination of salmeterol and formoterol owing to few exposures.
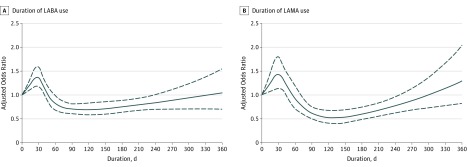
Figure 1 presents the results of duration-response analysis, which indicated that the cardiovascular risks peaked at around the 30th day after new initiation of LABA or LAMA therapy; waned from 31 to 60 days of therapies, and reduced to a level even lower than the baseline risk from 71 to 240 days.
Figure 1. Duration-Response Curves for the Adjusted Odds Ratios (95% CIs) of the Cardiovascular Risk as a Function of Duration of New LABA and New LAMA Therapy.
Duration-response curves for the adjusted odds ratios (solid line) and 95% CIs (dashed line) of the cardiovascular risk as a function of duration of new inhaled long-acting β2-agonists (LABAs) therapy (A) and duration of new inhaled antimuscarinic antagonists (LAMAs) therapy (B) by using a restricted cubic splines function in multiple conditional logistic regressions.
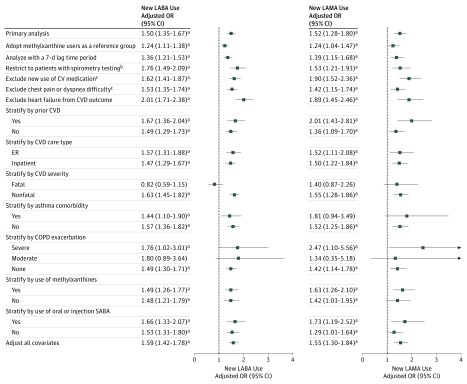
Our main findings remained robust in most of the sensitivity analyses (Figure 2, eTables 4 and 5, eFigures 2 and 3 in the Supplement). The increased risk of CVD with new LABA and new LAMA use persisted in a case-crossover analysis (eTables 4-5, eFigure 2 in the Supplement) and in comparison with new use of theophyllines. The results of the analyses performed to address protopathic bias corresponded closely to the main findings. The LABA- or LAMA-associated risk of CVDs remained significant regardless of patients’ CVD history and COPD exacerbations. In addition, an unmeasured confounder could fully account for our findings only if the confounder increased the CVD risk by 4-fold and was more prevalent in new LABA and LAMA users by 3.5 and 3.6 times, respectively, than in nonusers (eFigure 3 in the Supplement).
Figure 2. Sensitivity Analysis of Cardiovascular Disease (CVD) Risk With New Use of LABA and LAMA.
COPD indicates chronic obstructive pulmonary disease; OR, odds ratio; SABAs, short-acting β2-agonists.
aP < .05.
bWe confined analysis to patients who had been tested with spirometry for lung function in the 30 days before or on the date of the new prescription of inhaled long-acting β2-agonists (LABAs) and inhaled antimuscarinic antagonists (LAMAs).
cThese analyses were measured in the 30 days before the index date.
One severe CVD event requiring hospitalization or ER care occurred for every 406 (95% CI, 303-580) new LABA users and 391 (95% CI, 254-725) new LAMA users during the first 30 days of therapy (eTable 6 in the Supplement).
Discussion
This large observational study of more than 280 000 patients with COPD reported an approximate 1.5-fold increased risk of a severe CVD event within 30 days of initiation of LABA or LAMA therapy, but the risk was absent, or even reduced, with prevalent use of these medications. The findings were replicated in a case-crossover design analysis and unaffected by patients’ baseline CVD status. Collectively, we have provided the first evidence to indicate that new use and duration since initiation of inhaled long-acting bronchodilators are associated with the therapy-related risk of CVD in patients with COPD.
Previous studies that observed an increased risk of CVD from LABAs or LAMAs evaluated elderly patients with COPD, identified few CVD events, or included patients with prior LABA or LAMA usage. None of these studies examined drug use within a time period as short as 30 days after the initiation of therapy. The ability to observe an acute cardiovascular effect with inhaled long-acting bronchodilators in our study primarily resulted from the adoption of a new-user design and examination of a nationwide population of patients with COPD.
