Key Points
Question
Is 12 months of transdermal estradiol and intermittent micronized progesterone more effective than placebo in preventing the development of depressive symptoms in the menopause transition and early postmenopausal period?
Findings
In this randomized clinical trial that included 172 perimenopausal and early postmenopausal women, 32.3% of women receiving placebo developed clinically significant depressive symptoms, while 17.3% of women taking transdermal estradiol and intermittent micronized progesterone did so.
Meaning
If confirmed in future research, clinicians may consider prescribing hormone therapy to mitigate the increased risk of clinically significant depressive symptoms that accompany the menopause transition and early postmenopausal period.
Abstract
Importance
The menopause transition and early postmenopausal period are associated with a 2- to 4-fold increased risk for clinically significant depressive symptoms. Although a few studies suggest that hormone therapy can effectively manage existing depression during this time, to our knowledge, there have been no studies testing whether hormone therapy can prevent the onset of perimenopausal and early postmenopausal depressive symptoms.
Objective
To examine the efficacy of transdermal estradiol plus intermittent micronized progesterone (TE+IMP) in preventing depressive symptom onset among initially euthymic perimenopausal and early postmenopausal women. A secondary aim was to identify baseline characteristics predicting TE+IMP’s beneficial mood effects.
Design, Setting, and Participants
Double-blind, placebo-controlled randomized trial at the University of North Carolina at Chapel Hill from October 2010 to February 2016. Participants included euthymic perimenopausal and early postmenopausal women from the community, aged 45 to 60 years.
Interventions
Transdermal estradiol (0.1 mg/d) or transdermal placebo for 12 months. Oral micronized progesterone (200 mg/d for 12 days) was also given every 3 months to women receiving active TE, and identical placebo pills were given to women receiving placebo.
Main Outcome Measures
Scores on the Center for Epidemiological Studies–Depression Scale (CES-D), assessed at baseline and months 1, 2, 4, 6, 8, 10, and 12 after randomization, and the incidence of clinically significant depressive symptoms, defined as a CES-D score of at least 16.
Results
Of 172 participants, 130 were white (76%), and 70 were African American (19%), with a mean household income of $50 000 to $79 999. The mean age was 51 years, and 43 developed clinically significant depressive symptoms. Women assigned to placebo were more likely than those assigned to TE+IMP to score at least 16 on the CES-D at least once during the intervention phase (32.3% vs 17.3%; odds ratio [OR], 2.5; 95% CI, 1.1-5.7; P = .03) and had a higher mean CES-D score across the intervention period (P = .03). Baseline reproductive stage moderated the effect of treatment (β, −1.97; SEM, 0.80; P for the interaction = .03) such that mood benefits of TE+IMP vs placebo were evident among women in the early menopause transition (β, −4.2; SEM, 1.2; P < .001) but not the late menopause transition (β, −0.9; SEM, 0.3; P = .23) or among postmenopausal women (β, −0.3; SEM, 1.1; P = .92). Stressful life events in the 6 months preceding enrollment also moderated the effect of treatment on mean CES-D score such that the mood benefits of TE+IMP increased with a greater number of events (β, 1.22; SEM, 0.40; P = .003). Baseline estradiol levels, baseline vasomotor symptoms, history of depression, and history of abuse did not moderate treatment effects.
Conclusions
Twelve months of TE+IMP were more effective than placebo in preventing the development of clinically significant depressive symptoms among initially euthymic perimenopausal and early postmenopausal women.
Trial Registration
clinicaltrials.gov Identifier: NCT01308814
This randomized clinical trial examines the efficacy of transdermal estradiol plus intermittent micronized progesterone in preventing depressive symptom onset among initially euthymic perimenopausal and early postmenopausal women.
