Key Points
Question
Among stroke patients with intracranial atherosclerotic disease, what are the prevalence and prognostic associations of concomitant systemic atherosclerosis and overlapping stroke etiologies?
Findings
In this prospective registry of 403 patients who experienced strokes, intracranial atherosclerosis frequently coexisted with atherosclerotic lesions in different arteries (ie, extracranial carotid, aorta, femoral, and coronary) and other potential stroke etiologies (ie, cardioembolic pathology and small vessel disease). Concomitant extracranial carotid atherosclerosis, coronary atherosclerosis, and cardioembolic pathology increased 4-year vascular risk among patients with intracranial atherosclerosis.
Meaning
Evaluating coexisting diseases may help guide the prognosis and management of patients with strokes that are related to intracranial atherosclerosis.
Abstract
Importance
Patients who have experienced stroke with intracranial atherosclerotic disease (ICAD) may also have concomitant atherosclerosis in different arterial beds and other possible causes for ischemic stroke. However, little is known about the frequency and prognostic effect of such overlapping diseases.
Objectives
To describe the prevalence of systemic atherosclerotic burdens and overlapping stroke etiologies and their contributions to long-term prognoses among patients who have experienced stroke with ICAD.
Design, Setting, and Participants
The Asymptomatic Myocardial Ischemia in Stroke and Atherosclerotic Disease study is a single-center prospective study in which 405 patients with acute ischemic stroke within 10 days of onset were consecutively enrolled between June 2005 and December 2008 and followed up for 4 years. After excluding 2 patients because of incomplete investigations, 403 were included in this analysis.
Main Outcomes and Measures
Significant ICAD was defined as having 50% or greater stenosis/occlusion by contrast-enhanced/time-of-flight magnetic resonance angiography, computed tomography angiography, and/or transcranial Doppler ultrasonography. Systemic vascular investigations on atherosclerotic disease were performed with ultrasonography in carotid arteries, aorta and femoral arteries, and by angiography in coronary arteries. Coexistent stroke etiologies were assessed using the atherosclerosis, small-vessel disease, cardiac pathology, other cause, and dissection (ASCOD) grading system. We estimated the 4-year risk of major adverse cardiovascular events (MACE), including vascular death, nonfatal cardiac events, nonfatal stroke, and major peripheral arterial events.
Results
Of 403 participants, 298 (74%) were men and the mean (SD) age was 62.6 (13.1) years. Significant ICAD was found in 146 (36.2%). Patients with significant ICAD more often had aortic arch (70 [60.9%] vs 99 [49.0%]; P = .04) and coronary artery (103 [76.9%] vs 153 [63.2%]; P = .007) atherosclerosis than those without. Among patients with ICAD, concurrent stenosis in the extracranial carotid artery (24 [23.4%] vs 3 [9.0%]; P = .08; adjusted hazard ratio[aHR] = 2.12) and the coronary artery (19 [29.9%] vs 8 [12.8%]; P = .01; aHR = 1.90) increased the MACE risk. Furthermore, patients with ICAD who also had any cardiac pathology (ASCOD grade C1-3) were at a higher MACE risk than others (grade C0) (20 [28.2%] vs 7 [11.4%]; P = .01; aHR = 2.24). By contrast, patients with ICAD with any form of small vessel disease (grade S1-3) had a lower MACE risk than those without (grade S0) (20 [17.3%] vs 6 [34.6%]; P = .05; aHR = 0.23).
Conclusions and Relevance
Patients with ICAD often have coexisting systemic atherosclerosis and multiple potential stroke mechanisms that affect their prognosis, suggesting that extensive evaluations of overlapping diseases may allow better risk stratification.
This study explores the frequency of intracranial atherosclerotic disease in patients who have had a stroke and whether it coexists with systemic atherosclerotic lesions and other stroke etiologies.
Introduction
Intracranial atherosclerotic disease (ICAD) is a major cause of ischemic stroke. In autopsy studies, intracranial plaques and stenoses were identified in 45% to 62% of patients with ischemic stroke and were considered causal in approximately 10% of cases. Intracranial atherosclerotic disease has the highest risk of recurrent stroke compared with other stroke etiologies—as high as 15% per year even under the best medical treatment. Therefore, the need for a better understanding and management of this disease is pressing.
Intracranial atherosclerotic disease can manifest as part of concomitant systemic atherosclerotic disease that involves other arteries (eg, the aorta and the extracranial carotid, coronary, and lower extremity peripheral arteries), given that they share the same risk factors and genetic predispositions. The presence of atherosclerosis at one arterial site may encourage the clinician to look further for overlaps in different arterial beds and may prompt a global approach to reducing systemic vascular events. In addition, ICAD may coexist with other potential stroke etiologies (eg, cardioembolic source, small vessel disease [SVD]), which can be associated with their prognosis. However, to our knowledge, few studies have evaluated such overlapping diseases systematically in a large cohort. The aim of this study was to describe the prevalence of concomitant nonintracranial atherosclerotic diseases and overlap of other stroke mechanisms, and their associations with long-term vascular prognoses among patients with stroke related to ICAD. We adopted a phenotypic (but not a causative) stroke classification scheme to capture the etiological overlap and focused on stroke patients with ICAD, regardless of whether ICAD was considered as the most likely cause of the stroke.
