Key Points
Question
How are tau, β-amyloid, and cognitive status associated in Parkinson disease?
Findings
This cross-sectional positron emission tomographic imaging study examined β-amyloid and tau deposition in 15 cognitively normal patients with Parkinson disease, 14 cognitively impaired patients with Parkinson disease, and 49 cognitively normal older control participants. Tau deposition was not related to cognitive status in patients with Parkinson disease, but was significantly associated with age and β-amyloid regardless of diagnostic category.
Meaning
The presence of tau and β-amyloid in patients with Parkinson disease does not explain their cognitive functioning, although increased tau accumulation is seen in individuals with greater levels of brain β-amyloid.
This case-control study evaluates the associations between β-amyloid, tau pathology, and cognition by comparing cognitively normal and mildly cognitively impaired patients with Parkinson disease with healthy control participants.
Abstract
Importance
Multiple disease processes are associated with cognitive impairment in Parkinson disease (PD), including Lewy bodies, cerebrovascular disease, and Alzheimer disease. It remains unknown whether tau pathology relates to cognition in patients with PD without dementia.
Objective
To compare tau aggregation in patients with PD who are cognitively normal (PD-CN), patients with PD with mild cognitive impairment (PD-MCI), and healthy control participants, and evaluate the relationships between β-amyloid (Aβ), tau, and cognition in patients with PD who did not have dementia.
Design, Setting, and Participants
This cross-sectional study recruited 30 patients with Parkinson disease (15 with PD-CN and 15 with PD-MCI) from a tertiary care medical center and research institutions from July 2015 through October 2016. One patient with PD-MCI did not receive a magnetic resonance imaging scan and thus was excluded from all analyses; 29 patients with PD were included in the present study. Participants underwent tau positron emission tomographic (PET) scanning with fluorine 18–labeled AV-1451, Aβ PET scanning with carbon 11–labeled Pittsburgh compound B, magnetic resonance imaging, cognitive testing, and neurologic evaluation. Imaging measures were compared with 49 healthy control participants.
Main Outcomes and Measures
Outcomes were tau PET measurements of groups of patients with PD-CN and PD-MCI. We hypothesized that tau aggregation across groups would be related to age and Aβ status.
Results
Of the 78 participants, 47 (60%) were female, and the mean (SD) age was 71.1 (6.6) years. Six patients with PD (21%) were Aβ-positive, of whom 1 was mildly cognitively impaired; 23 were Aβ-negative (79%). (Of the 49 healthy controls, 25 were Aβ-negative and 24 Aβ-positive.) Voxelwise contrasts of whole-brain tau PET uptake between patients with PD-CN and patients with PD-MCI, and additionally between all patients with PD and Aβ-negative controls, did not reveal significant differences. Tau PET binding did not differ between patients with PD-MCI and PD-CN in brain regions reflecting Alzheimer disease Braak stages 1/2, 3/4, or 5/6, and did not differ from Aβ-negative healthy older adults. Mean (SD) tau PET binding was significantly elevated in Aβ-positive patients with PD relative to Aβ-negative patients with PD within brain regions reflecting Alzheimer disease Braak stage 3/4 (1.22 [0.07] vs 1.14 [0.07]; P = .03) and Braak stage 5/6 (1.20 [0.07] vs 1.11 [0.08]; P = .02).
Conclusions and Relevance
These findings suggest that patterns of cortical Aβ and tau do not differ in people with PD-CN, people with PD-MCI, and healthy older adults. Age, Aβ, and tau do not differentiate patients with PD-CN and PD-MCI. Tau deposition is related to Aβ status and age in both people with PD and healthy older adults. Cognitive deficits in people with PD without dementia do not appear to reflect measureable Alzheimer disease.
Introduction
Mild cognitive impairment is estimated to affect approximately 25% to 30% of patients with Parkinson disease (PD). Postmortem studies suggest that cognitive decline in PD is related to the combined influence of α-synuclein (a protein associated with presynaptic terminal function and the regulation of dopamine), the development of Alzheimer disease (AD), and cerebrovascular factors. Work in animal and cellular models has demonstrated that a synergistic relationship between α-synuclein and AD might accelerate cognitive decline. Despite this evidence for the contribution of multiple disease processes to cognitive impairment in PD, recent studies using positron emission tomographic (PET) imaging to measure AD biomarkers in patients with PD have reported mixed findings.
Studies of deposits of tau protein, the dysfunction of which is implicated in dementia, have used PET imaging and the tracer fluorine 18–labeled AV-1451 ([18F]AV-1451) to show elevated binding in patients with dementia with Lewy bodies and Parkinson disease dementia relative to healthy controls. The relationship between cortical tau aggregation and cognitive dysfunction seen in AD has also been observed in Lewy body diseases. However, patients with PD who had mild cognitive impairment (PD-MCI) showed no differences in tau PET scan results compared with patients with PD who were cognitively normal (PD-CN). Crucially, this study left unresolved whether β-amyloid (Aβ) plaques are associated with tau in the brains of people with PD, and whether the 2 disease processes might jointly contribute to cognitive impairment in PD. Positron emission tomography measures of cortical Aβ in patients with PD who do not have dementia have demonstrated an association between longitudinal cognitive decline and cortical Aβ burden despite a lack of relationships between Aβ and baseline cognition.