Introduction
Depression risk is known to increase among women in the menopause transition and early postmenopausal period, with rates of major depressive disorder and clinical elevations in depressive symptoms roughly doubling to tripling compared with premenopausal and late postmenopausal rates. Increased sensitivity to the extreme estradiol fluctuation that characterizes the menopause transition has been implicated. Although a few trials suggest that estrogen therapy, with or without progesterone, which might act to minimize estradiol fluctuation and/or withdrawal, is an effective treatment for perimenopausal depression, to our knowledge, this is the first study to examine the efficacy of hormone therapy, in this case, transdermal estradiol plus intermittent micronized progesterone (TE+IMP), in preventing the development of depressive symptoms in initially euthymic perimenopausal and early postmenopausal women.
This study further sought to identify baseline characteristics predicting TE+IMP’s effect on mood. Several factors known to be associated with an increased vulnerability to perimenopausal depression and/or other reproductive mood disorders, including recent life stress, a positive history of depression, a history of physical or sexual abuse, and more severe vasomotor symptoms, were therefore considered as potential moderators of TE+IMP’s effects. Indicators of the preintervention hormonal environment, including menopausal stage, were also considered.
Methods
Participants
Between October 2010 and January 2015, 172 women aged 45 to 60 years, medically healthy and perimenopausal or early postmenopausal according to the Stages of Reproductive Aging Workshop criteria, were self-referred in response to advertisements posted throughout the community and on social media. The final participant completed the trial in February 2016. Data were collected at the University of North Carolina at Chapel Hill. Women were euthymic on study enrollment according to the Structured Clinical Interview for DSM-IV. The trial protocol was approved by the University of North Carolina’s institutional review board. All participants provided informed, written consent prior to participating and received up to $1425 in compensation for participating in full compliance.
Trial Design
The Perimenopausal Estrogen Replacement Therapy study was designed to examine the mood and cardiovascular benefits of TE+IMP among perimenopausal and early postmenopausal women and to investigate several mechanisms underlying estradiol’s effects. In this article, only TE+IMP’s effects on the study’s primary mood outcome, depressive symptoms, are reported. The Perimenopausal Estrogen Replacement Therapy study used a randomized, double-blind, placebo-controlled design in which 172 women were enrolled and randomly assigned to treatment with either patches of 0.1 mg of 17β-estradiol or placebo patches (developed by 3M pharmaceuticals) for 12 months. Oral micronized progesterone (200 mg/d for 12 days) was also given every 2 to 3 months to women receiving active TE+IMP to protect the endometrium, and an identical schedule of placebo pills was implemented for women receiving the placebo. Postrandomization study visits occurred at months 1, 2, 4, 6, 8, 10, and 12. To allow for the examination of the effects of estradiol without the confound of progesterone, no visits occurred while women were taking progesterone. The trial protocols can be found in Supplement 1 and additional details regarding the trial design can be found in the eMethods of Supplement 2.
Measures
Depressive symptoms were assessed at each study visit using the Center for Epidemiologic Studies–Depression Scale (CES-D). Vasomotor symptom bother was measured at each visit using the Vasomotor Subscale of the Greene Climacteric Scale. Baseline estradiol levels were measured, in serum, at the study enrollment session. The assay procedure is described elsewhere. For women with an intact uterus, reproductive stage, assessed at enrollment, was defined as follows: early perimenopause, defined as menstrual cycle length at least 7 days longer than usual; late perimenopause, defined as at least 2 skipped cycles and an interval of amenorrhea of at least 60 days but within 1 year of the last menstrual period; and early postmenopause, defined as an interval of amenorrhea between 1 and 2 years. This classification scheme is very similar to the Stages of Reproductive Aging Workshop criteria. A history of a major depressive episode was identified using the Structured Clinical Interview for DSM-IV at the study enrollment session. Stressful life events during the 6 months before the baseline assessment were measured using the Life Events Survey interview, modified to include only those events that are considered moderate to severely stressful based on previous studies with interviewer-based objectively rated stresses. Experiences of sexual and physical abuse were assessed using a validated interview developed by Leserman et al. Criteria for establishing sexual or physical abuse are described elsewhere.