Methods
Study Population
The design of the Asymptomatic Myocardial Ischemia in Stroke and Atherosclerotic Disease (AMISTAD) study has been published elsewhere. In brief, AMISTAD is a prospective, single-center registry of patients hospitalized with acute ischemic stroke that is designed to assess the prevalence and association of systemic atherosclerosis with vascular risk. All patients 18 years or older with a Rankin scale score of less than 5 were offered to participate, and were enrolled within 10 days of symptom onset, after providing written informed consent. Patients with stroke caused by cervicocerebral artery dissection, or secondary to a revascularization procedure, were not included.
Among 785 patients consecutively assessed for eligibility between June 2005 and December 2008, 405 were enrolled (patients who were not enrolled either had exclusion criteria [eg, had no documented cerebral infarction on neuroimaging results or were bedridden with a Rankin scale score of ≥5] or refused to sign an informed consent document). Of these, 403 patients underwent intracranial arterial investigations and were included in the current analysis (eFigure 1 in the Supplement).
The research protocol was approved by the ethics committee of Paris Bichat-Claude Bernard (September 5, 2004) and of Ile de France No1 Hotel Dieu (November 24, 2006).
Evaluation of Atherosclerotic Disease Burden
Intracranial arteries were examined by contrast-enhanced/time-of-flight magnetic resonance angiography (n = 289), computed tomography angiography (n = 53), and/or transcranial Doppler ultrasound (n = 399). For angiography, the narrowest diameter of each stenosed vessel was measured and divided by the diameter of the normal vessel proximal to the lesion or distal to the lesion if the proximal artery was diseased. Transcranial Doppler evaluations were made by senior ultrasonographers according to previously published criteria (eMethods in the Supplement). Significant ICAD was defined as a 50% or greater stenosis or occlusion. Intracranial atherosclerotic disease was considered symptomatic if the stenosis was ipsilateral to the index stroke and asymptomatic if not.
Extracranial carotid atherosclerosis was evaluated by ultrasonography. Results were categorized into absence of plaques, plaques with or without stenosis of any degree, and luminal stenosis at the most stenosed segment of 1% to 49%, 50% to 69%, or 70% to 100%. We defined extracranial carotid artery stenosis (ECAS) as the presence of atherosclerotic stenosis af 50% or greater or occlusion. We used the term plaque to describe the anatomical lesion of an artery that was produced by atherosclerotic disease, regardless of whether it induced arterial stenosis or lumen narrowing.
Coronary atherosclerosis was evaluated by catheter angiography in patients with no known history of coronary events. We performed cardiac catheterization for research purposes after obtaining consent from every participant. Results were categorized into absence of plaques, plaques with or without stenosis of any degree, and stenosis of 1% to 49%, 50% to 69%, or 70% to 100%. Patients who had a known history of the coronary events did not undergo coronary angiography, and were grouped based on previous angiography records. We defined coronary artery disease (CAD) as having a stenosis of 50% or greater, occlusion on coronary angiography results, or a known history of any acute coronary event.
Aortic atherosclerosis was evaluated by transesophageal echocardiography (aortic arch and thoracic descending aorta) and abdominal ultrasonography (abdominal aorta, including measurement of infrarenal aortic diameter). Femoral artery atherosclerosis was evaluated by lower extremity ultrasonography. Patients were categorized into presence or absence of plaques, regardless of severity.
Stroke Etiology Phenotyping
To capture and weigh the overlap between diseases underlying ischemic stroke, an etiologic phenotyping of stroke was performed using the atherosclerosis (A), SVD (S), cardiac pathology (C), other definite causes (O), and dissection (D) (ASCOD) grading system. The ASCOD system categorizes 5 predefined phenotypes, and each of the phenotypes is graded according to (1) when the disease is potentially causal, (2) when causality is uncertain, (3) when the disease is present but is unlikely to be causal, and (0) when the disease is absent.
Follow-up
Follow-up visits were scheduled between 3 and 6 months after enrollment and thereafter every year for 4 years. At each follow-up visit, treatment, blood pressure, lipid profile, and any occurrence of clinical events were recorded. The primary outcome was a composite of major adverse cardiovascular events (MACE), including vascular death, nonfatal cardiac events, nonfatal stroke, and major peripheral arterial events.
Statistical Analysis
Quantitative variables were expressed as mean (SD) in cases of normal distribution or median (interquartile range [IQR]) otherwise. Qualitative variables were expressed as frequencies (percentages). The normality of distributions was assessed graphically and using the Shapiro-Wilk test. Bivariate comparisons were made using the t test or analysis of variance for quantitative variables (after log-transformation for skewed data), the χ2 test (or Fisher exact test when the expected cell frequency was <5) for categorical variables, and the log-rank test for censored variables. Patient characteristics, the presence of non-ICADs, and 4-year outcomes were compared between patients with and without significant ICAD. Further comparisons were made for subgroups according to the symptomaticity and localization of ICAD. The patient characteristics described in Table 1 (except for ASCOD grades because of multicollinearity) that remained significantly associated with ICAD (P < .10) were considered for entrance into the multivariable logistic regression model, and the full model was simplified with a backward selection procedure by using a removal criteria of 0.10. Characteristics that remained in the model were subsequently used to adjust the association of significant ICAD with non-ICAD in a multiple logistic regression, and the association of ICAD with 4-year outcomes in a Cox proportional hazards model. We then compared the 4-year outcomes between patients with and without significant ICAD according to ECAS, CAD, and ASCOD grades for cardiac pathology and SVD. For a given outcome, patients who died of causes other than the outcome were censored at the time of death. The proportional hazard assumptions were checked using log-log survival plots and by introducing a time-dependent variable into the models.
