Key Points
Question
How early can we detect changes in presymptomatic carriers of the C9orf72 mutation?
Findings
In this multicenter cross-sectional study of 80 individuals, praxis impairment, cortico-subcortical atrophy, and white matter alterations were detected in C9orf72 mutation carriers younger than 40 years.
Meaning
While cortico-subcortical atrophy appears diffuse, white matter changes predominate in frontal regions and corticospinal tracts, thus being more reflective of the expected phenotype of frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
This multicenter cross-sectional study assesses the occurrence of cognitive, structural, and microstructural changes in presymptomatic carriers of C9orf72 mutation.
Abstract
Importance
Presymptomatic carriers of chromosome 9 open reading frame 72 (C9orf72) mutation, the most frequent genetic cause of frontotemporal lobar degeneration and amyotrophic lateral sclerosis, represent the optimal target population for the development of disease-modifying drugs. Preclinical biomarkers are needed to monitor the effect of therapeutic interventions in this population.
Objectives
To assess the occurrence of cognitive, structural, and microstructural changes in presymptomatic C9orf72 carriers.
Design, Setting, and Participants
The PREV-DEMALS study is a prospective, multicenter, observational study of first-degree relatives of individuals carrying the C9orf72 mutation. Eighty-four participants entered the study between October 2015 and April 2017; 80 (95%) were included in cross-sectional analyses of baseline data. All participants underwent neuropsychological testing and magnetic resonance imaging; 63 (79%) underwent diffusion tensor magnetic resonance imaging. Gray matter volumes and diffusion tensor imaging metrics were calculated within regions of interest. Anatomical and microstructural differences between individuals who carried the C9orf72 mutation (C9+) and those who did not carry the C9orf72 mutation (C9−) were assessed using linear mixed-effects models. Data were analyzed from October 2015 to April 2017.
Main Outcomes and Measures
Differences in neuropsychological scores, gray matter volume, and white matter integrity between C9+ and C9− individuals.
Results
Of the 80 included participants, there were 41 C9+ individuals (24 [59%] female; mean [SD] age, 39.8 [11.1] years) and 39 C9− individuals (24 [62%] female; mean [SD] age, 45.2 [13.9] years). Compared with C9− individuals, C9+ individuals had lower mean (SD) praxis scores (163.4 [6.1] vs 165.3 [5.9]; P = .01) and intransitive gesture scores (34.9 [1.6] vs 35.7 [1.5]; P = .004), atrophy in 8 cortical regions of interest and in the right thalamus, and white matter alterations in 8 tracts. When restricting the analyses to participants younger than 40 years, compared with C9− individuals, C9+ individuals had lower praxis scores and intransitive gesture scores, atrophy in 4 cortical regions of interest and in the right thalamus, and white matter alterations in 2 tracts.
Conclusions and Relevance
Cognitive, structural, and microstructural alterations are detectable in young C9+ individuals. Early and subtle praxis alterations, underpinned by focal atrophy of the left supramarginal gyrus, may represent an early and nonevolving phenotype related to neurodevelopmental effects of C9orf72 mutation. White matter alterations reflect the future phenotype of frontotemporal lobar degeneration/amyotrophic lateral sclerosis, while atrophy appears more diffuse. Our results contribute to a better understanding of the preclinical phase of C9orf72 disease and of the respective contribution of magnetic resonance biomarkers.
Trial Registration
clinicaltrials.gov Identifier: NCT02590276
Introduction
Frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) are neurodegenerative diseases with common genetic causes, the most frequent being a GGGGCC repeat expansion in the chromosome 9 open reading frame 72 (C9orf72) gene. This expansion may lead to a loss of C9orf72 function and causes abnormal neuronal aggregation of nuclear RNA foci, dipeptides repeats (DPR), and transactive response DNA-binding protein 43 (TDP-43) inclusions. Recent preclinical development of disease-modifying drugs, such as antisense oligonucleotides that target mutant RNA, offer promising therapeutic perspectives in C9orf72 disease.
Presymptomatic carriers of genetic mutation represent the optimal target population for the development of new disease-modifying treatments against FTLD and ALS. It is now established that neurodegenerative diseases cause biological and morphological changes decades before symptom onset; the presymptomatic stage represents the best time for therapeutic interventions because it allows the possibility of stopping the neurodegenerative process before irreversible brain damage occurs. Thus, establishing the chronology of structural and microstructural changes during the presymptomatic stage is crucial to identify markers of disease progression and monitor the effect of treatments. Three studies have suggested that atrophy, studied with anatomical magnetic resonance imaging (MRI), could be detected years before symptom onset in presymptomatic individuals who carry the C9orf72 mutation (C9+) but were limited by the small number of participants. One study also detected alterations of white matter integrity using diffusion tensor MRI (DTI), whereas another study failed to identify such changes. The present work aims to assess cognitive, structural, and microstructural changes in a large cohort of asymptomatic C9+ individuals to characterize the presymptomatic course of the disease and to identify potential neuroimaging biomarkers of preclinical disease progression.
Methods
Participants
Eighty-four first-degree relatives of C9orf72 mutation carriers from 48 families were enrolled in a national multicentric study (PREV-DEMALS) between October 2015 and April 2017. This study was approved by the Comité de Prévention des Personnes Ile de France VI of the Hôpital Pitié-Salpêtrière, and written informed consent was obtained from all participants.
At inclusion, asymptomatic status of participants was ascertained based on relative’s interview, neurological examination, and the normality of behavioral scales and neuropsychological scores, taking into account age and educational level. Neuropsychological tests are detailed in eMethods 1 in the Supplement. Two participants were excluded from the analysis because mild cerebellar syndrome or cognitive impairment were detected during the visit; 2 other participants were excluded because of incomplete MRI protocol data. Eighty neurologically healthy participants were finally included in the analyses. C9orf72 genetic status was determined by repeat-primed polymerase chain reaction on lymphocytes DNA. Forty-one C9+ participants carried a pathogenic expansion (>23 GGGGCC repeats); 39 control participants did not carry this expansion (C9−). Expected ages at onset of C9orf72 carriers were estimated by averaging the ages at onset of affected relatives, similar to previous studies.