Statistical Analysis
Effect of Treatment
Unless otherwise specified, an intent-to-treat analysis was performed. First, we tested the main effect of treatment (placebo vs TE+IMP) on the risk of developing clinically significant depressive symptoms (CES-D score ≥16), the primary outcome. A score of 16 or greater on the CES-D is commonly used as a cutoff for identifying potential clinical depression and is associated with major depression. Second, we tested the moderating effect of several baseline characteristics on the effect of treatment: reproductive stage at enrollment, baseline estradiol levels, baseline vasomotor symptom bother, history of a major depressive episode, stressful life events, and history of physical or sexual abuse. Estradiol levels, vasomotor symptom bother, and stressful life events were treated as continuous variables; baseline estradiol level was standardized to have a mean of 0 and a standard deviation of 1.
All analyses were conducted using SAS, version 9.2 (SAS Institute Inc). Logistic regression was used to examine the effect of treatment (and potential moderation by baseline characteristics) on the risk of developing a CES-D score of at least 16. Poisson regression using PROC GENMOD examined the effect of treatment and potential moderation of treatment on the number of visits during which a CES-D score of at least 16 was obtained (0 to 7). To test for potential moderating effects, the variable of interest and a treatment-by-variable of interest interaction term were added as predictors in the analyses described here. To ensure that any mood benefits observed were not owing to a reduction in vasomotor symptoms, mean change in vasomotor symptom bother averaged across the entire study was included as a covariate, as was prerandomization enrollment CES-D score.
Secondary analyses examined the effect of treatment (placebo vs TE+IMP) on continuous CES-D scores, a repeated-measures regression analysis using PROC MIXED (for mixed models) with 8 repeated measures (prerandomization [denoted visit −1] and months 1, 2, 4, 6, 8, 10, and 12). Change in vasomotor symptom bother relative to