Table 1. Baseline Characteristics of Patients With and Without Significant ICAD.
| Characteristic | Intracranial Stenosis ≥50% or Occlusion | P Value | |
|---|---|---|---|
| No (n = 257) |
Yes (n = 146) |
||
| Age, mean (SD), y | 61 (13) | 65 (13) | .007 |
| Men, No. (%) | 190 (73.9) | 108 (74.0) | .99 |
| Body mass index (calculated as weight in kilograms divided by height in meters squared), mean (SD) | 25.6 (4.3) | 26.7 (4.5) | .02 |
| Medical history, No. (%) | |||
| Hypertension | 207 (80.5) | 121 (82.9) | .56 |
| Diabetes | 56 (21.8) | 32 (21.9) | .98 |
| Dyslipidemia | 103 (40.1) | 70 (47.9) | .12 |
| Current smokers | 101 (39.4) | 53 (36.3) | .53 |
| History of stroke | 17 (6.6) | 15 (10.3) | .20 |
| History of coronary heart disease | 36 (14.0) | 26 (17.8) | .31 |
| History of peripheral artery disease | 22 (8.6) | 15 (10.3) | .57 |
| History of atrial fibrillation | 10 (3.9) | 14 (9.6) | .02 |
| Examination findings | |||
| Systolic BP, mean (SD), mm Hg | 137 (17) | 142 (20) | .01 |
| Diastolic BP, mean (SD), mm Hg | 79 (11) | 79 (11) | .67 |
| Total cholesterol, mean (SD), mg/dL | 200 (49) | 198 (45) | .80 |
| LDL-C, mean (SD), mg/dL | 117 (40) | 121 (41) | .42 |
| HDL-C, mean (SD), mg/dL | 55 (18) | 52 (15) | .06 |
| TG, median (IQR), mg/dL | 112 (81-161) | 118 (88-158) | .68a |
| TG/HDL-C ratio, median (IQR) | 2.2 (1.4-3.3) | 2.3 (1.5-3.6) | .30a |
| Glucose, median (IQR), mg/dL | 95 (86-108) | 97 (88-110) | .60a |
| HbA1c, median (IQR), % | 5.7 (5.4-6.2) | 5.8 (5.4-6.4) | .39a |
| Metabolic syndrome, No. (%) | 38 (17.3) | 32 (25.0) | .08 |
| Atherogenic dyslipidemia, No. (%) | 26 (10.1) | 15 (10.3) | .96 |
| Main ASCOD grades,b No. (%) | |||
| Atherothrombosis | |||
| A0 | 36 (14.1) | 0 (0.0) | <.001 |
| A3 | 103 (40.4) | 28 (19.2) | |
| A2 | 48 (18.8) | 13 (8.9) | |
| A1 | 66 (25.9) | 105 (71.9) | |
| Cardiac pathology | |||
| C0 | 108 (42.3) | 65 (44.5) | .77 |
| C3 | 40 (15.7) | 21 (14.4) | |
| C2 | 40 (15.7) | 17 (11.6) | |
| C1 | 54 (21.2) | 36 (24.7) | |
| Small vessel disease | |||
| S0 | 47 (18.4) | 19 (13.0) | .005 |
| S3 | 156 (61.2) | 113 (77.4) | |
| S2 | 7 (2.7) | 0 (0.0) | |
| S1 | 39 (15.3) | 7 (7.8) | |
Abbreviations: ASCOD, atherosclerosis, small-vessel disease, cardiac pathology, other definite cause, and dissection; BP, blood pressure; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; ICAD, intracranial atherosclerotic disease; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
SI conversion factors: To convert total cholesterol, LDL-C, and HDL-C to millimoles per liter, multiply by 0.0259; TG to millimoles per liter, multiply by 0.0113; glucose to millimoles, multiply by 0.0555.
Calculated after log-transformation of data.
ASCOD phenotyping assigns a degree of causality between the index stroke and each category as follows: 1, potential cause; 2, causality is uncertain; 3, disease is present but is unlikely a direct cause; 0, the disease is absent.
Statistical testing was done with a 2-tailed α level of .05. Data were analyzed using SAS, version 9.3 (SAS Institute).
Results
Among 403 patients (mean age [SD], 62.6 [13.1] years; 298 men [74.0%]), significant ICAD was found in 146 (36.2%), of whom 72 (17.9%) were symptomatic and 74 (18.4%) were asymptomatic. eTable 1 in the Supplement shows the affected sites and severity of ICAD.
Baseline Characteristics
Patients with significant ICAD were older and had a higher body mass index (calculated as weight in kilograms divided by height in meters squared), higher systolic blood pressure, and a higher rate of atrial fibrillation than those without (Table 1). There were significant differences in the distribution of A and S grades but no differences in C grades by ASCOD phenotyping. Seventy-four (50.5%) and 120 (85.2%) patients with ICAD had any degree of cardioembolic pathology (grade C1, C2, or C3) and SVD (grade S1, S2, or S3), respectively. eTable 2 in the Supplement shows baseline characteristics of patients with symptomatic and asymptomatic significant ICAD. eTable 3 in the Supplement shows baseline characteristics according to ICAD location.