MRI Acquisition
All MRI acquisitions were performed on a 3-T MRI system (64 on Siemens Prisma Syngo 3-T, 9 on Philips Achieva 3-T, and 7 on GE Discovery 3-T) in 3 imaging centers belonging to the harmonized national network of the Centre d’Acquisition et de Traitement d’Images (http://cati-neuroimaging.com/). The Centre d’Acquisition et de Traitement d’Images performs on-site visits for the setup of imaging protocols and regular follow-up. Three-dimensional T1 sequence parameters were similar for the 3 centers, while the DTI sequence was performed in only 1 center (eMethods 2 in the Supplement). Systematic quality checks of MRI results were performed by the Centre d’Acquisition et de Traitement d’Images using a dedicated software program with quantitative and qualitative indices, which allowed us to check for (1) protocol consistency (MRI scanner, software version, type of reception coil, acquisition slab position, sequence parameters, and sequence order), (2) presence and localization of artifacts (motion artifacts, spike artifacts, or other), and (3) overall image quality based on signal-to-noise ratio, contrast-to-noise ratio, and intensity nonuniformity. Among the 80 MRI data sets, 75 (94%) were considered good quality and 5 (6%) were acceptable quality.
Anatomical MRI Processing
FreeSurfer image analysis software version 5.3 was used to process the T1-weighted images. The processing pipeline included nonuniformity and intensity correction, skull stripping, gray/white matter segmentation, reconstruction of the cortical surface, extraction of cortical region of interest (ROI) volumes using the Desikan-Killiany atlas, and subcortical ROI volumes and total intracranial volumes (TIV) using the aseg atlas. We used for analyses the normalized volume of each ROI (VROI), defined as normalized VROI = (TIVm × VROI)/TIV, where TIVm indicates the average total intracranial volume computed across all participants, which is constant, and VROI indicates the volume of the ROI. The role of the constant multiplicative factor TIVm is simply to preserve the order of magnitude of normalized VROI similar to that of VROI.
Diffusion MRI Processing
All raw diffusion-weighted imaging volumes were aligned to the average b0 image, with the first 6 df to correct for head motion, and diffusion directions were appropriately updated. A registration with 12 df was used to correct for eddy current distortions. These registrations were done using the Functional MRI of the Brain Software Library flirt tool (http://www.fmrib.ox.ac.uk/fsl). Field map imaging was used to correct for echo-planar imaging–induced susceptibility artifacts with the Functional MRI of the Brain Software Library prelude/fugue tools. Diffusion-weighted imaging volumes were corrected for nonuniform intensity using Advanced Normalization Tools N4 bias correction algorithm. A single multiplicative bias field from the averaged b0 images was estimated. The diffusion-weighted imaging data sets were upsampled at 1 mm to improve the registration between the T1-weighted imaging and the diffusion-weighted imaging. A diffusion tensor model was fitted at each voxel to calculate fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity maps. White matter tracts were defined using the John Hopkins University white matter tractography atlas with a 25% probabilistic threshold. For each participant, the fractional anisotropy map was registered onto the John Hopkins University atlas template with the Advanced Normalization Tools SyN algorithm. Then, the estimated nonlinear deformation was applied to the parametric maps, and in each patient, we extracted the average values of DTI metrics (fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity) within each tract of the John Hopkins University atlas.
Statistical Analysis
Statistical analyses were performed using R version 3.4.0 (The R Foundation) and GraphPad Prism 7.0 (GraphPad Software). Demographic characteristics and clinical tests were compared between groups using χ2 test for dichotomous and categorical variables or Mann-Whitney test for numerical variables. Structural and microstructural differences between C9+ and C9− participants were assessed using linear mixed-effects models. We used real age and group (ie, mutation status) as fixed effects and family membership as random effect, with the following model:
| Yik(j) = μ + β × Genderi + λ × Agei + η × Groupi + Uk + Εik(j), |
where Yik(j) is the response of the jth region of interest for the ith participant and the kth family; Genderi, Agei, and Groupi are the fixed effects; μ, β, λ, and η are their estimated parameters; Uk is the random effect measuring the difference between the average response in the family and in the whole population; and Εik(j) is the random error.
The correlation between real age and expected years to onset was assessed using Pearson correlation coefficient. Correlations between clinical scores and structural or microstructural measures in presymptomatic carriers were assessed using Spearman correlation coefficient. All P values were 2-tailed, and statistical significance was set at P < .05. Corrections for multiple comparisons were performed using Benjamini-Hochberg method. All statistical analyses were performed independently by 2 scientists (J.W. and M.H.).