We investigated the contributions of AD processes to cognitive impairment in PD by collecting both tau and Aβ PET scan data from 14 mildly cognitively impaired and 15 cognitively normal patients with PD, as well as 49 healthy control participants. In examining the contributions of both tau and Aβ to cognitive status in patients with PD who do not have dementia, we hypothesized that tau aggregation across both groups would be related to age and Aβ status.
Methods
Participants
We recruited 30 patients with PD through the University of California, San Francisco Memory and Aging Center, including 15 with mild cognitive impairment (PD-MCI) and 15 cognitively normal individuals with PD (PD-CN).
All patients with PD met the clinical diagnostic criteria of the UK Parkinson’s Disease Society Brain Bank. In addition, this participant group underwent neuropsychological assessments, including the Montreal Cognitive Assessment and additional assessments spanning 5 cognitive domains known to be sensitive to idiopathic PD: attention and working memory, executive function, language, episodic memory, and visuospatial function. Patients with PD met criteria for mild cognitive impairment by demonstrating level 2 clinical categorization via comprehensive assessment and by self-reported or informant-reported cognitive decline on interview, as described by Litvan et al; in other words, patients were characterized as having PD-MCI if they performed 1 to 2 SDs below age-matched and sex-matched norms on 2 or more neuropsychological tests. Patients underwent medical history and neurological examination by a qualified neurologist. Severity of motor symptoms was measured using the Unified Parkinson’s Disease Rating Scale version 3. All patients were diagnosed by clinicians who were blinded to PET imaging at a consensus case conference.
Additionally, 49 cognitively normal control participants were recruited from the Berkeley Aging Cohort Study, an ongoing longitudinal study of cognitive aging. Control participants underwent cognitive assessment via the Mini-Mental State Examination and were included in the study if they scored 25 or more, indicating normal cognitive function.
In addition to detailed neuropsychological assessment, all study participants underwent structural magnetic resonance imaging (MRI) and [18F]AV-1451 PET imaging. All participants with PD and 46 of the 49 control participants also underwent carbon 11–labeled Pittsburgh compound B ([11C]PiB) PET imaging. Control participants were included in the study based on their Aβ status, which was determined by PET scan. In addition, 3 control participants 60 years or younger did not receive these scans but were included in the Aβ-negative analysis group because cognitively healthy individuals are unlikely to have cortical Aβ pathology before age 60 years.
For this study, exclusion criteria of people with PD and control participants included the presence of a non-PD neurological disorder, major systemic illness, contraindications to MRI or PET, or history of a substance use disorder. In addition, 1 person with PD-MCI did not receive a magnetic resonance imaging (MRI) scan and thus was excluded from all analyses. Therefore 29 patients with PD were included in this study along with 49 control participants.
The study was approved by institutional review boards at all participating institutions. Written informed consent was obtained from all participants.
Magnetic Resonance Imaging
The research team acquired 1 × 1 × 1-mm-resolution T1-weighted magnetization prepared rapid gradient echo images for every participant on a 1.5-T MRI scanner at Lawrence Berkeley National Laboratory (using techniques described previously). These scans were processed with FreeSurfer version 5.3.0 (Massachusetts General Hospital) (http://surfer.nmr.mgh.harvard.edu/) to derive regions of interest (ROIs) in the native space of each participant using the Desikan-Killiany atlas, a feature of FreeSurfer software. FreeSurfer ROIs were used to calculate regional tau PET measures in the native space of each participant (after partial volume correction).
Magnetic resonance images were also segmented into tissue types using Statistical Parametric Mapping software, also known as SPM12 (Wellcome Trust Centre for Neuroimaging). The segments derived from SPM12 for noncerebral tissues were subsequently used for partial volume correction. Segments for gray and white matter were warped to Montreal Neurological Institute (MNI) 152 T1-weighted template at 2-mm isotropic resolution, and these were used to warp tau PET images into MNI space.
PET Imaging
A detailed description of the acquisition process for [11C]PiB PET for the detection of Aβ and [18F]AV-1451 PET for quantification of tau has been published previously. In this study, [11C]PiB and [18F]AV-1451 PET data were acquired at Lawrence Berkeley National Laboratory on a Siemens Biograph 6 PET and computed tomography scanner.
PET Imaging for Determination of β-Amyloid Status
Using a cerebellar gray matter reference region, we generated distribution volume ratios for [11C]PiB images with Logan graphical analysis on [11C]PiB frames corresponding to 35 minutes to 90 minutes postinjection. Global cortical [11C]PiB distribution volume ratios were calculated for all participants as weighted means across FreeSurfer-derived frontal, temporal, parietal, and posterior cingulate cortical regions.