Systemic Atherosclerosis Burden
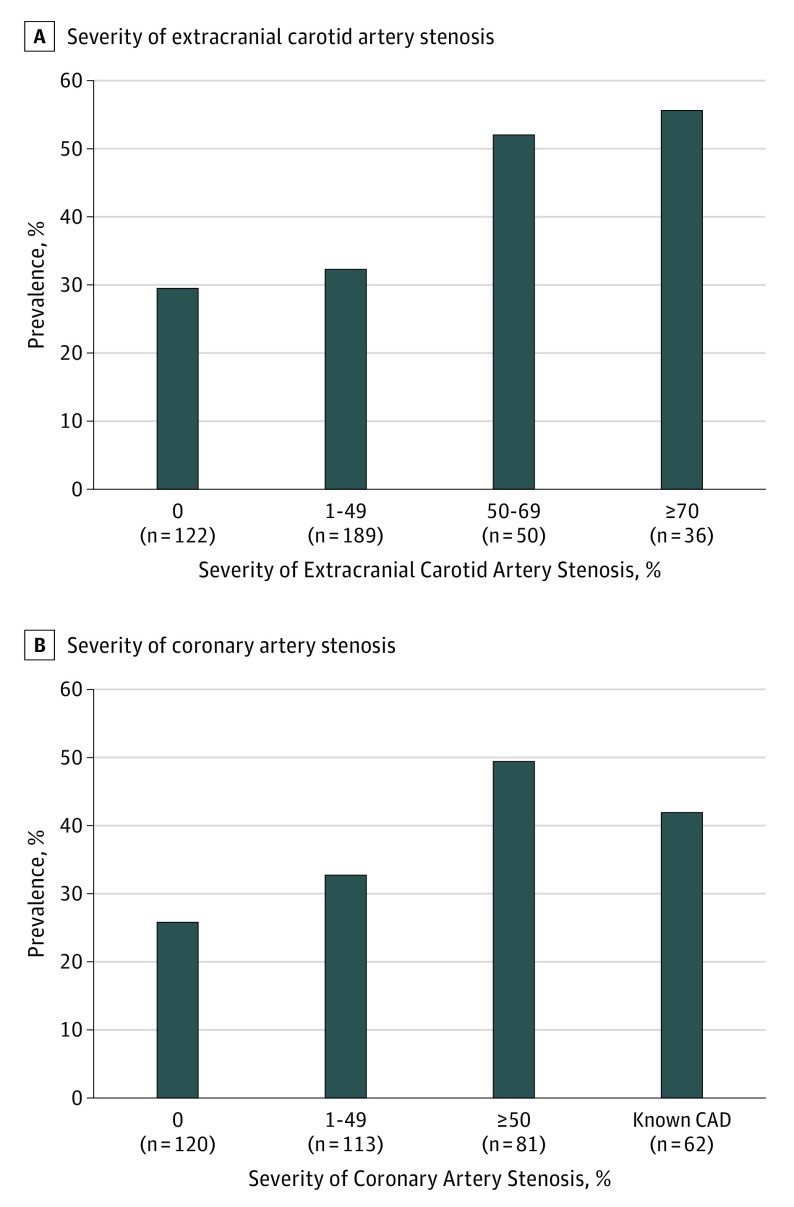
As shown in Table 2, significant ICAD frequently involved concurrent atherosclerotic diseases in other arterial territories, particularly in the extracranial carotid (n = 107, 74.8%), femoral (n = 101, 76.5%), and coronary (n = 103, 76.9%) arteries. Patients with significant ICAD were more likely to have atherosclerotic plaques in the aortic arch (60.9% [n = 70] vs 49.0% [n = 99]; P = .04) and in coronary arteries (76.9% [n = 103] vs 63.2% [n = 153]; P = .007) than those without. The prevalence of ICAD increased with the severity of both extracranial carotid and coronary artery lesions (Figure 1). The prevalence of systemic atherosclerosis did not differ according to whether ICAD was symptomatic (eTable 4 in the Supplement) or according to its location (eTable 5 in the Supplement).
Table 2. Prevalence of Significant Intracranial Atherosclerotic Disease According to the Presence of Nonintracranial Atherosclerosis.
| Characteristic | Intracranial Stenosis ≥50% or Occlusion | P Value | |
|---|---|---|---|
| No (n = 257) |
Yes (n = 146) |
||
| No. (%) with atherosclerosis | |||
| Extracranial carotid artery | 168 (66.2) | 107 (74.8) | .07 |
| Aortic arch | 99 (49.0) | 70 (60.9) | .04 |
| Descending aorta | 41 (20.3) | 30 (26.1) | .23 |
| Abdominal aorta | 53 (23.7) | 39 (31.2) | .13 |
| Femoral artery | 169 (72.8) | 101 (76.5) | .44 |
| Coronary artery | 153 (63.2) | 103 (76.9) | .007 |
Figure 1. Prevalence of Significant Intracranial Atherosclerosis Disease (ICAD).
A, Prevalences of intracranial stenosis of 50% or greater or occlusion are shown according to the severity of extracranial carotid atherosclerosis. The P value for the trend was .002 and was calculated including the severity as the ordinal variable. B, Prevalences of intracranial stenosis of 50% or greater or occlusion are shown according to the severity of coronary atherosclerosis. The P value for the trend was .004 and was calculated including the severity as the ordinal variable.