Results
Participants
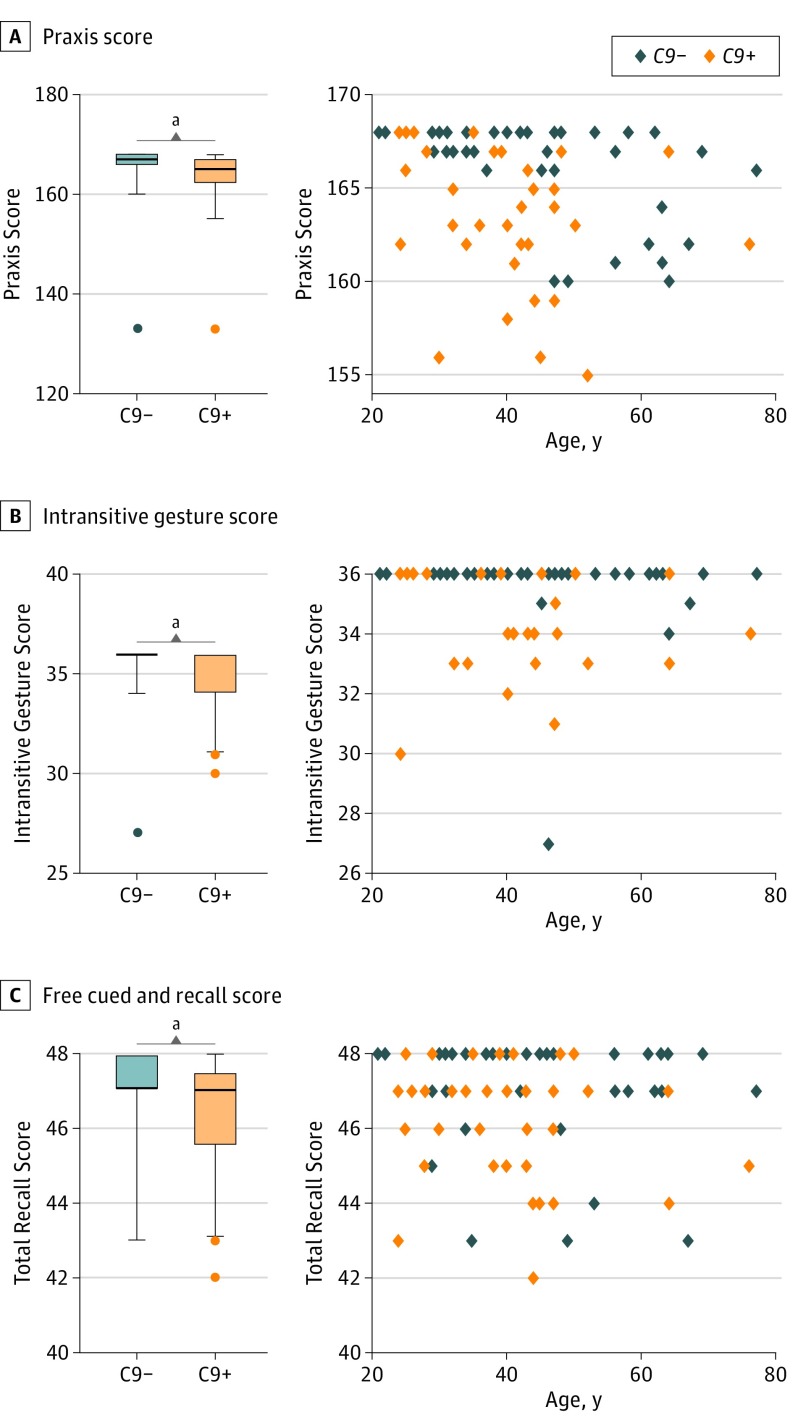
There were no statistical differences between C9+ and C9− participants regarding age at evaluation and demographic characteristics (Table). In C9+ participants, real age and expected years to onset were strongly correlated (r2 = 0.802; 95% CI, 0.659-0.890; P < .001; eFigure 1 in the Supplement), with a mean (SD) estimated age at onset of 58.9 (4.9) years. C9+ participants had significantly lower praxis scores; this difference remained statistically significant in participants younger than 40 years (mean [SD] praxis score, 165.2 [3.4] vs 167.6 [0.6]; P = .04), who had a mean (SD) time to onset of 25.4 (8.1) years. Praxis score was significantly correlated with age in both C9+ and C9− individuals (Figure 1A). When analyzing the subscores of the praxis test, all were lower for the C9+ group, but statistical significance was reached only for the subscore of nontransitive gestures (Figure 1B); this difference remained statistically significant in participants younger than 40 years (mean [SD] nontransitive gesture score, 35.0 [1.7] vs 36.0 [0.0]; P = .04). Lastly, the total recall score of the free cued and recall test was significantly lower in C9+ individuals compared with C9− individuals (Figure 1C) but with a large overlap of scores between the 2 groups, and there was no significant difference among participants younger than 40 years (mean [SD] free cued and recall test score, 46.8 [1.3] vs 47.2 [1.4]; P = .08).
Table. Study Group Characteristics.
| Characteristic | Mean (SD) | P Value | |
|---|---|---|---|
| C9− (n = 39) | C9+ (n = 41) | ||
| Demographic characteristics | |||
| Age, y | 45.2 (13.9) | 39.8 (11.1) | .08 |
| <40 y, No. (%) | 16 (41) | 22 (54) | NA |
| Female, No. (%) | 24 (62) | 24 (59) | .78 |
| Right laterality, No. (%) | 33 (85) | 35 (85) | .92 |
| Expected time to onset, y | NA | 19.3 (11.2) | NA |
| Familial phenotype, No. (%) | .77 | ||
| FTLD | 15 (38) | 18 (44) | |
| ALS | 2 (5) | 3 (7) | |
| Mixed | 21 (54) | 20 (49) | |
| Unavailable | 1 (3) | 0 | |
| Neuropsychological scores | |||
| MMSE score (maximum, 30) | 28.8 (1.5) | 28.6 (1.3) | .34 |
| MDRS score | |||
| Total score (maximum, 144) | 141.5 (3.2) | 141.4 (2.6) | .54 |
| Initiation (maximum, 37) | 36.5 (1.3) | 36.6 (1.1) | .72 |
| Concept (maximum, 39) | 38.3 (1.2) | 38.3 (1.1) | .75 |
| Attention (maximum, 37) | 36.7 (0.8) | 36.7 (0.6) | .95 |
| Construction (maximum, 6) | 5.9 (0.2) | 6.0 (0.0) | .23 |
| Memory (maximum, 25) | 24.0 (1.4) | 23.8 (1.6) | .81 |
| FBI | 0.8 (1.8) | 1.3 (2.6) | .54 |
| FAB score (maximum, 18) | 16.8 (1.4) | 17.1 (0.9) | .39 |
| Mini-SEA | |||
| Emotion recognition test (maximum, 35) | 29.9 (2.7) | 29.8 (2.5) | .73 |
| Faux pas test (maximum, 30) | 26.2 (4.7) | 25.6 (3.5) | .13 |
| Praxis score | |||
| Total score (maximum, 168) | 165.3 (5.9) | 163.4 (6.1) | .01 |
| Finger dexterity (maximum, 36) | 35.5 (1.2) | 35.4 (1.2) | .57 |
| Melokinetic apraxia (maximum, 24) | 23.2 (1.5) | 22.8 (2.3) | .37 |
| Nonrepresentational gestures (maximum, 36) | 35.7 (0.9) | 35.4 (1.2) | .16 |
| Intransitive gestures (maximum, 36) | 35.7 (1.5) | 34.9 (1.6) | .004 |
| Transitive gestures (maximum, 36) | 35.2 (2.0) | 34.9 (2.9) | .79 |
| Benson figure | |||
| Copy (maximum, 17) | 16.5 (0.8) | 16.6 (0.6) | .92 |