enrollment levels and prerandomization enrollment CES-D score were included as covariates. A first-order autoregressive covariance structure was specified for within-person error. The Kenward-Roger method was used for computing degrees of freedom for tests of fixed effects. For significant interaction effects, simple effects of treatment condition on the least squares means of the outcome variable at key levels of the moderating variable were examined. Sensitivity analyses were used to identify extreme outliers, defined as values 3 or more interquartile ranges lower than the first quartile or greater than the third quartile (SAS Institute Inc, 2011). The effect of treatment on CES-D score was reexamined with these outliers removed. Because PROC MIXED does not delete missing data listwise, all available data were used. A 2-sided value of P less than .05 was considered statistically significant.
Results
Participant Characteristics
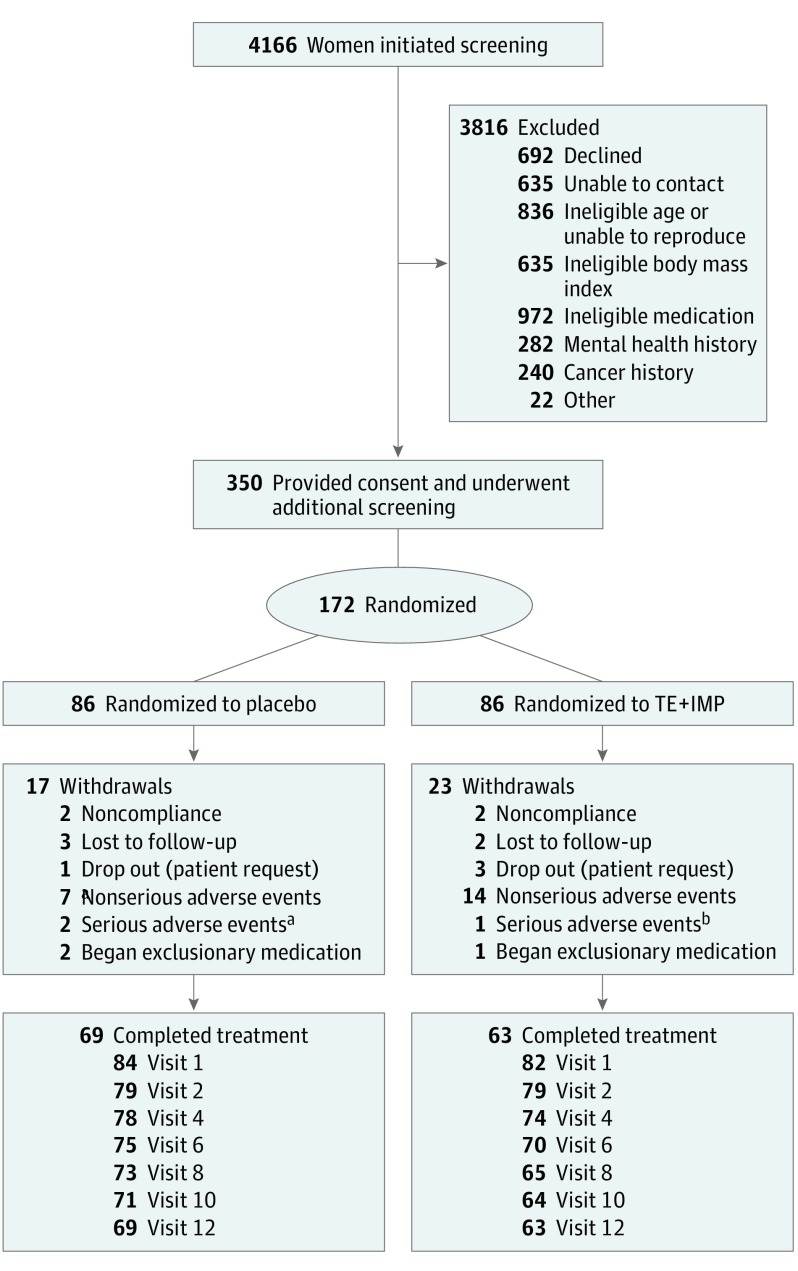
One hundred seventy-two women entered the trial (Figure 1). As seen in Table 1, women randomized to TE+IMP and placebo did not significantly differ in terms of any baseline demographic or psychosocial variables. By visit 12, 17% of women assigned to placebo were early perimenopausal (n = 12 of 69), 49% were late perimenopausal (n = 34), and 32% were early postmenopausal (n = 22). Twenty-five percent of participants (n = 43 of 172) obtained a score of at least 16 at least once during the course of the study. The mean (SD) CES-D score among visits in which a score of at least 16 was obtained was 22.0 (6.5). Additional details regarding baseline participant characteristics and adherence can be found in the eResults of Supplement 2.
Figure 1. Flow of Participants Through the Study.
Seventy-six percent of participants attended all 7 postrandomization study visits, and 85% attended at least 4 visits. The number of visits attended in the placebo and transdermal estradiol plus intermittent micronized progesterone (TE+IMP) groups (M [SD], 6.3 [1.7] vs 6.0 [1.9], respectively) did not significantly differ (P = .25).
aTwo cases of major depressive disorder.