PET Imaging for Measurement of Tau
To measure tau protein, we created [18F]AV-1451 standardized uptake volume ratio (SUVR) images based on the mean uptake over 80 to 100 minutes postinjection, normalized by the mean inferior cerebellar gray matter uptake. The SUVR images were coregistered and resliced into structural magnetic resonance images. To account for partial volume effects due to atrophy and signal spillover, we used the Geometric Transfer Matrix approach for partial volume correction based on FreeSurfer-derived ROIs, including corrections for extracerebral tissue as previously described. Voxelwise analyses used SUVR images (without partial volume correction) that were transformed into MNI-152 space and were smoothed prior to analysis using an 8-millimeter isotropic, full-width at half-maximum gaussian kernel.
We quantified [18F]AV-1451 uptake by grouping together ROIs that corresponded to the pathologic stages of tau protein tangle deposition in AD described by Braak and Braak. The regions grouped in each Braak stage ROI have been published previously. Specifically, we calculated weighted mean SUVR in native space (after partial volume correction) from 3 composite ROIs that roughly correspond to anatomical definitions of AD Braak stages 0 (an informal stage included for the purpose of this study in which no AD lesions are observed by PET scan), stage 1/2 (which show tau-related disease processes in the transentorhinal portion of the brain), stage 3/4 (which expands in which tau pathology is present to encompass the limbic system), and stage 5/6 (which displays the same processes in neocortical regions). Participants were then categorized into AD Braak stages according to thresholds that were based on Braak ROI-specific tracer uptake in different clinical groups, which were previously derived using conditional inference regression tree analysis. Participants with a mean SUVR of more than 1.129 met the classification for stage 1/2; those with a mean SUVR of more than 1.304 were judged to be at stage 3/4, and those whose mean SUVR was more than 1.873 were categorized as stage 5/6. Participants were classified at the highest stage for which they exceeded the cutoff.
Additionally, we compared the mean SUVRs of FreeSurfer-derived bilateral inferior temporal lobe and precuneus ROIs to investigate [18F]AV-1451 uptake differences, which have previously been reported in patients with severe PD and dementia with Lewy bodies. We also looked at [18F]AV-1451 uptake in a FreeSurfer-derived bilateral putamen ROI. The putamen has been reported as a site of high [18F]AV-1451 uptake, which is presumed to be off target; however, the pattern of uptake in putamen has been found to demonstrate elevation in people with other movement disorders, such as corticobasal degeneration.
Statistical Analysis
Voxelwise 2-sample t tests were performed in SPM12 to test for differences in [18F]AV-1451 uptake between groups with PD-CN and PD-MCI, as well as between both groups with PD and the [11C]PiB-negative normal control group. A whole-brain explicit mask was used to test only voxels within the intracranial space, and we also controlled for participant age in analysis. To examine [18F]AV-1451 uptake in the substantia nigra, which has been previously reported to differ in PD and controls, we used a bilateral ROI from an MNI-space version of the Talairach atlas to contrast [18F]AV-1451 uptake between patients with PD and control participants who were Aβ-negative as measured by [11C]PiB PET scan.
Differences between groups in native-space Braak-stage ROIs and MNI-space substantia nigra ROIs were assessed using nonparametric Kruskal-Wallis tests, followed by post hoc Mann-Whitney U groupwise comparisons. Nonparametric tests were applied because of small group sizes and because they do not require the data to be normally distributed. Age and education associations were examined with Pearson correlation coefficients.
Results
Participant characteristics are summarized in the Table. The mean age did not differ between PD-CN and PD-MCI patient groups, but the mean (SD) age of the Aβ-negative control group (71.7 [6.7] years) and the Aβ-positive control group (75.9 [3.0] years) were significantly higher than the groups of patients with PD-CN (66.6 [6.6] years) and PD-MCI (66.5 [4.9] years). People with PD-CN had significantly higher mean (SD) years of education than people with PD-MCI and Aβ-positive controls (17.9 [1.9] years vs 16.1 [2.0] years vs 15.8 [1.8] years, respectively; P < .01), and Aβ-negative controls had significantly higher mean (SD) education levels than Aβ-positive controls (17.2 [1.9] years vs 15.8 [1.8] years, respectively; P = .01). Mean years of education were not related to mean [18F]AV-1451 binding in any of the AD Braak stage ROIs across all participant groups.