| Recall (maximum, 17) | 12.8 (2.2) | 13.0 (2.5) | .52 |
| Free and cued recall test | |||
| Free recall (maximum, 48) | 35.6 (4.8) | 32.9 (5.5) | .06 |
| Total recall (maximum, 48) | 47.1 (1.5) | 46.4 (1.5) | .005 |
| Delayed free recall (maximum, 16) | 13.2 (2.1) | 13.0 (2.2) | .88 |
| Delayed total recall (maximum, 16) | 15.5 (1.8) | 15.6 (0.9) | .70 |
| Boston naming test (maximum, 30) | 27.2 (2.0) | 27.2 (2.2) | .93 |
| Fluency tasks | |||
| Categories (animals) | 36.1 (10.3) | 36.3 (7.1) | .82 |
| Letter (P) | 24.7 (8.0) | 23.5 (6.5) | .23 |
Abbreviations: ALS, amyotrophic lateral sclerosis; C9−, individuals without the C9orf72 mutation; C9+, individuals with the C9orf72 mutation; FAB, frontal assessment battery; FBI, Frontal Behavioural Inventory; FTLD, frontotemporal lobar degeneration; MMSE, Mini-Mental State Examination; MDRS, Mattis dementia rating scale; NA, not applicable; SEA, Social Cognition and Emotional Assessment.
Figure 1. Early Cognitive Changes in C9orf72 Mutation Carriers .
Compared with individuals who did not carry the C9orf72 mutation (C9−), those who carried the C9orf72 mutation (C9+) showed significantly lower mean (SD) praxis scores (163.4 [6.1] vs 165.3 [5.9]; P = .01 [Mann-Whitney test]) (A), significantly lower mean (SD) intransitive gesture subscores (34.9 [1.6] vs 35.7 [1.5]; P = .004 [Mann-Whitney test]) (B), and significantly lower mean (SD) free cued and recall scores (46.4 [1.5] vs 47.1 [1.5]; P = .005 [Mann-Whitney test]) (C). Praxis score was significantly correlated with age in C9+ individuals (r = –0.387; P = .01) and C9− individuals (r = –0.508; P = .001) (Spearman correlation coefficient; correlation assessed after removal of the 2 outliers with a score of 133). Other scores did not correlate with age. The box indicates the interquartile range; error bars, the fifth to 95th percentiles. Outliers are presented as individual data points. The exact age of individuals is not provided to prevent individuals from identifying their mutation status.
aStatistically significant at P < .01.
Association of C9orf72 Mutation With Cortical Structures
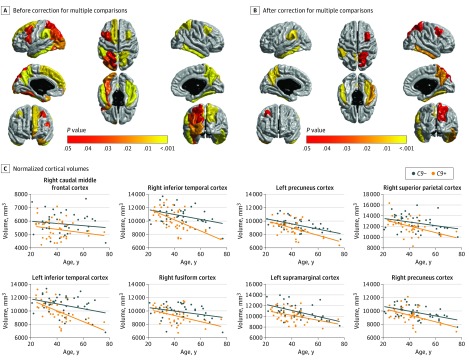
C9+ participants showed diffuse cortical atrophy within the associative cortex, with a sparring of primary sensorimotor and visual cortex, frontobasal cortex, and superior temporal cortex (Figure 2A). After correction for multiple comparisons, this association remained significant for 1 frontal, 3 inferior temporal, and 4 parietal ROIs (Figure 2B) (eTable 1 in the Supplement). In these 8 ROIs, we performed the same analyses restricted to participants younger than 40 years and found significant atrophy within the right caudal middle frontal cortex, left and right precuneus cortex, and left supramarginal cortex.
Figure 2. Cortical Atrophy in C9orf72 Mutation Carriers .
Color-coded representation of P values corresponding to the association of C9orf72 mutation with the volume of cortical regions of interest before (A) and after (B) correction for multiple comparisons. C, Graphs of normalized cortical volumes as a function of age in individuals who carried the C9orf72 mutation (C9+) and individuals who did not carry the C9orf72 mutation (C9−). The exact age is not provided to prevent individuals from identifying their mutation status.