bAn acute deep vein thrombosis.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Placebo (n = 86) | TE+IMP (n = 86) | P Value | |
| Age (SD), y | 51.0 (3.2) | 51.0 (3.0) | .71 |
| Race/ethnicity | |||
| White | 60 (70) | 70 (81) | .20 |
| African American | 20 (23) | 70 (81) | |
| Other | 6 (7) | 3 (4) | |
| Educationa, mean (SD) | 7.0 (1.1) | 6.8 (1.1) | .40 |
| Incomeb, mean (SD) | 9.0 (3.1) | 8.7 (3.3) | .52 |
| Reproductive stage | |||
| Early perimenopausal | 18 (21) | 18 (21) | .79 |
| Late perimenopausal | 48 (56) | 51 (59) | |
| Early postmenopausal | 20 (23) | 17 (20) | |
| Plasma estradiol, pg/mL | 90.1 (55.2) | 104.7 (102.2) | .56 |
| BMI, mean (SD) | 25.8 (3.7) | 25.5 (3.6) | .62 |
| Current smoker | 6 (7) | 4 (5) | .70 |
| History of MDD | 28 (33) | 28 (33) | .87 |
| Any physical or sexual abuse | 29 (34) | 29 (34) | >.99 |
| Prestudy CES-D score, mean (SD) | 5.5 (4.9) | 5.3 (5.1) | .92 |
| Prestudy Greene Climacteric Scale, mean (SD) | |||
| Psychological subscale | 4.1 (3.4) | 4.0 (3.5) | .93 |
| Vasomotor subscale | 2.2 (1.6) | 2.0 (1.8) | .58 |
| Somatic subscale | 1.4 (1.6) | 1.3 (1.7) | .98 |
| Total stressful life events | |||
| 0 | 34 (40) | 36 (42) | .64 |
| 1 | 25 (29) | 21 (24) | |
| 2 | 27 (31) | 29 (34) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiological Studies–Depression Scale; MDD, major depressive disorder; TE+IMP, transdermal estradiol plus intermittent micronized progesterone.
SI conversion factor: To convert estradiol to picomoles per liter, multiply by 3.671.
6 = some college (including completion of junior college); 7 = graduated from 4-year college.
8 = $50 000 to $79 999; 9 = $80 000 to $99 999.
Main Effect of Treatment
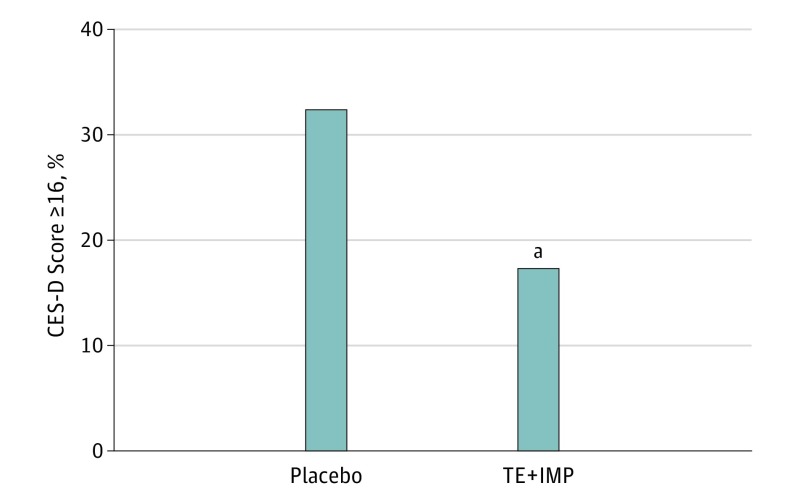
Women assigned to placebo were more likely than those assigned to TE+IMP to score at least 16 on the CES-D at least once during the intervention phase (OR , 2.5; 95% CI, 1.1-5.7; P = .03; Figure 2) and scored at least 16 on the CES-D during more visits compared with those assigned to TE+IMP (β, .7; SEM, 0.2; P = .002).Women receiving placebo also exhibited a significantly higher mean CES-D score across the 12-month intervention compared with those assigned to TE+IMP (Table 2). Mean (SD) unadjusted CES-D scores of the placebo and TE+IMP groups were 5.6 (5.7) and 4.2 (5.3) at visit 6, respectively, and 5.7 (7.6) and 4.0 (5.0) at visit 12. The effect of treatment on CES-D scores remained significant when 6 outliers were removed from the analysis (β, 1.1; SEM, 0.5; P = .03). Additional sensitivity analyses confirmed that these results also remained significant when adjusting for participants’ beliefs about their treatment assignment.
Figure 2. Rate of Clinically Significant Depressive Symptoms by Treatment, Adjusting For Baseline Center for Epidemiological Studies–Depression Scale (CES-D) Score and Mean Change in Vasomotor Symptom Bother.
aP < .05.
TE+IMP indicates transdermal estradiol plus intermittent micronized progesterone.