Table. Subject Demographics, β-Amyloid Status, and Tau Protein.
| Characteristic | Participant Subgroup | P Value | |||
|---|---|---|---|---|---|
| Participants With Parkinson Disease–Cognitively Normal | Participants With Parkinson Disease–Mildly Cognitively Impaired | β-Amyloid–Negative Control Participants |
β-Amyloid–Positive Control Participants | ||
| Total | 15 | 14 | 25 | 24 | NA |
| Age, mean (SD), y | 66.6 (6.6) | 66.5 (4.9) | 71.7 (6.7) | 75.9 (3.0) | < .001a |
| Female, No. (%) | 8 (53) | 4 (29) | 14 (56) | 16 (67) | NA |
| Education, mean (SD), y | 17.9 (1.9) | 16.1 (2.0) | 17.2 (1.9) | 15.8 (1.8) | .01b |
| Cognitive assessments, mean (SD)c | 28.3 (1.3) | 27.2 (2.3) | 29.0 (1.0) | 28.9 (1.1) | .14d |
| UPDRS Motor subscores, mean (SD) | 24.9 (12.7) | 28.6 (10.9) | NA | NA | .40e |
| AD Braak staging 0, 1/2, 3/4, 5/6 | 5,8,2,0 | 6,8,0,0 | 9,16,0,0 | 4,14,6,0 | NA |
| β-Amyloid-positivef | 5 | 1 | 0 | 24 | NA |
Abbreviations: AD, Alzheimer disease; NA, not applicable; UPDRS, Unified Parkinson’s Disease Rating Scale.
P value compares all patients with Parkinson disease with all controls.
P value compares β-amyloid–positive control participants with all patients with Parkinson disease and β-amyloid–negative control participants.
Cognitive assessments were via the Montreal Cognitive Assessment Scale for patients with Parkinson disease and via the Mini-Mental State Examination for control participants.
P value compares patients with Parkinson disease who were cognitively normal with patients with Parkinson disease who were mildly cognitively impaired.
P value compares β-amyloid–positive control participants with all patients with Parkinson disease plus β-amyloid–negative control participants.
Three normal control participants 60 years or younger did not receive positron emission tomography scans to determine β-amyloid status, but were included in β-amyloid–negative group.
Clinical Characterization of Cognition in Patients With PD-MCI
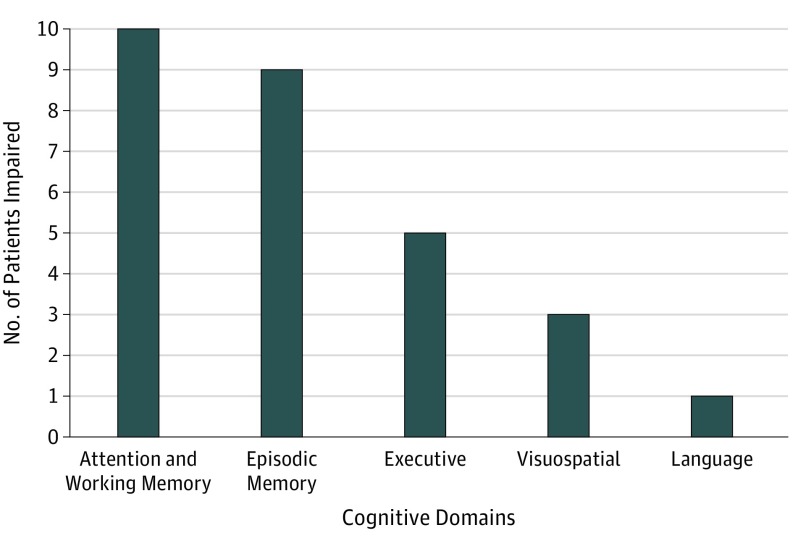
Unified Parkinson’s Disease Rating Scale scores of motor impairment and performance on the Montreal Cognitive Assessment did not differ between patients with PD-CN and patients with PD-MCI (mean [SD], 24.9 [12.7] vs 28.6 [10.97]; P = .40; Table). Impairment was defined as performance at or below 1 SD from age-matched and sex-matched norms on 2 or more neuropsychological tests. Of 14 patients with PD-MCI, 2 were impaired in a single cognitive domain (14%), 9 were impaired in 2 domains (64%), and 3 were impaired in 3 domains (21%). The distribution of impaired cognitive domains is illustrated in Figure 1. The 2 most common impairments were in attention and working memory and in episodic memory.
Figure 1. Characterization of Cognitive Impairment in Patients With Parkinson Disease With Mild Cognitive Impairment (PD-MCI).
Patients with PD-MCI were characterized as performing at 1 to 2 SD below published age-matched and sex-matched norms on 2 or more neuropsychological tests. The bar plot shows the number of patients with PD-MCI who were impaired in each cognitive domain; 2 patients were impaired in a single domain, 9 across 2 domains, and 3 across 3 domains.
β-Amyloid Burden
Of the 29 patients with PD, 6 (21%) were Aβ-positive (Table). One Aβ-positive patient met criteria for PD-MCI, and 5 were cognitively normal. Of the Aβ-negative patients with PD, 13 met criteria for PD-MCI and 10 were cognitively normal.
Of the 46 control participants who received [11C]PiB scans, 22 were found to be Aβ-negative and 24 Aβ-positive based on a threshold of a [11C]PiB distribution volume ratio of 1.065. (An additional 3 control participants were included in the Aβ-negative subgroup, as noted.)