Association of C9orf72 Mutation With Subcortical Structures
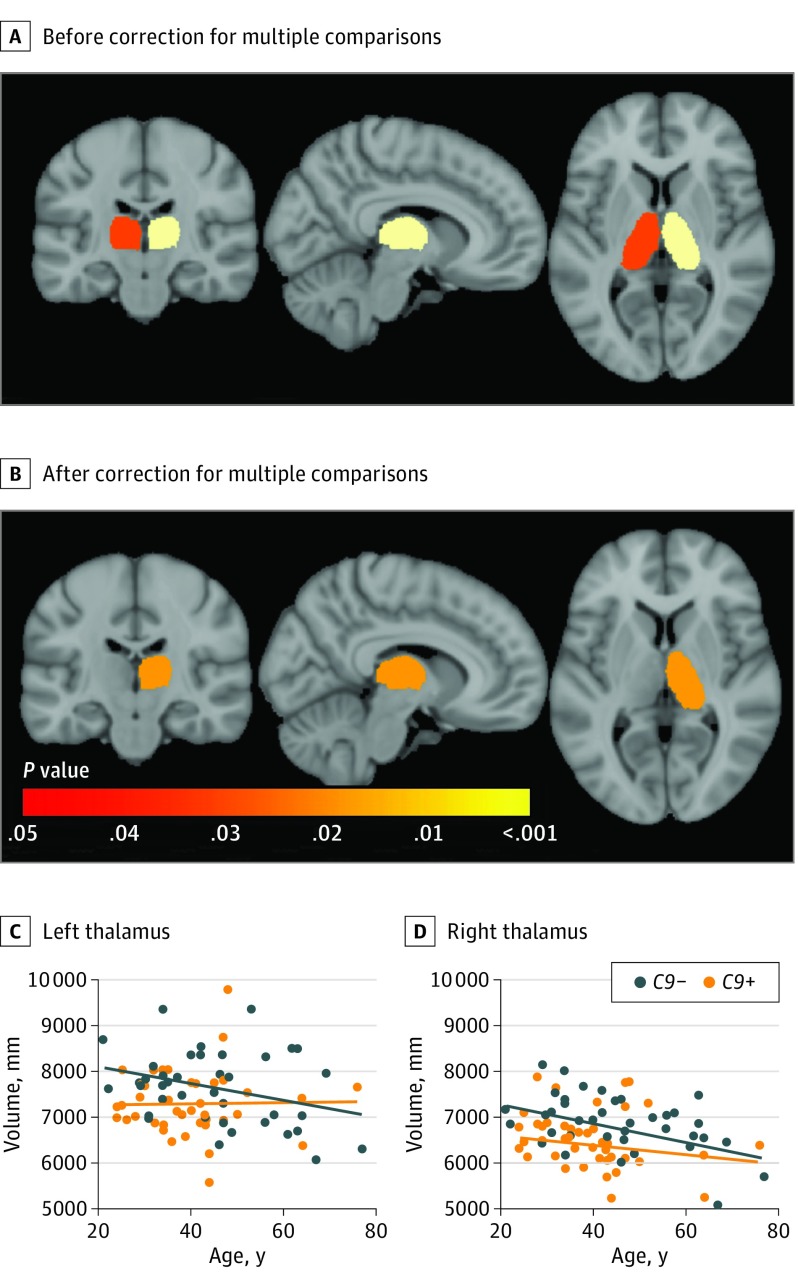
C9+ participants showed significant atrophy in the left and right thalamus compared with C9− participants (Figure 2A). After correction for multiple comparisons, this association remained significant for the right thalamus (Figure 2B) (eTable 2 in the Supplement) and persisted when restricting the analysis to participants younger than 40 years.
Association of C9orf72 Mutation With White Matter Microstructure
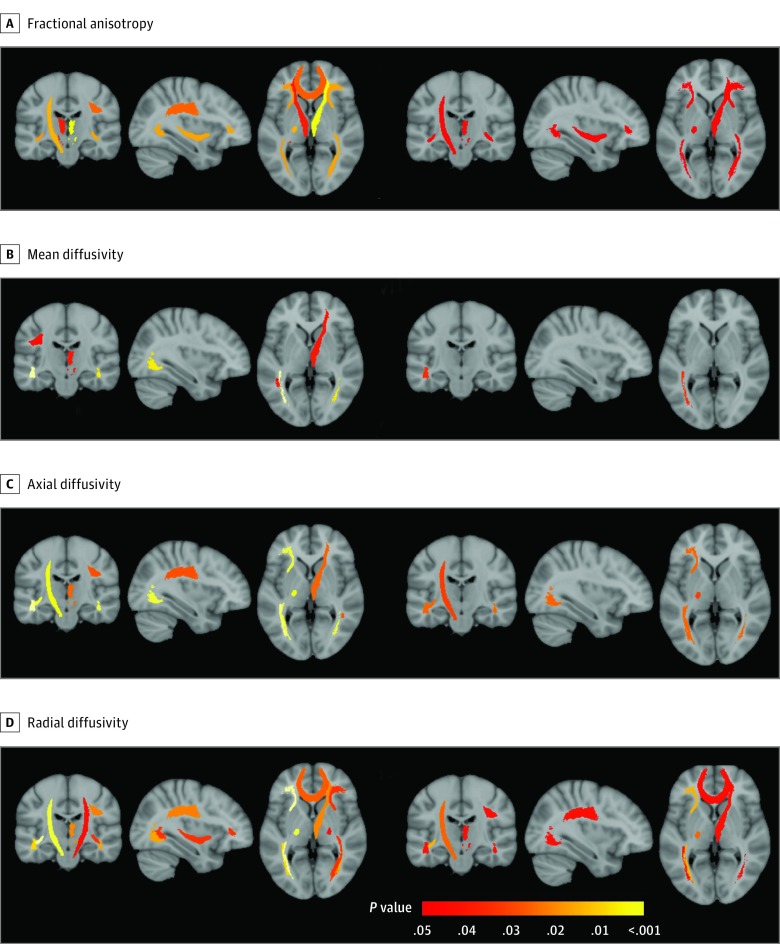
C9+ participants showed diffuse alteration of white matter microstructure (ie, decreased fractional anisotropy, increased mean diffusivity, axial diffusivity, and radial diffusivity), predominating in frontal regions and affecting corticospinal tracts bilaterally (Figure 3) (eTable 3 and eFigure 2 in the Supplement). Only for this modality, we observed that the oldest C9+ participant was an outlier for some DTI metrics (eFigure 2 in the Supplement); to make sure that results were not driven by this outlier, we performed the same analyses without this participant and still found significant differences in 23 DTI metrics (instead of 27) within the same white matter tracts. After correction for multiple comparisons, 8 tracts remained significantly altered: the left corticospinal tract, the right anterior thalamic radiation, 4 tracts connected to the frontal lobes (ie, forceps minor, bilateral inferior fronto-occipital fasciculus, and right superior longitudinal fasciculus), and 2 tracts connected to the temporal lobes (ie, bilateral inferior longitudinal fasciculus). In these tracts, we performed the same analyses restricted to participants younger than 40 years and still found significantly increased radial diffusivity and decreased fractional anisotropy within the right anterior thalamic radiation and increased radial diffusivity within the right forceps minor.