Table 2. Multilevel Regression Models Predicting Follow-up CES-D Scores From Baseline CES-D Score, Treatment, and Moderators.
| Parameter | Moderators of Treatment Effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main Effect of Treatment | SLEs | Depression History | Reproductive Stage | Abuse History | Baseline Estradiol | Baseline VMS | ||||||||
| Estimate (SE) | P Value | Estimate (SE) | P Value | Estimate (SE) | P Value | Estimate (SE) | P Value | Estimate (SE) | P Value | Estimate (SE) | P Value | Estimate (SE) | P Value | |
| Intercept | 2.25 (0.49) | <.001 | 2.39 (0.55) | <.001 | 3.25 (0.75) | <.001 | 2.65 (1.29) | .04 | 2.53 (0.75) | <.001 | 2.19 (0.55) | <.001 | 2.08 (0.57) | <.001 |
| Baseline CES-D | 0.47 (0.05) | <.001 | 0.42 (0.05) | <.001 | 0.44 (0.05) | <.001 | 0.45 (0.06) | <.001 | 0.43 (0.06) | <.001 | 0.44 (0.06) | <.001 | 0.48 (0.05) | <.001 |
| Change in VMS | −0.005 (0.13) | .97 | 0.01 (0.12) | .95 | 0.02 (0.12) | .88 | −0.03 (0.13) | .79 | −0.05 (0.14) | .92 | −0.02 (0.14) | .87 | NA | NA |
| Treatmenta | −1.18 (0.51) | .02 | −0.33 (0.64) | .61 | −1.58 (0.85) | .07 | −5.33 (1.70) | .005 | −2.26 (0.93) | .04 | −1.49 (0.55) | <.01 | −0.16 (0.76) | .83 |
| Moderatorb | NA | NA | 0.13c (0.33) | .69 | 1.20 (0.76) | .12 | −0.16d (0.58) | .78 | 0.51 (0.82) | .69 | −0.17 (0.30) | .56 | 0.17e (0.20) | .6 |
| Moderator × treatment | NA | NA | 1.22 (0.40) | .003 | −0.63 (1.04) | .55 | −1.97 (0.80) | .02 | −0.91 (1.05) | .39 | 0.91 (0.58) | .12 | 0.47 (0.29) | .1 |
| Simple effects of treatment (PBO vs TE+IMP) at key levels of significant moderators | ||||||||||||||
| 0 SLEs | NA | NA | −0.5 (0.8) | .53 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 1 SLE | NA | NA | −0.4 (1.0) | .7 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2+ SLEs | NA | NA | −2.4f (0.9) | <.01 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Early perimenopausal | NA | NA | NA | NA | NA | NA | −4.2 (1.2) | <.001 | NA | NA | NA | NA | NA | NA |
| Late perimenopausal | NA | NA | NA | NA | NA | NA | −0.9 (0.7) | .23 | NA | NA | NA | NA | NA | NA |
| Early postmenopausal | NA | NA | NA | NA | NA | NA | −0.3 (1.1) | .92 | NA | NA | NA | NA | NA | NA |
Abbreviations: CES-D, Center for Epidemiological Studies–Depression Scale; PBO, placebo; SLE, stressful life event; TE+IMP, transdermal estradiol plus intermittent micronized progesterone; VMS, vasomotor symptom bother.
Indicates that being in the TE+IMP group is associated with a CES-D score decrease of 1.18 compared with the placebo group.
See eResults in Supplement 2 for more information about the effect of moderating variables on CES-D score.
Score increase per additional life event.
Score decrease per advancement in reproductive status (early perimenopause to late perimenopause to early postmenopause).
Score increase per 1 SD increase in baseline VMS.
Among women with at least 2 life events, the TE+IMP group has a mean CES-D score that is 2.4 points lower than that of the placebo group.