Tau Burden and Distribution
MNI–Space Tau Analyses
Voxelwise [18F]AV-1451 SUVR comparisons between patients with PD-CN and patients with PD-MCI, and additionally between all patients with PD and Aβ-negative normal controls, revealed no significant clusters after controlling for age (P > .05 after family-wise error correction to reduce type I error). The mean [18F]AV-1451 uptake in bilateral substantia nigra template-space ROI was significantly lower in patients with PD than in Aβ-negative controls (in PD: SUVR mean [SD], 1.30 [0.10]; in controls, 1.35 [0.09]; P = .03 by Mann-Whitney U test). Motor impairment as measured by Unified Parkinson Disease Rating Scale was not related to [18F]AV-1451 binding in the substantia nigra (r = −0.22; P = .24) or global cognition as measured by the Montreal Cognitive Assessment (r = −0.04; P = .85).
Native-Space Tau Analyses
Of 29 patients with PD, most were categorized as being in AD Braak stage 0 or 1/2, with only 2 in stage 3/4 (Table). The 25 Aβ-negative control participants were all categorized in stages 0 or 1/2, while 6 of the 24 Aβ-positive control participants were categorized in stage 3/4 and the remaining 18 Aβ-positive control participants were categorized in stages 0 or 1/2. A χ2 test of Braak staging distributions showed no significant differences across diagnostic groups (Pearson χ2 = 11.89; P = .06), with the Aβ-positive control group showing a higher proportion of participants in Braak 3/4 stage relative to the other groups.
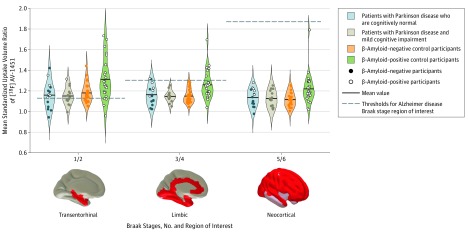
After partial volume correction, the mean [18F]AV-1451 SUVR differed significantly across groups for AD Braak stage ROIs; control participants who were Aβ-positive exhibited more tau tracer binding across each AD Braak-stage ROI than groups with PD-CN, PD-MCI, and control participants who were Aβ-negative. In specific, the mean (SD) SUVRs of the Aβ-positive control participants differed significantly from those of Aβ-negative control participants, people with PD-MCI, and people with PD-CN with respect to ROIs for AD Braak stage 1/2 (1.31 [0.20] vs 1.17 [0.10] vs 1.15 [0.09] vs 1.16 [0.13], respectively), Braak stage 3/4 (1.26 [0.14] vs 1.15 [0.07] vs 1.15 [0.05] vs 1.16 [0.10], respectively), and Braak stage 5/6 (1.22 [0.15] vs 1.12 [0.07] vs 1.13 [0.08] vs 1.14 [0.08], respectively) (for all comparisons, P < .05; further information is shown in Figure 2). The mean [18F]AV-1451 SUVR did not differ significantly between patients with PD-CN and those with PD-MCI in any of the AD Braak-stage ROIs; additionally, tracer binding in these ROIs did not differ significantly between both subgroups with PD and the Aβ-negative controls.
Figure 2. Standardized Uptake Volume Ratios of Fluorine 18–Labeled AV-1451 in Patients With Parkinson Disease and Control Participants, Stratified by Alzheimer Disease Braak Stage.
Violin plots show [18F]AV-1451 positron emission tomography mean standardized uptake volume ratios in regions of interest derived from Alzheimer disease Braak stages. In each region of interest, significant differences were found between β-amyloid–positive controls and β-amyloid–negative controls, β-amyloid–positive controls and patients with Parkinson disease with mild cognitive impairment, and β-amyloid–positive controls and patients with Parkinson disease who were cognitively normal.
The mean [18F]AV-1451 SUVR differed after partial volume correction across groups in the inferior temporal lobe ROI and the precuneus ROI (inferior temporal lobe, P < .001; precuneus, P = .30). Differences in bilateral inferior temporal lobe and precuneus ROIs were not significant between patients with PD-CN, patients with PD-MCI, and Aβ-negative control participants. Control participants who were Aβ-positive had significantly elevated inferior temporal lobe binding values (mean [SD], 1.38 [0.20]) relative to Aβ-negative controls, patients with PD-MCI, and patients with PD-CN (1.23 [0.10] vs 1.25 [0.07] vs 1.26 [0.11], respectively; P < .05 for all comparisons). Significantly elevated precuneus tau binding was also seen in the mean (SD) values of Aβ-positive control participants (1.23 [0.15]) relative to those of Aβ-negative control participants (1.13 [0.08]) and people with PD-CN (1.14 [0.11]) (for each comparison, P < .05; Figure 2), but not those of people with PD-MCI (1.16 [0.10]).
Uncorrected ROI mean analyses showed a similar pattern to partially volume-corrected ROI means. However, the observed elevated mean (SD) [18F]AV-1451 binding in Aβ-positive controls was only significant in AD Braak stage 1/2 (Aβ-positive controls, 1.24 [0.13]; Aβ-negative controls, 1.16 [0.07]; people with PD-MCI, 1.13 [0.07]; people with PD-CN, 1.15 [0.10]; P < .05 for each pairwise comparison).