Figure 3. Alterations of White Matter in C9orf72 Mutation Carriers .
Color-coded representation of P values corresponding to the association of C9orf72 mutation with the diffusion tensor magnetic resonance imaging scalars of white matter regions of interest, both before (left 3) and after (right 3) correction for multiple comparisons.
Correlation Between Structural Changes and Clinical Scores
We looked for possible correlations between the neuropsychological scores altered in C9+ participants (ie, praxis, intransitive gestures, and free cued and recall test scores) and the markers of structural and microstructural alterations in C9+ participants (ie, volume of cortical and subcortical regions and DTI metrics significantly altered in C9+ individuals after correction for multiple comparisons). No correlation was found between the 3 scores and structural or microstructural changes.
Discussion
Using a large cohort of presymptomatic C9orf72 carriers, this study reveals unexpected results. We show that cognitive, structural, and microstructural changes can be detected very early in C9+ individuals aged 20 to 40 years, corresponding to a mean (SD) time to expected onset of 25.4 (8.1) years. We also show that praxis score appears as the first cognitive domain to be altered in young C9+ individuals. Lastly, we show that presymptomatic C9+ individuals display distinct patterns of atrophy and white matter alterations; cortico-subcortical atrophy appears as a diffuse process, while white matter microstructural changes predominate in the areas specifically affected during FTLD and ALS.
In this study, we chose to model the association of C9orf72 mutation with atrophy and white matter microstructure using the real age of participants. Instead of real age, some authors have used the distance to mean age at onset in affected relatives as an estimation of expected years to onset in presymptomatic carriers of C9orf72. However, age at onset is highly variable, even within individuals of the same family, one of the possible reasons being a possible anticipation phenomenon. Thus, it must be highlighted that quantification of the effects of C9orf72 mutation on brain structure remains currently limited by the difficulty to accurately estimate expected time to onset in presymptomatic carriers.
Cognitive, Structural, and Microstructural Changes Are Detected in Young C9+ Individuals
During the preclinical course of neurodegenerative diseases, structural changes are expected 10 to 15 years and clinical changes 5 years before expected symptom onset, according to studies of the largest presymptomatic FTLD cohort and Alzheimer disease cohort. However, the pace of progression varies depending on the underlying mutation. In C9orf72-carrying patients with FTLD, disease duration can be remarkably long, and atrophy progresses at a slow rate compared with other genetic or sporadic forms. Thus, it is conceivable that the preclinical phase of C9orf72 disease would last particularly long. Our study evidences that subtle cognitive, structural, and microstructural alterations can be detected in young C9orf72 carriers younger than 40 years. This finding suggests that young individuals may represent the optimal target population for future disease-modifying interventions. Previous studies have suggested that atrophy emerges in young C9orf72 carriers, either based on group differences obtained on extrapolated measures or because no acceleration of atrophy was detected during aging in C9orf72 carriers. Our results confirm this hypothesis by showing significant differences of metrics directly measured in young C9+ and C9− individuals.
Praxis Impairment Is an Early Feature of C9orf72 Disease
The evidence of subtle praxis alterations in young C9+ individuals is a surprising result. One study has suggested that cognitive and behavioral changes could occur 10 to 15 years from symptom onset in presymptomatic C9orf72 carriers, based on extrapolated data; however, praxis evaluation was not reported. Our result is particularly striking because a clear separation was visible between the praxis scores of young C9+ and C9− individuals (Figure 1A). Praxis score has been reported to decrease during normal aging; similarly, it was inversely correlated with age in both C9− and C9+ individuals in our study (Figure 1A). The difficulty of this task may explain its sensitivity to detect subtle preclinical changes in C9+ individuals. The observed impairment in nontransitive gestures (symbolic gestures without the use of an object) is a feature of ideomotor apraxia, which involves the posterior part of the left parietal lobe, mainly the left supramarginal gyrus. Consistently, the impairment in nontransitive gestures in young C9+ individuals was associated with a focal atrophy of this region (ie, the left supramarginal cortex). No correlation was detected between volume of left supramarginal cortex and nontransitive gesture score in C9+ individuals; this lack of correlation was likely related to the relatively low variance of the score, which was only slightly altered in C9+ individuals (1 to 6 points less than the normal score of 36) (Figure 1B). Praxis alteration was unexpected because it is not a salient feature of C9orf72 FTLD; although it has been occasionally reported, it is usually less marked than executive and behavioral dysfunction. Thus, praxis impairment may represent an early-expressed and nonevolving phenotype of C9orf72 mutation. These intriguing findings stress the need to characterize C9orf72 mutation alterations on the scale of the entire lifespan of mutation carriers, including childhood, to disentangle possible developmental alterations from potential preclinical prognostic markers of C9orf72 disease. It also emphasizes the fact that neuropsychological features of C9orf72 mutation may extend well beyond the classical spectrum of FTLD and require extensive neuropsychological characterization.
Additionally, we also observed a slight decrease in recall performance in C9+ individuals. Interestingly, C9orf72 mutation is associated with abnormal deposition of TDP-43, DPR, and RNA foci in the hippocampus. However, the slight memory impairment we observed appeared less striking than praxis impairment; there was a large overlap of values between C9+ and C9− individuals, and the difference did not persist when restricting the analysis to participants younger than 40 years. Moreover, we did not detect any significant atrophy in the hippocampus of C9+ individuals.