Our observed duration-response associations may explain why previous relevant RCTs did not find an increased risk of CVD with LABA or LAMA use. We report herein that the greatest risk of CVD emerged at around the 30th day following initiation of LABA and LAMA therapy. Nevertheless, a substantial proportion of participants with prior LABA or LAMA usage was included in previous RCTs. For instance, more than one-third of the enrolled patients received LABAs at baseline in the TORCH trial; therefore, cardiovascular events arising following first use of LABAs or LAMAs, if any, could not be observed. In addition, patients with severe CVD or life-threatening cardiovascular events at baseline were excluded in prior trials. Both factors could have led to the inclusion of patients with tolerability to cardiovascular events in the RCTs.
The phenomenon of depletion of susceptibles effect may contribute to the observed duration-response associations. Depletion of susceptibles effect has been described as an increased rate of an event from the initial exposure to a medication, followed by a decreased incidence rate with a longer drug exposure, such as the temporal sequence of hormone replacement therapy and its relationship with venous thromboembolism. We suspect that there may exist a subgroup of patients with COPD who are particularly at risk of CVD with initial exposure to LABAs or LAMAs owing to predisposing factors that could amplify sympathetic overactivation or systemic inflammation with inhaled long-acting bronchodilators. Future studies should identify and characterize the most vulnerable subgroups of patients with COPD. We also suspect that after depletion of susceptible patients who experienced CVD events early on, the remaining patients with COPD who continued to use LABAs or LAMAs may exhibit improved systemic inflammation, or may have a more stable lung function status, which could lessen the CVD burden in these patients.
Our findings contribute significantly to investigation of the cardiovascular safety of inhaled long-acting bronchodilators, in which the risk window of adverse cardiovascular events from inhalation therapies is specified. Based on our findings, we suggest that the use of inhaled long-acting bronchodilators in COPD need to be carefully assessed, and a thorough cardiovascular physical examination, especially heart rate measurement and electrocardiograms, need to be performed when prescribing LABAs and LAMAs to patients. Health care professionals should be vigilant for any cardiovascular symptoms during the first 30 days of inhalation therapy. Given that CVD is highly prevalent among patients with COPD, clinicians should also pay attention to the management of CVD risk factors throughout the duration of LABA or LAMA therapy. We also suggest that physicians assess patients’ cardiovascular risk before initiation of LABAs or LAMAs, and, if needed, a preventive therapy for CVD should be considered during the initial treatment of inhaled long-acting bronchodilators.
Two potential mechanisms have been proposed to underpin the cardiovascular risk arising from LABA and LAMA use. Acting as autonomous nervous system agents, LABAs and LAMAs are believed to cause sympathetic overactivation by activating sympathetic β2-adrenergic receptors (β2R) and suppressing parasympathetic muscarinic-3 receptors (M3R), respectively. However, LABA and LAMA use in COPD has been observed to increase the inflammatory cytokine levels, such as an increased level of interleukin-8, which could lead to an increased risk of CVD.
Strengths and Limitations
Several important strengths of our study merit discussion. We investigated the whole spectrum of timing and duration effects of LABA and LAMA usage on the development of severe CVD events in a population with COPD, which is a clinically important aspect that, to our knowledge, no study has addressed previously. In addition, this study was the first to examine the effect of LABA-LAMA regimens with various concomitant COPD medications, which provided pivotal information given that COPD care usually involves multiple therapies. Furthermore, a new-user design served to minimize tolerance effects in prevalent drug users. Moreover, our findings can be generalized to the entire COPD population with multiple coexisting comorbidities.
Several study limitations of the current report need to be addressed. First, worsening of COPD could have prompted the use of LABAs or LAMAs and caused the observed CVD. To control for this confounding effect, we adjusted for multiple proxies of COPD severity and compared new LABA users and new LAMA users, respectively, with new theophylline users, which yielded consistent results. Second, selection bias may have been present, although this bias was expected to be minimal, because we balanced all covariates at cohort entry between cases and controls, treated them as time-varying effects, and obtained consistent findings with an adopted case-only design. Third, protopathic bias could have existed. Nevertheless, we reached the same conclusions when adopting multiple approaches to address this bias, such as the use of a lag-time approach. Fourth, patients’ CVD status at baseline may have confounded our findings. However, we believe this confounding effect not to be substantial because all baseline CVD statuses and CVD medications were balanced between the 2 groups, and the LABA- or LAMA-associated CVD risk remained significant among patients without a CVD history. Fifth, several important determinants of CVD, for example, smoking and alcohol consumption, were unavailable, which could have confounded our findings. However, their effects were unlikely to fully explain our findings, as assessed by rule-out analysis, especially for highly prevalent confounders such as smoking. Sixth, newer LABAs or LAMAs were unable to be assessed, such as aclidinium.