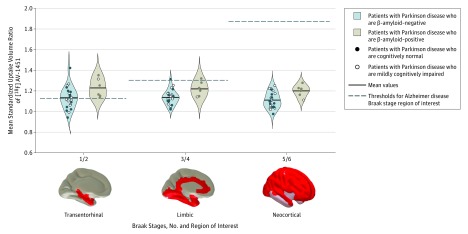
The mean (SD) [18F]AV-1451 SUVR was significantly elevated in Aβ-positive patients with PD compared with Aβ-negative participants with PD in AD Braak stages 3/4 (1.22 [0.07] vs 1.14 [0.07]; P = .03) and stage 5/6 (1.20 [0.07] vs 1.11 [0.08]; P = .02) (Figure 3), with no significant difference in stage 1/2. The binding of [18F]AV-1451 in patients with PD who were Aβ-positive was not significantly different from that of Aβ-positive control participants, while binding in patients with PD who were Aβ-negative was not significantly different from that of Aβ-negative control participants at any all AD Braak-stage ROI.
Figure 3. Standardized Uptake Volume Ratios of Fluorine 18–Labeled AV-1451 ([18F]AV-1451) in Patients With β-Amyloid–Positive and β-Amyloid–Negative Patients With Parkinson Disease, Stratified by Alzheimer Disease Braak Stage.
Violin plots show [18F]AV-1451 positron emission tomography mean standardized uptake volume ratios in regions of interest derived from Alzheimer disease Braak stages. Significant differences were found between the β-amyloid–positive and β-amyloid–negative groups in AD Braak stages 3/4 and 5/6 (P < .05) but not stage 1/2.
Tau, β-Amyloid, and Age
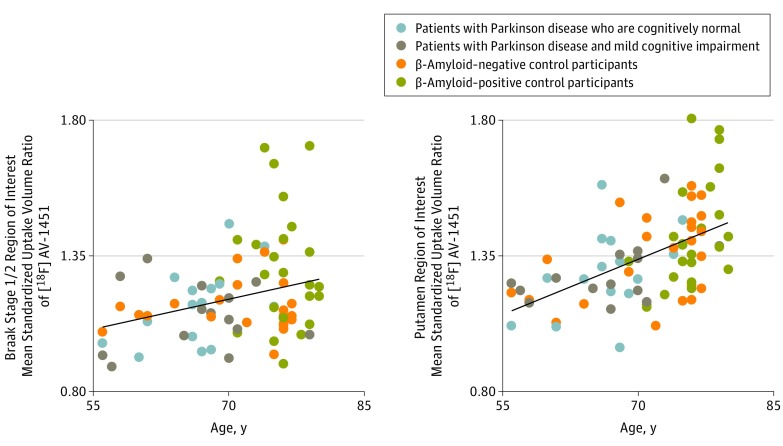
Across patients with PD and control participants, the mean [18F]AV-1451 binding was correlated with age (by AD Braak-stage ROIs: stage 1/2, r = 0.35, P = .002; stage 3/4, r = 0.33, P = .003; stage 5/6, r = 0.28, P = .01) (Figure 4). Tracer binding in the bilateral putamen, believed to reflect off-target tracer binding, also correlated with age (Figure 4; r = 0.52, P < .001).
Figure 4. Age and Regional Fluorine 18–Labeled AV-1451 ([18F]AV-1451) Binding.
Scatterplots show correlations between age and [18F]AV1451 positron emission tomography mean standardized uptake volume ratios in regions of interest derived from Alzheimer disease Braak stage 1/2 (left, R2 = 0.12; P = .002) and the bilateral putamen region of interest (right, R2 = 0.27; P < .001).
Discussion
To our knowledge, this is the first study to use PET scans to examine Aβ and tau in relationship to cognitive status in patients with PD but not dementia, and the first finding that tau binding observed via PET scan is related to Aβ status in patients with PD without dementia. We found that patterns of tau and Aβ did not differ between patients with PD-MCI and those with PD-CN, and that the binding of tau PET tracer in patients with PD did not differ from Aβ-negative healthy controls. Furthermore, Aβ status in patients with PD was not related to cognitive status, and the proportion of patients with PD in this study who were Aβ-positive (21%) was comparable with healthy controls of the same age range. Additionally, we found that tau observable via PET scan had similar associations with age and Aβ status in patients with PD as in healthy control participants.