C9orf72 Mutation Is Associated With Early Thalamic Atrophy
Thalamic atrophy appears as a reliable effect of C9orf72 mutation. Thalamic atrophy has been previously reported in smaller cohorts of presymptomatic C9orf72 carriers and also in symptomatic C9orf72 carriers with FTLD or ALS. Thalamic atrophy may be related to the presence of pathological deposits, ie, TDP-43 and/or DPR, but it can also be caused by deafferentation processes secondary to the diffuse cortical atrophy owing to the high number of connections between the hemispheric cortex and the thalamus. These mechanisms are not exclusive and may be associated, which would explain the high sensitivity of previous studies for detecting early thalamic atrophy in C9+ individuals.
White Matter Microstructural Changes but Not Cortical Atrophy Reflects the Expected Topography of FTLD and ALS in C9+ Individuals
Our study demonstrates a major difference of pattern between atrophy and white matter alterations in C9+ individuals. Atrophy appears as a widespread phenomenon with a relative sparing of primary motor cortex and frontobasal cortex, areas that are preferentially involved during ALS and FTLD, respectively (Figure 4). Conversely, white matter alterations seem to preferentially target corticospinal tracts and frontal white matter (Figure 3). These tendencies suggest that, in C9+ individuals, white matter changes may be more predictive of future cognitive and motor deficits than cortical atrophy. These different patterns are reminiscent of the topography of the 2 histopathological hallmarks of C9orf72 mutation, DPR and TDP-43. Even if this is still debated, DPR deposits have a diffuse distribution unrelated to the clinical phenotype of patients and seem to precede TDP-43 deposition. Conversely, TDP-43 deposits may represent a downstream process more correlated with clinical symptoms. Furthermore, TDP-43 deposits are present both in cortical neurons and white matter glial cells; thus, white matter changes, possibly more reflective of future clinical deficits, may relate more to TDP-43 pathology than to DPR in presymptomatic C9orf72 disease.
Figure 4. Subcortical Atrophy in C9orf72 Mutation Carriers .
Color-coded representation of P values corresponding to the association of C9orf72 mutation with the volume of subcortical structures before (A) and after (B) correction for multiple comparisons. C, Graph of normalized thalamic volumes in the left thalamus as a function of age in individuals who carried the C9orf72 mutation (C9+) and individuals who did not carry the C9orf72 mutation (C9−). D, Graph of normalized thalamic volumes in the right thalamus as a function of age in C9+ and C9− individuals. The exact age is not provided to prevent individuals from identifying their mutation status.
Limitations
The main limitation of our study relies on its cross-sectional design; our hypotheses need to be confirmed on longitudinal data sets. Additionally, studies in young children carrying C9orf72 mutation could help clarify how early praxis impairment can be detected during neurodevelopment.
Conclusions
This study demonstrates that pathological processes emerge during early adulthood in C9orf72 mutation carriers. Early and subtle praxis alterations in young C9+ individuals, underpinned by a focal atrophy of the left supramarginal gyrus, may represent a nonevolving phenotype, which highlights the possible overlaps and intricacy between neurodevelopmental and neurodegenerative processes. The distinct patterns of atrophy and white matter changes observed in C9+ individuals suggest that white matter integrity might be more reflective of the future FTLD or ALS phenotype than atrophy. Our results contribute to a better understanding of the spectrum of C9orf72 disease and of the respective contribution of magnetic resonance biomarkers in assessing disease-related changes.
eMethods 1. Neuropsychological and behavioral tests.
eMethods 2. Magnetic resonance imaging sequence parameters.
eFigure 1. Correlation between age and expected years to onset.
eFigure 2. Diffusion tensor magnetic resonance imaging metrics.
eTable 1. Effect of C9orf72 mutation on volume of cortical regions of interest.
eTable 2. Effect of C9orf72 mutation on volume of subcortical structures.
eTable 3. Effect of C9orf72 mutation on diffusion tensor magnetic resonance imaging metrics.
eReferences.
References
- 1.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. . Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renton AE, Majounie E, Waite A, et al. ; ITALSGEN Consortium . A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci. 2013;36(8):450-459. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly CJ, Zhang P-W, Pham JT, et al. . RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention [published correction appears in Neuron. 2013;80(4):1102]. Neuron. 2013;80(2):415-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang J, Zhu Q, Gendron TF, et al. . Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron. 2016;90(3):535-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ, Xiong C, Benzinger TLS, et al. ; Dominantly Inherited Alzheimer Network . Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohrer JD, Nicholas JM, Cash DM, et al. . Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis [published correction appears in Lancet Neurol. 2015;14(12):1151]. Lancet Neurol. 2015;14(3):253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walhout R, Schmidt R, Westeneng H-J, et al. . Brain morphologic changes in asymptomatic C9orf72 repeat expansion carriers. Neurology. 2015;85(20):1780-1788. [DOI] [PubMed] [Google Scholar]
- 9.Lee SE, Sias AC, Mandelli ML, et al. . Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clin. 2016;14:286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Operto G, Chupin M, Batrancourt B, et al. ; CATI Consortium . CATI: a large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14(3):253-264. [DOI] [PubMed] [Google Scholar]
- 11.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009;61(6):1336-1349. [DOI] [PubMed] [Google Scholar]
- 12.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65-73. [DOI] [PubMed] [Google Scholar]
- 13.Tustison NJ, Avants BB. Explicit B-spline regularization in diffeomorphic image registration. Front Neuroinform. 2013;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411-426. [DOI] [PubMed] [Google Scholar]
- 15.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of Human White Matter. 1st ed Amsterdam, the Netherlands: Elsevier Science; 2005. [Google Scholar]