Risk of MACE
At discharge, 391 (97.1%) received antithrombotic therapy (339 [84.1%] and 111 [27.6%] received any antiplatelet and anticoagulant agents, respectively). At 4 years, the mean (SD) systolic blood pressure level was 130 (17) mm Hg and the mean (SD) low-density lipoprotein cholesterol concentration was 82 (30) mg/dL (to convert to millimoles per liter, multiply by 0.0259) (eFigure 2 in the Supplement).
Among 403 patients, 71 (4.2%) had at least 1 vascular event within 4 years, giving an event rate of 18.3% (95% CI, 14.8-22.5). As shown in Table 3 and eFigure 3 in the Supplement, significant ICAD was not associated with a 4-year risk of MACE, cerebrovascular events, or mortality. The differences remained nonsignificant even when patients were stratified by blood pressure level (≥140 or <140 mm Hg) and low-density lipoprotein cholesterol concentration (≥100 or <100 mg/dL) (data not shown). There were no differences in MACE risk between symptomatic ICAD (n = 12, 17.5%) and asymptomatic ICAD (n = 16, 22.5%).
Table 3. Four-Year Risk of Recurrent Vascular Events Associated With Significant Intracranial Atherosclerotic Disease.
| No. (%) | Log-Rank P Value | Hazard Ratio (95% CI)a | P Value | |
|---|---|---|---|---|
| MACE | ||||
| No stenosis ≥50% or occlusion | 43 (17.3) | .53 | 1 [Reference] | NA |
| Asymptomatic stenosis ≥50% or occlusion | 16 (22.5) | 1.24 (0.65-2.38) | .51 | |
| Symptomatic stenosis ≥50% or occlusion | 12 (17.5) | 0.88 (0.44-1.74) | .71 | |
| Any stenosis ≥50% or occlusion | 28 (20.1) | .43 | 1.04 (0.62-1.77) | .87 |
| Stroke + TIA | ||||
| No stenosis ≥50% or occlusion | 28 (11.5) | .88 | 1 [Reference] | NA |
| Asymptomatic stenosis ≥50% or occlusion | 8 (11.5) | 0.87 (0.36-2.13) | .76 | |
| Symptomatic stenosis ≥50% or occlusion | 9 (13.2) | 1.10 (0.49-2.48) | .82 | |
| Any stenosis ≥50% or occlusion | 17 (12.4) | .69 | 0.99 (0.50-1.93) | .97 |
| Any death | ||||
| No stenosis ≥50% or occlusion | 21 (8.3) | .32 | 1 [Reference] | NA |
| Asymptomatic stenosis ≥50% or occlusion | 10 (14.0) | 1.65 (0.72-3.81) | .24 | |
| Symptomatic stenosis ≥50% or occlusion | 6 (8.5) | 0.90 (0.35-2.29) | .82 | |
| Any stenosis ≥50% or occlusion | 16 (11.3) | .31 | 1.23 (0.60-2.49) | .57 |
| Vascular death | ||||
| No stenosis ≥50% or occlusion | 9 (3.6) | .40 | 1 [Reference] | NA |
| Asymptomatic stenosis ≥50% or occlusion | 5 (7.2) | 2.40 (0.66-8.76) | .18 | |
| Symptomatic stenosis ≥50% or occlusion | 4 (5.9) | 1.57 (0.43-5.71) | .49 | |
| Any stenosis ≥50% or occlusion | 9 (6.6) | .19 | 1.91 (0.65-5.63) | .24 |
Abbreviations: MACE, major adverse cardiovascular events; NA, not applicable; TIA, transient ischemic attack.
Adjusted for age, baseline systolic blood pressure, baseline high-density lipoprotein cholesterol, body mass index (calculated as weight in kilograms divided by height in meters squared), and history of atrial fibrillation.
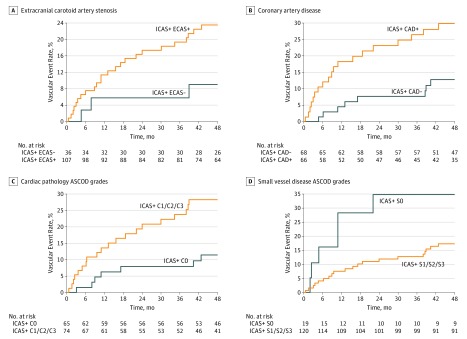
Among patients with significant ICAD, concurrent ECAS was associated with an increased MACE risk by a borderline of significance (23.4% vs 9.0%; log-rank P = .08) (Figure 2A and eTable 6 in the Supplement). Furthermore, as shown in Figure 2B, the MACE risk was significantly higher in patients with concurrent CAD than in those without (29.9% vs 12.8%; log-rank P = .01).
Figure 2. Cumulative Incidence Curves for Major Vascular Events.
Major vascular events among patients with significant intracranial atherosclerotic disease, stratified by the presence of extracranial carotid artery stenosis (log-rank, P = .08) (A), coronary artery disease (log-rank, P = .01) (B), cardiac pathology ASCOD grades (log-rank, P = .01) (C), and small vessel disease ASCOD grades (log-rank, P = .05) (D). CAD indicates coronary artery disease; ECAS, extracranial carotid artery stenosis; ICAS, intracranial artery stenosis.