Conclusions
New use of LABAs or LAMAs was associated with a 1.5-fold increased cardiovascular risk in patients with COPD within 30 days of therapy initiation. We caution physicians to closely monitor new users of LABAs or LAMAs for cardiovascular symptoms.
eMethods 1. Development of a disease risk score-matched nested case-control study
eMethods 2. Modeling a nonlinear duration-response association using a restricted cubic splines function model.
eMethods 3. A case-crossover study for assessing the association between the CVD risk and use of LABAs and LAMAs
eTable 1. Diagnosis codes used to define the comorbidities and individual drugs of comedications
eTable 2. Comparison of CVD risk between new LAMA use and new LABA use
eTable 3. Risk of each primary cardiovascular outcome with new LABA and LAMA use
eTable 4. Case-crossover analysis for the risk of CVDs with use of LABA and LAMA within 30 days
eTable 5. Clinical characteristics of CVD patients during the case and control periods in an alternative case-crossover study
eTable 6. Numbers needed to harm for patients who had an increased CVD risk from using LABAs and LAMAs in our primary and secondary analyses
eFigure 1. Overview for the adopted nested case-control study design. CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; ER, emergency department; NHI represents National Health Insurance
eFigure 2. Specification of case and control periods in an alternative case-crossover study
eFigure 3. The impact of unmeasured confounders assessed by the rule-out method
eFigure 4. Study flow diagram outlining the selection of study cohort
References
- 1.World Health Organization Chronic Obstructive Pulmonary Disease (COPD). http://www.who.int/respiratory/copd/burden/en/. Accessed June 23, 2016.
- 2.Vestbo J, Hurd SS, Agustí AG, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. [DOI] [PubMed] [Google Scholar]
- 3.Terzano C, Conti V, Di Stefano F, et al. . Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung. 2010;188(4):321-329. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631-639. [DOI] [PubMed] [Google Scholar]
- 5.Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir Med. 2011;105(10):1516-1522. [DOI] [PubMed] [Google Scholar]
- 6.Wood-Baker R, Cochrane B, Naughton MT. Cardiovascular mortality and morbidity in chronic obstructive pulmonary disease: the impact of bronchodilator treatment. Intern Med J. 2010;40(2):94-101. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstructive Lung Disease Pocket Guide to COPD Diagnosis, Management, and Prevention. http://goldcopd.org/download/361/. Accessed February 22, 2017.
- 8.National Institute for Health and Care Excellence Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (Partial Update). http://www.nice.org.uk/guidance/cg101. Accessed June 23, 2016.
- 9.Calverley PM, Anderson JA, Celli B, et al. ; TORCH investigators . Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775-789. [DOI] [PubMed] [Google Scholar]
- 10.Tashkin DP, Celli B, Senn S, et al. ; UPLIFT Study Investigators . A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543-1554. [DOI] [PubMed] [Google Scholar]
- 11.Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP. Cardiovascular safety of tiotropium in patients with COPD. Chest. 2010;137(1):20-30. [DOI] [PubMed] [Google Scholar]
- 12.Xia N, Wang H, Nie X. Inhaled long-acting β2-agonists do not increase fatal cardiovascular adverse events in COPD: a meta-analysis. PLoS One. 2015;10(9):e0137904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TA, Pickard AS, Au DH, Bartle B, Weiss KB. Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med. 2008;149(6):380-390. [DOI] [PubMed] [Google Scholar]
- 14.de Luise C, Lanes SF, Jacobsen J, Pedersen L, Sørensen HT. Cardiovascular and respiratory hospitalizations and mortality among users of tiotropium in Denmark. Eur J Epidemiol. 2007;22(4):267-272. [DOI] [PubMed] [Google Scholar]
- 15.Gershon A, Croxford R, Calzavara A, et al. . Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175-1185. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Choi S, Jang EJ, et al. . Inhaled bronchodilators and the risk of tachyarrhythmias. Int J Cardiol. 2015;190:133-139. [DOI] [PubMed] [Google Scholar]
- 17.Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. 2012;142(2):305-311. [DOI] [PubMed] [Google Scholar]
- 18.Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD, part 1: Saskatchewan cohort study. Chest. 2012;142(2):298-304. [DOI] [PubMed] [Google Scholar]
- 19.National Health Insurance Administration, Ministry of Health and Welfare Enrollment status alteration and termination file. https://www.nhi.gov.tw/Content_List.aspx?n=579D59A24BD2297C&topn=CB563D844DBDA35A&upn=F987E40C7FB25AD9. Accessed October 11, 2017.