This study replicates a report that patients with PD-MCI and patients with PD who were cognitively normal do not show significant differences in levels of brain tau. Previous work with tau observed via PET scan has shown elevated tracer binding in the brains of patients with PD-MCI relative to healthy controls, specifically in the precuneus and inferior temporal gyrus. We did not find elevated tau in these brain regions in the present cohort of patients with PD-MCI. It is likely that the contrast in tau between cognitively impaired and patients with PD who were cognitively normal (as reported in Gomperts et al) was driven by increases observed in patients with PD who had dementia, who were not included in this study. Cognitive status was not related to Aβ status in the present cohort of patients with PD, which was unsurprising given the previous finding that, while [11C]PiB-indexed Aβ burden may contribute to longitudinal cognitive decline, it does not distinguish between patients with PD-MCI and patients with PD who were cognitively normal at baseline. Our analyses also replicated a finding by Hansen et al demonstrating the lower mean binding of [18F]AV-1451 in the substantia nigra in patients with PD relative to a control group. This contrast has been suggested to reflect off-target tracer binding to neuromelanin-containing dopaminergic neurons in the substantia nigra; progressive loss of nigral neurons may be related to disease progression in PD. In this study, we did not find an association between [18F]AV-1451 binding in the substantia nigra and motor impairment or global cognition.
While α-synuclein pathology is considered the hallmark feature of PD, post-mortem studies of people with PD-MCI suggest that the pathological processes of AD and cerebrovascular factors additionally contribute to cognitive decline. The onset and severity of dementia in Lewy body diseases is also associated with pathologies beyond changes in α-synuclein, and work in animal and cellular models has suggested a synergistic relationship between changes in α-synuclein and AD processes that accelerates cognitive decline. Providing further evidence for these multipathology interactions are postmortem findings that pathological tau colocalizes to α-synuclein in Lewy bodies, particularly in brain regions known to be vulnerable to both Lewy bodies and neurofibrillary tangle disease processes.
Current tools do not allow us to measure the distribution of unhealthy α-synuclein protein in the bodies of living patients. However, our findings suggest that any presence of α-synuclein in the patients with PD included in this study has not influenced the spread of tau protein, as detectable by [18F]AV-1451 PET scan. While this result may be surprising given the postmortem and animal evidence supporting synergy and colocalization of α-synuclein and tau, it may be that these relationships are not detectable until patients express symptoms of dementia.
Abnormal developments of tau and Aβ did not explain cognitive status in this cohort of patients with PD. While abnormalities in α-synuclein and a decline in dopaminergic functioning are thought to be the main contributors to disease severity in PD, the search for in vivo physiological markers of cognitive impairment in PD has not been straightforward. Studies of Aβ PET scans, cerebrospinal fluid markers, gray matter volume, white matter integrity, and loss of dopaminergic neurons have not yielded consistent results associating biomarkers with cognitive status, although a PET study using [18F]fluorodeoxyglucose demonstrated abnormal metabolic network activity in patients with PD-MCI relative to patients with PD who were cognitively normal. The lack of a relationship between cognitive impairment and AD-related PET biomarkers in PD highlights the need for an α-synuclein ligand for PET scanning, which would be a crucial tool for understanding the interaction between pathology and symptoms in PD.
Importantly, mood and lifestyle factors may additionally contribute to cognitive status, as suggested by reports of increased depressive symptoms in cognitively impaired patients as well as evidence that sleep may mediate cognitive symptoms of PD. Thus, health care professionals and/or family caregivers may wish to address mood and lifestyle factors in cognitively impaired patients, and further research should determine whether lifestyle factor interventions can improve cognitive function in these patients.
Limitations
Limitations of the present study include a small sample size. A sample that included more patients with PD-MCI would allow for contrasts between different domains of cognitive impairment. The nonspecificity of cognitive impairment as defined by current PD-MCI criteria may explain the lack of uniform patterns of AD across patients. It is also important to note that [18F]AV-1451 does not bind to all forms of pathological tau protein, but preferentially binds to paired-helical filament tau, allowing for the possibility that other forms of abnormal tau accumulation are not measured by this tracer and therefore not represented in this study.
Conclusions
The results of the present study suggest that deposits of tau in patients with PD without dementia are related to age and Aβ-status. Positron emission tomography scans to measure Aβ and tau did not distinguish patients with PD who were cognitively normal from patients with PD-MCI. The observed similarities between Aβ and tau PET binding in patients with PD and healthy control participants demonstrate that the pathological processes of AD are not exacerbated in people with PD without dementia.