With regard to the etiologic overlap assessed by ASCOD, patients with significant ICAD and graded as C1, C2, or C3 (ie, presence of any cardiac pathology) had a significantly higher risk of MACE than those graded as C0 (ie, no clinical, structural, or rhythm cardiac abnormality) (28.2% vs 11.4%; log-rank P = .01) (Figure 2C). The difference became borderline significant after a multivariable adjustment (adjusted hazard ratio, 2.24; 95% CI, 0.87-5.73; P = .009). On the other hand, patients with SVD graded as S1, S2, or S3 (ie, any form of SVD) tended to have a lower risk of MACE than those graded as S0 (ie, no clinical or magnetic resonance imaging evidence of SVD) (17.3% vs 34.6%; log-rank P = .05) (Figure 2D). The multivariable-adjusted hazard ratio was 0.23 (95% CI, 0.08-0.68; P = .008) for patients with grades S1 to S3.
Discussion
We found that ICAD is not a rare condition among patients who had a stroke and that it frequently coexisted with systemic atherosclerotic lesions and other stroke etiologies. Furthermore, concurrent ECAS, CAD, cardiac pathology, or SVD affected prognoses in patients who had a stroke with significant ICAD. Our results suggest that screening for such coexistent diseases can yield important supplemental information for identifying patients with ICAD who are at excess vascular risk. Also, this study highlighted that the ASCOD system can be useful not only in classifying strokes but also in predicting a prognosis.
Our study builds on earlier studies by achieving a comprehensive and rigorous search for various concurrent diseases in patients with ICAD as well as by focusing on their prognostic effect. It was not surprising that patients with ICAD frequently had atherosclerosis in other systemic arteries, given their shared pathophysiological mechanisms. Several studies reported significant associations of ICAD with concurrent ECAS, CAD, aortic atheroma, or lower extremity peripheral artery disease in patients who had a stroke. However, to our knowledge, none of these studies thoroughly performed systemic examinations on different arteries within a single cohort. In addition, prior studies did not use vascular imaging modalities to diagnose coronary or peripheral arterial stenosis, possibly overlooking asymptomatic (or early-stage) diseases. For example, the prevalence of CAD in patients with ICAD was 25% and 52% when diagnosed by cardiac stress test results and myocardial perfusion single-photon emission computed tomography results, respectively. The 77% prevalence of coronary atherosclerosis in our study, which was angiographically documented, was higher than in these studies but was similar to autopsy series that reported an around 80% prevalence. Meanwhile, overlapping stroke etiologies have been less well studied among patients with ICAD. This might be because of the difficulties in capturing and weighing the various underlying diseases simultaneously, but our study resolved this issue with the ASCOD system. Conventional causative classification systems (eg, trial of ORG 10172 in acute stroke treatment classification) would restrict our analysis because they only consider diseases that are considered directly causally related to the stroke event, neglecting other underlying diseases that are not considered causally related. Further, little is known about the prognostic effect of these wide-ranging comorbid diseases, including atherosclerotic and nonatherosclerotic ones, on ICAD. One of our aims was to identify patients at sufficiently high risk of experiencing vascular events who may benefit from more aggressive treatments and can be a target group for subsequent randomized clinical trials.
The risk of MACE in patients with symptomatic ICAD was 17.5% at 4 years, which is similar to the risk estimated in other studies. In the Stenting Versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS) randomized clinical trial, symptomatic ICAD with 70% to 90% stenosis had a 15% risk of stroke or death at 3 years, even when treated by controlling aggressive vascular risk factors. The blood pressure levels and lipid concentrations achieved in our study were similar to those in the SAMMPRIS trial. The MACE risk in patients with asymptomatic ICAD was as high as in patients with symptomatic ICAD in our study (22.5%) despite that the prognosis of asymptomatic stenosis has been reported as being relatively benign, with around 3% per year of stroke risk. This inconsistency may be explained by differences in the definition of “asymptomatic” patients between the studies. Namely, these prior studies included stroke-free populations, or excluded patients with other potential causes of stroke (eg, cardioembolic sources or ECAS). On the other hand, we included patients who had a stroke with ICAD altogether, irrespective of whether ICAD was a direct cause of stroke among mixed underlying diseases. When the AMISTAD patients with ICAD were considered “asymptomatic,” we meant stroke with contralateral stenosis. Therefore, all our “asymptomatic” patients had strokes due to various etiologies, including high-risk cases, which could result in a higher event rate.
The MACE risk in ICAD patients was borderline increased with ECAS and was significantly increased with CAD by 14% and 17%, respectively, in absolute risk. Such high risks may be related to the synergistic effect of the systemic atherosclerotic burden, and agree with the Reduction of Atherothrombosis for Continued Health (REACH) registry that demonstrated a high global vascular risk in patients with multiple atherosclerotic diseases across different vascular beds. Also, our results agree with previous reports that concurrent atherosclerosis of the extracranial and intracranial arteries have a poorer vascular outcome compared with those with 1 or none.