- 20.Cheng CL, Chien HC, Lee CH, Lin SJ, Yang YH. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol. 2015;201:96-101. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CY, Chen CH, Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. 2015;114(3):254-259. [DOI] [PubMed] [Google Scholar]
- 22.Wu CY, Chan FK, Wu MS, et al. . Histamine2-receptor antagonists are an alternative to proton pump inhibitor in patients receiving clopidogrel. Gastroenterology. 2010;139(4):1165-1171. [DOI] [PubMed] [Google Scholar]
- 23.Wang MT, Tsai CL, Lin CW, Yeh CB, Wang YH, Lin HL. Association between antipsychotic agents and risk of acute respiratory failure in patients with chronic obstructive pulmonary disease. JAMA Psychiatry. 2017;74(3):252-260. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol. 2014;24(6):500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai RJ, Glynn RJ, Wang S, Gagne JJ. Performance of disease risk score matching in nested case-control studies: a simulation study. Am J Epidemiol. 2016;183(10):949-957. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender R, Blettner M. Calculating the “number needed to be exposed” with adjustment for confounding variables in epidemiological studies. J Clin Epidemiol. 2002;55(5):525-530. [DOI] [PubMed] [Google Scholar]
- 28.Arfè A, Corrao G. The lag-time approach improved drug-outcome association estimates in presence of protopathic bias. J Clin Epidemiol. 2016;78:101-107. [DOI] [PubMed] [Google Scholar]
- 29.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291-303. [DOI] [PubMed] [Google Scholar]
- 30.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309-2321. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(12):1439-1450. [DOI] [PubMed] [Google Scholar]
- 32.Vestbo J, Anderson JA, Brook RD, et al. ; SUMMIT Investigators . Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817-1826. [DOI] [PubMed] [Google Scholar]
- 33.Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47(7):731-737. [DOI] [PubMed] [Google Scholar]
- 34.Renoux C, Dell’Aniello S, Brenner B, Suissa S. Bias from depletion of susceptibles: the example of hormone replacement therapy and the risk of venous thromboembolism. Pharmacoepidemiol Drug Saf. 2017;26(5):554-560. [DOI] [PubMed] [Google Scholar]
- 35.Barnes NC, Qiu YS, Pavord ID, et al. ; SCO30005 Study Group . Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;173(7):736-743. [DOI] [PubMed] [Google Scholar]
- 36.Casaburi R, Mahler DA, Jones PW, et al. . A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217-224. [DOI] [PubMed] [Google Scholar]
- 37.Bristow MR, Ginsburg R, Umans V, et al. . Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59(3):297-309. [DOI] [PubMed] [Google Scholar]
- 38.Hellgren I, Mustafa A, Riazi M, Suliman I, Sylvén C, Adem A. Muscarinic M3 receptor subtype gene expression in the human heart. Cell Mol Life Sci. 2000;57(1):175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavelaars A, van de Pol M, Zijlstra J, Heijnen CJ. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J Neuroimmunol. 1997;77(2):211-216. [DOI] [PubMed] [Google Scholar]
- 40.King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med. 2015;4(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Development of a disease risk score-matched nested case-control study
eMethods 2. Modeling a nonlinear duration-response association using a restricted cubic splines function model.
eMethods 3. A case-crossover study for assessing the association between the CVD risk and use of LABAs and LAMAs
eTable 1. Diagnosis codes used to define the comorbidities and individual drugs of comedications
eTable 2. Comparison of CVD risk between new LAMA use and new LABA use
eTable 3. Risk of each primary cardiovascular outcome with new LABA and LAMA use
eTable 4. Case-crossover analysis for the risk of CVDs with use of LABA and LAMA within 30 days
eTable 5. Clinical characteristics of CVD patients during the case and control periods in an alternative case-crossover study
eTable 6. Numbers needed to harm for patients who had an increased CVD risk from using LABAs and LAMAs in our primary and secondary analyses
eFigure 1. Overview for the adopted nested case-control study design. CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; ER, emergency department; NHI represents National Health Insurance
eFigure 2. Specification of case and control periods in an alternative case-crossover study
eFigure 3. The impact of unmeasured confounders assessed by the rule-out method
eFigure 4. Study flow diagram outlining the selection of study cohort