References
- 1.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11(8):697-707. [DOI] [PubMed] [Google Scholar]
- 2.Adler CH, Caviness JN, Sabbagh MN, et al. . Heterogeneous neuropathological findings in Parkinson’s disease with mild cognitive impairment. Acta Neuropathol. 2010;120(6):827-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jellinger K. Heterogenous mechanisms of mild cognitive impairment in Parkinson’s disease. J Neural Transm (Vienna). 2012;119(3):381-382. [DOI] [PubMed] [Google Scholar]
- 4.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic interactions between aβ, tau, and α-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30(21):7281-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin DJ, Lee VM-Y, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14(9):626-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waxman EA, Giasson BI. Induction of intracellular tau aggregation is promoted by α-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J Neurosci. 2011;31(21):7604-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62(4):389-397. [DOI] [PubMed] [Google Scholar]
- 8.Gomperts SN, Locascio JJ, Makaretz SJ, et al. . Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol. 2016;73(11):1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarci K, Lowe VJ, Boeve BF, et al. . AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol. 2017;81(1):58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AK, Damholdt MF, Fedorova TD, et al. . In vivo cortical tau in Parkinson’s disease using 18F-AV-1451 positron emission tomography. Mov Disord. 2017;32(6):922-927. [DOI] [PubMed] [Google Scholar]
- 11.Brier MR, Gordon B, Friedrichsen K, et al. . Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8(338):338ra366-338ra366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KA, Schultz A, Betensky RA, et al. . Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schöll M, Lockhart SN, Schonhaut DR, et al. . PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomperts SN, Locascio JJ, Rentz D, et al. . Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80(1):85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litvan I, Goldman JG, Tröster AI, et al. . Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz CG, Tilley BC, Shaftman SR, et al. ; Movement Disorder Society UPDRS Revision Task Force . Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”—A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351-357. [DOI] [PubMed] [Google Scholar]
- 20.Ossenkoppele R, Schonhaut DR, Schöll M, et al. . Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(Pt 5):1551-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maass A, Landau S, Baker SL, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834-840. [DOI] [PubMed] [Google Scholar]
- 23.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358-368. [DOI] [PubMed] [Google Scholar]
- 24.Baker SL, Lockhart SN, Price JC, et al. . Reference tissue-based kinetic evaluation of 18f-av-1451 for tau imaging. J Nucl Med. 2017;58(2):332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39(5):904-911. [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. [DOI] [PubMed] [Google Scholar]
- 27.Vemuri P, Lowe VJ, Knopman DS, et al. . Tau-PET uptake: regional variation in average SUVR and impact of amyloid deposition. Alzheimers Dement (Amst). 2016;6:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josephs KA, Whitwell JL, Tacik P, et al. . [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 2016;132(6):931-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen AK, Knudsen K, Lillethorup TP, et al. . In vivo imaging of neuromelanin in Parkinson’s disease using 18F-AV-1451 PET. Brain. 2016;139(pt 7):2039-2049. [DOI] [PubMed] [Google Scholar]
- 30.Lancaster JL, Woldorff MG, Parsons LM, et al. . Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mormino EC, Brandel MG, Madison CM, et al. . Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59(2):1152-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, et al. . Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138(Pt 7):2020-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe VJ, Curran G, Fang P, et al. . An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm (Vienna). 2002;109(3):329-339. [DOI] [PubMed] [Google Scholar]
- 36.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115(4):427-436. [DOI] [PubMed] [Google Scholar]
- 37.Hurtig HI, Trojanowski JQ, Galvin J, et al. . Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54(10):1916-1921. [DOI] [PubMed] [Google Scholar]
- 38.Mattila PM, Rinne JO, Helenius H, Dickson DW, Röyttä M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000;100(3):285-290. [DOI] [PubMed] [Google Scholar]
- 39.Ito K, Nagano-Saito A, Kato T, et al. . Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: a 6-[18F]fluoro-L-dopa PET study. Brain. 2002;125(Pt 6):1358-1365. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Cholerton B, Shi M, et al. ; Parkinson Study Group DATATOP Investigators . CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(3):271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMillan CT, Wolk DA. Presence of cerebral amyloid modulates phenotype and pattern of neurodegeneration in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87(10):1112-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melzer TR, Watts R, MacAskill MR, et al. . Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83(2):188-194. [DOI] [PubMed] [Google Scholar]
- 43.Yarnall AJ, Breen DP, Duncan GW, et al. ; ICICLE-PD Study Group . Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology. 2014;82(4):308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan GW, Firbank MJ, Yarnall AJ, et al. . Gray and white matter imaging: a biomarker for cognitive impairment in early Parkinson’s disease? Mov Disord. 2016;31(1):103-110. [DOI] [PubMed] [Google Scholar]
- 45.Song I-U, Kim Y-D, Cho H-J, Chung S-W, Chung Y-A. An FP-CIT PET comparison of the differences in dopaminergic neuronal loss between idiopathic Parkinson disease with dementia and without dementia. Alzheimer Dis Assoc Disord. 2013;27(1):51-55. [DOI] [PubMed] [Google Scholar]
- 46.Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70(16, pt 2):1470-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pushpanathan ME, Loftus AM, Thomas MG, Gasson N, Bucks RS. The relationship between sleep and cognition in Parkinson’s disease: A meta-analysis. Sleep Med Rev. 2016;26:21-32. [DOI] [PubMed] [Google Scholar]
- 48.Scullin MK, Trotti LM, Wilson AG, Greer SA, Bliwise DL. Nocturnal sleep enhances working memory training in Parkinson’s disease but not Lewy body dementia. Brain. 2012;135(pt 9):2789-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marquié M, Normandin MD, Vanderburg CR, et al. . Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787-800. [DOI] [PMC free article] [PubMed] [Google Scholar]