Our group previously reported that the A, S, and C categories of ASCOD often coexist. In the present study, 74 (51%) and 120 (85%) patients with ICAD simultaneously had potential cardioembolic pathology and SVD, respectively. Coincidence of ICAD and atrial fibrillation was not rare (10%), presumably because they share many risk factors. Although few studies have achieved systematic evaluations, specific relationships between cerebral atherosclerosis and atrial fibrillation, cerebral atherosclerosis and SVD, or atrial fibrillation and SVD have been reported. These findings imply that concurrent etiology is a frequent situation among all stroke subtypes. Importantly, we found a significant increase in MACE risk in patients with ICAD who also had any form of cardioembolic pathology; the absolute risk increase of 17% was considered substantial. A subanalysis of the REACH registry found comparable results that patients with atherothrombotic stroke with atrial fibrillation were at a higher risk of MACE than those without. By contrast, the MACE risk tended to be lower in patients with ICAD with coexisting SVD than in those without. A subanalysis of the SAMMPRIS trial reported conflicting findings that patients with symptomatic ICAD with concurrent SVD had a higher stroke risk than those without by a nearly significant difference. The association of SVD in patients with ICAD may warrant further discussion, given the relatively low event rates in our analysis. Nevertheless, a more sophisticated and global evaluation of underlying stroke mechanisms may allow better risk stratification because certain combinations appeared to have higher or lower vascular risk.
Limitations
First, our study was not population-based, but was hospital-based in one of the largest stroke units in Paris, France, that works as a primary care referral center for acute stroke admissions with a dedicated catchment area. The baseline characteristics of the participants were typical of the general stroke population and seem representative of patients with stroke. Although single-center cohort studies tend to be less generalizable than population-based multicenter studies, this is less of a concern when examining internal correlations with outcomes that were collected during prospective follow-up than it is in cross-sectional analyses. Second, we did not include the patients who experienced the most severe strokes with a Rankin scale score of more than 4 who might be likely to die from the index event or have a further recurrent event. Third, the methods for determining ICAD could be another limitation. Conventional catheter angiography is considered the gold standard for ICAD measurements but was not considered feasible to maintain the consecutive inclusion of patients. Magnetic resonance angiography and transcranial Doppler ultrasonography may be prone to overdiagnosis, although data on interrater reproducibility for ICAD diagnoses were not available in our study. Finally, owning to the few events and the multiplicity of tests within a study, our results should be interpreted carefully and be replicated by future studies.
Conclusions
Systemic atherosclerotic disease and overlapping stroke etiologies are common and are associated with prognosis in patients with stroke and ICAD. Extensive evaluations of coexisting diseases may be essential from the perspectives of treatment, follow-up, and prognosis. It is noteworthy that concomitant ECAS, CAD, and cardioembolic diseases as defined by ASCOD increased the vascular risk in these patients.
eMethods
eTable 1. Prevalence of ICAD by Site and Severity
eTable 2. Baseline Characteristics of Patients with Symptomatic and Asymptomatic Significant ICAD
eTable 3. Baseline Characteristics According to Localization of Significant ICAD
eTable 4. Prevalence of Nonintracranial Atherosclerosis in Patients with Symptomatic and Asymptomatic Significant ICAD
eTable 5. Prevalence of Nonintracranial Atherosclerosis According to Localization of Significant ICAD
eTable 6. Four-Year Risk of Recurrent Vascular Events Associated with Coexisting Diseases in Patients with Significant ICAD
eFigure 1. Study Flow Chart
eFigure 2. Mean Systolic Blood Pressure Levels and Low-Density Lipoprotein Cholesterol Concentrations at Baseline and Follow-Up Visits
eFigure 3. Cumulative Incidence Curves of Major Vascular Events According to the Presence of Significant ICAD
eReferences
References
- 1.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12(11):1106-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis. 2008;25(1-2):74-80. [DOI] [PubMed] [Google Scholar]
- 3.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39(4):1142-1147. [DOI] [PubMed] [Google Scholar]
- 4.Wong KS, Li H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke. 2003;34(10):2361-2366. [DOI] [PubMed] [Google Scholar]
- 5.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. ; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators . Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305-1316. [DOI] [PubMed] [Google Scholar]
- 6.Mazighi M, Tanasescu R, Ducrocq X, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66(8):1187-1191. [DOI] [PubMed] [Google Scholar]
- 7.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. ; SAMMPRIS Trial Investigators . Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt DL, Steg PG, Ohman EM, et al. ; REACH Registry Investigators . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180-189. [DOI] [PubMed] [Google Scholar]
- 9.Arnett DK, Baird AE, Barkley RA, et al. ; American Heart Association Council on Epidemiology and Prevention; American Heart Association Stroke Council; Functional Genomics and Translational Biology Interdisciplinary Working Group . Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: a scientific statement from the American Heart Association Council on Epidemiology and Prevention, the Stroke Council, and the Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2007;115(22):2878-2901. [DOI] [PubMed] [Google Scholar]
- 10.Amarenco P, Lavallée PC, Labreuche J, et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. 2011;42(1):22-29. [DOI] [PubMed] [Google Scholar]