Moderators of the Effect of Treatment
Reproductive Stage
A significant interaction between treatment and reproductive stage at study enrollment was found for mean CES-D scores (β, −1.97; SEM, 0.80, P = .03) such that mood benefits of TE+IMP vs placebo were evident among women in the early (β, −4.2; SEM, 1.2; P < .001) but not the late (β, −0.9; SEM, 0.3; P = .23) menopause transition or among postmenopausal women (β, −0.3; SEM, 1.1; P = .92) (Table 2; eFigure in Supplement 2). A similar interaction was seen between treatment and reproductive stage in predicting the number of visits during which a CES-D score of at least 16 was obtained (χ2 = 6.9; P = .03): TE+IMP was associated with 2 fewer instances of clinically significant depressive symptoms compared with placebo among early perimenopausal (β [SE], 2.0 [0.6]; P = .001) but not among late perimenopausal (β, 0.3; SEM, 0.3; P = .30) or early postmenopausal (β, 0.7; SEM, 0.7; P = .35) women. The interaction between treatment and reproductive stage did not reach significance in predicting the likelihood of experiencing clinically significant depressive symptoms (CES-D ≥16) (χ2 = 4.1; P = .13).
Stressful Life Events
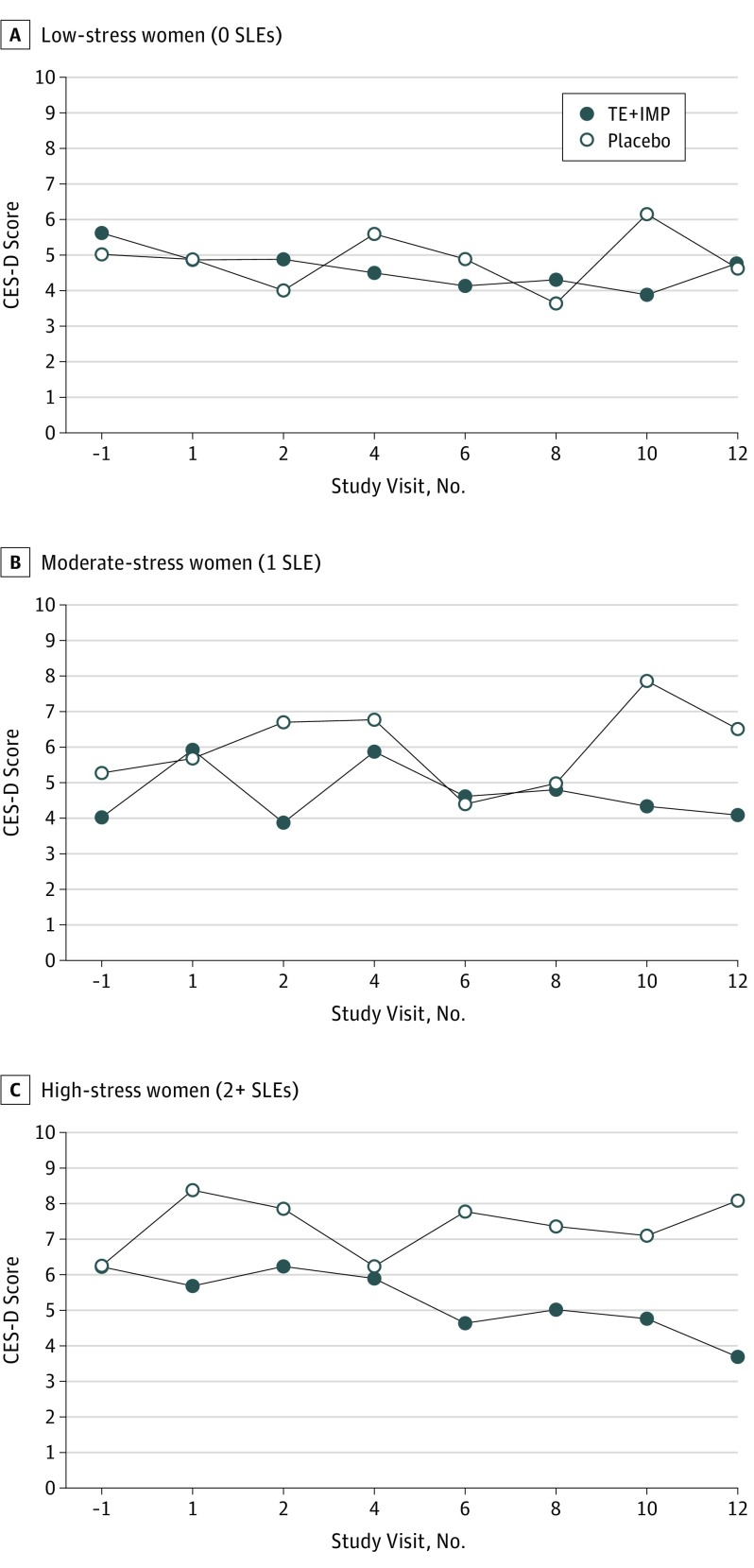
A significant interaction between treatment and number of stressful life events in the 6 months preceding study enrollment on mean CES-D score was observed (Table 2) such that a beneficial effect of treatment was more apparent in those with a greater number of stressful life events. Figure 3 depicts the effect of treatment on CES-D scores throughout the study among women reporting 0, 1, or 2 or more stressful life events at baseline. Although stressful life events did not moderate the effect of treatment on the likelihood of obtaining a CES-D score of at least 16 (β, 0.04; SEM, 0.17; P = .79), there was a marginally significant interaction between treatment and number of stressful life events on the total number of instances of clinically significant elevations in depressive symptoms experienced over the intervention period (β, 0.3; SEM, 0.2; P = .06) such that TE+IMP was associated with 1 less instance of a CES-D score of at least 16 among women with 2 or more recent events compared with placebo (β [SE], 1.0 [0.4];P = .007) but not among women with 0 (β, −0.2; SEM, 0.4, P = .67) or 1 (β, 0.5; SEM, 0.5, P = .33) stressful life event.