- 16.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benzinger TLS, Blazey T, Jack CR Jr, et al. . Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110(47):E4502-E4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gómez-Tortosa E, Gallego J, Guerrero-López R, et al. . C9ORF72 hexanucleotide expansions of 20-22 repeats are associated with frontotemporal deterioration. Neurology. 2013;80(4):366-370. [DOI] [PubMed] [Google Scholar]
- 19.Khan BK, Yokoyama JS, Takada LT, et al. . Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J Neurol Neurosurg Psychiatry. 2012;83(4):358-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suhonen N-M, Kaivorinne A-L, Moilanen V, et al. . Slowly progressive frontotemporal lobar degeneration caused by the C9ORF72 repeat expansion: a 20-year follow-up study. Neurocase. 2015;21(1):85-89. [DOI] [PubMed] [Google Scholar]
- 21.Whitwell JL, Boeve BF, Weigand SD, et al. . Brain atrophy over time in genetic and sporadic frontotemporal dementia: a study of 198 serial magnetic resonance images. Eur J Neurol. 2015;22(5):745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peigneux P, van der Linden M. Influence of ageing and educational level on the prevalence of body-part-as-objects in normal subjects. J Clin Exp Neuropsychol. 1999;21(4):547-552. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues Cavalcante K, Caramelli P. Evaluation of the performance of normal elderly in a limb praxis protocol: influence of age, gender, and education. J Int Neuropsychol Soc. 2009;15(4):618-622. [DOI] [PubMed] [Google Scholar]
- 24.Króliczak G, Piper BJ, Frey SH. Specialization of the left supramarginal gyrus for hand-independent praxis representation is not related to hand dominance. Neuropsychologia. 2016;93(pt B):501-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floris G, Borghero G, Cannas A, et al. . Constructional apraxia in frontotemporal dementia associated with the C9orf72 mutation: broadening the clinical and neuropsychological phenotype. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1-2):8-15. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney CJ, Beck J, Rohrer JD, et al. . Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135(pt 3):736-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahoney CJ, Downey LE, Ridgway GR, et al. . Longitudinal neuroimaging and neuropsychological profiles of frontotemporal dementia with C9ORF72 expansions. Alzheimers Res Ther. 2012;4(5):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Langenhove T, van der Zee J, Gijselinck I, et al. . Distinct clinical characteristics of C9orf72 expansion carriers compared with GRN, MAPT, and nonmutation carriers in a Flanders-Belgian FTLD cohort. JAMA Neurol. 2013;70(3):365-373. [DOI] [PubMed] [Google Scholar]
- 29.Devenney E, Hornberger M, Irish M, et al. . Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol. 2014;71(3):331-339. [DOI] [PubMed] [Google Scholar]
- 30.Floeter MK, Traynor BJ, Farren J, et al. . Disease progression in C9orf72 mutation carriers. Neurology. 2017;89(3):234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sha SJ, Takada LT, Rankin KP, et al. . Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology. 2012;79(10):1002-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suhonen N-M, Haanpää RM, Korhonen V, et al. . Neuropsychological profile in the C9ORF72 associated behavioral variant frontotemporal dementia. J Alzheimers Dis. 2017;58(2):479-489. [DOI] [PubMed] [Google Scholar]
- 33.Mackenzie IRA, Frick P, Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2014;127(3):347-357. [DOI] [PubMed] [Google Scholar]
- 34.Floeter MK, Bageac D, Danielian LE, Braun LE, Traynor BJ, Kwan JY. Longitudinal imaging in C9orf72 mutation carriers: relationship to phenotype. Neuroimage Clin. 2016;12:1035-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agosta F, Ferraro PM, Riva N, et al. . Structural and functional brain signatures of C9orf72 in motor neuron disease. Neurobiol Aging. 2017;57:206-219. [DOI] [PubMed] [Google Scholar]
- 36.Davidson Y, Robinson AC, Liu X, et al. . Neurodegeneration in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9orf72 is linked to TDP-43 pathology and not associated with aggregated forms of dipeptide repeat proteins. Neuropathol Appl Neurobiol. 2016;42(3):242-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davidson YS, Barker H, Robinson AC, et al. . Brain distribution of dipeptide repeat proteins in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol Commun. 2014;2:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez-Deza J, Lee Y-B, Troakes C, et al. . Dipeptide repeat protein inclusions are rare in the spinal cord and almost absent from motor neurons in C9ORF72 mutant amyotrophic lateral sclerosis and are unlikely to cause their degeneration. Acta Neuropathol Commun. 2015;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackenzie IRA, Frick P, Grässer FA, et al. . Quantitative analysis and clinico-pathological correlations of different dipeptide repeat protein pathologies in C9ORF72 mutation carriers. Acta Neuropathol. 2015;130(6):845-861. [DOI] [PubMed] [Google Scholar]
- 40.Vatsavayai SC, Yoon SJ, Gardner RC, et al. . Timing and significance of pathological features in C9orf72 expansion-associated frontotemporal dementia. Brain. 2016;139(pt 12):3202-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann M, Kwong LK, Truax AC, et al. . TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66(3):177-183. [DOI] [PubMed] [Google Scholar]
- 42.Van Mossevelde S, van der Zee J, Gijselinck I, et al. ; Belgian Neurology (BELNEU) Consortium . Clinical evidence of disease anticipation in families segregating a C9orf72 repeat expansion. JAMA Neurol. 2017;74(4):445-452. [DOI] [PubMed] [Google Scholar]
- 43.Neumann M, Kwong LK, Truax AC, et al. . TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66(3):177-183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Neuropsychological and behavioral tests.
eMethods 2. Magnetic resonance imaging sequence parameters.
eFigure 1. Correlation between age and expected years to onset.
eFigure 2. Diffusion tensor magnetic resonance imaging metrics.
eTable 1. Effect of C9orf72 mutation on volume of cortical regions of interest.
eTable 2. Effect of C9orf72 mutation on volume of subcortical structures.
eTable 3. Effect of C9orf72 mutation on diffusion tensor magnetic resonance imaging metrics.
eReferences.