- 11.Amarenco P, Lavallée PC, Labreuche J, et al. Coronary artery disease and risk of major vascular events after cerebral infarction. Stroke. 2013;44(6):1505-1511. [DOI] [PubMed] [Google Scholar]
- 12.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643-646. [PMC free article] [PubMed] [Google Scholar]
- 13.Meseguer E, Lavallée PC, Mazighi M, et al. Yield of systematic transcranial Doppler in patients with transient ischemic attack. Ann Neurol. 2010;68(1):9-17. [DOI] [PubMed] [Google Scholar]
- 14.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36(1):1-5. [DOI] [PubMed] [Google Scholar]
- 15.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852-1866. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Cho SJ, Moon HS, et al. Combined extracranial and intracranial atherosclerosis in Korean patients. Arch Neurol. 2003;60(11):1561-1564. [DOI] [PubMed] [Google Scholar]
- 17.Liu HM, Tu YK, Yip PK, Su CT. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke. 1996;27(4):650-653. [DOI] [PubMed] [Google Scholar]
- 18.Chimowitz MI, Poole RM, Starling MR, Schwaiger M, Gross MD. Frequency and severity of asymptomatic coronary disease in patients with different causes of stroke. Stroke. 1997;28(5):941-945. [DOI] [PubMed] [Google Scholar]
- 19.Arenillas JF, Candell-Riera J, Romero-Farina G, et al. Silent myocardial ischemia in patients with symptomatic intracranial atherosclerosis: associated factors. Stroke. 2005;36(6):1201-1206. [DOI] [PubMed] [Google Scholar]
- 20.Nam HS, Han SW, Lee JY, et al. Association of aortic plaque with intracranial atherosclerosis in patients with stroke. Neurology. 2006;67(7):1184-1188. [DOI] [PubMed] [Google Scholar]
- 21.Manzano JJ, De Silva DA, Pascual JL, Chang HM, Wong MC, Chen CP. Associations of ankle-brachial index (ABI) with cerebral arterial disease and vascular events following ischemic stroke. Atherosclerosis. 2012;223(1):219-222. [DOI] [PubMed] [Google Scholar]
- 22.Barreto-Neto N, Barros AD, Jesus PA, et al. Low ankle-brachial index is a simple physical exam sign predicting intracranial atherosclerotic stenosis in ischemic stroke patients. J Stroke Cerebrovasc Dis. 2016;25(6):1417-1420. [DOI] [PubMed] [Google Scholar]
- 23.Gongora-Rivera F, Labreuche J, Jaramillo A, Steg PG, Hauw JJ, Amarenco P. Autopsy prevalence of coronary atherosclerosis in patients with fatal stroke. Stroke. 2007;38(4):1203-1210. [DOI] [PubMed] [Google Scholar]
- 24.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. ; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators . Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahab F, Cotsonis G, Lynn M, et al. ; WASID Study Group . Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke. 2008;39(3):1039-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern R, Steinke W, Daffertshofer M, Prager R, Hennerici M. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005;65(6):859-864. [DOI] [PubMed] [Google Scholar]
- 27.Steg PG, Bhatt DL, Wilson PW, et al. ; REACH Registry Investigators . One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197-1206. [DOI] [PubMed] [Google Scholar]
- 28.Man BL, Fu YP, Chan YY, et al. Use of magnetic resonance angiography to predict long-term outcomes of ischemic stroke patients with concurrent stenoses in Hong Kong. Cerebrovasc Dis. 2009;28(2):112-118. [DOI] [PubMed] [Google Scholar]
- 29.Sirimarco G, Lavallée PC, Labreuche J, et al. Overlap of diseases underlying ischemic stroke: the ASCOD phenotyping. Stroke. 2013;44(9):2427-2433. [DOI] [PubMed] [Google Scholar]
- 30.Goto S, Bhatt DL, Röther J, et al. ; REACH Registry Investigators . Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156(5):855-863, 863.e2. [DOI] [PubMed] [Google Scholar]
- 31.Kim YD, Cha MJ, Kim J, et al. Increases in cerebral atherosclerosis according to CHADS2 scores in patients with stroke with nonvalvular atrial fibrillation. Stroke. 2011;42(4):930-934. [DOI] [PubMed] [Google Scholar]
- 32.Kwon HM, Lynn MJ, Turan TN, et al. ; SAMMPRIS Investigators . Frequency, risk factors, and outcome of coexistent small vessel disease and intracranial arterial stenosis: results from the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. JAMA Neurol. 2016;73(1):36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chutinet A, Biffi A, Kanakis A, Fitzpatrick KM, Furie KL, Rost NS. Severity of leukoaraiosis in large vessel atherosclerotic disease. AJNR Am J Neuroradiol. 2012;33(8):1591-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horstmann S, Möhlenbruch M, Wegele C, et al. Prevalence of atrial fibrillation and association of previous antithrombotic treatment in patients with cerebral microbleeds. Eur J Neurol. 2015;22(10):1355-1362. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi A, Iguchi M, Shimizu S, Uchiyama S. Silent cerebral infarcts and cerebral white matter lesions in patients with nonvalvular atrial fibrillation. J Stroke Cerebrovasc Dis. 2012;21(4):310-317. [DOI] [PubMed] [Google Scholar]
- 36.Feldmann E, Wilterdink JL, Kosinski A, et al. ; Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators . The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68(24):2099-2106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Prevalence of ICAD by Site and Severity
eTable 2. Baseline Characteristics of Patients with Symptomatic and Asymptomatic Significant ICAD
eTable 3. Baseline Characteristics According to Localization of Significant ICAD
eTable 4. Prevalence of Nonintracranial Atherosclerosis in Patients with Symptomatic and Asymptomatic Significant ICAD
eTable 5. Prevalence of Nonintracranial Atherosclerosis According to Localization of Significant ICAD
eTable 6. Four-Year Risk of Recurrent Vascular Events Associated with Coexisting Diseases in Patients with Significant ICAD
eFigure 1. Study Flow Chart
eFigure 2. Mean Systolic Blood Pressure Levels and Low-Density Lipoprotein Cholesterol Concentrations at Baseline and Follow-Up Visits
eFigure 3. Cumulative Incidence Curves of Major Vascular Events According to the Presence of Significant ICAD
eReferences