Figure 3. Model-Based Estimates of Treatment Effect on CES-D Score Over Time in Women With 0, 1, 2, or More Stressful Life Events (SLEs).
A significant effect of treatment is evident among the high-stress (n = 55; P = .005) but not low-stress (n = 72; P = .53) or moderate-stress (n = 46, P = .70) women. CES-D indicates Center for Epidemiological Studies–Depression Scale; TE+IMP, transdermal estradiol plus intermittent micronized progesterone.
Nonsignificant Moderators
Baseline estradiol levels, baseline vasomotor symptom bother, depression history, and abuse history were not found to be significant moderators of TE+IMP’s effects on depressive symptoms, the likelihood of obtaining a CES-D score of at least 16, or on the number of times a CES-D score of at least 16 occurred.
Adverse Effects
As would be expected given the purpose of the progesterone regimen was to induce vaginal bleeding, adverse effects were reported more commonly by participants assigned to TE+IMP than those assigned to placebo, as determined by χ2 analyses including spotting (64% vs 34%; P < .01), mild or moderate bleeding (80% vs 44%; P < .01), heavy bleeding (37% vs. 13%; P < .01), and prolonged bleeding (15% vs 1%; P < .01). The treatment groups did not differ in their reporting of breast tenderness, bloating, headache, migraine headaches, leg pain, fatigue, gastrointestinal symptoms, shortness of breath, skin irritation, hypertension, weight gain, breast lumps, or vision changes. There were 3 severe adverse events requiring study termination and medical treatment: 2 cases of major depressive disorder in the placebo group and 1 case of an acute deep vein thrombosis in the TE+IMT group.
Discussion
To our knowledge, this study is the first to longitudinally examine the prophylactic mood benefits of TE+IMP in initially euthymic women during the menopause transition and early postmenopausal period. During the 12-month intervention, rates of clinically significant depressive symptoms (CES-D score ≥16) were found to be 17% in the TE+IMP group and 32% in the placebo group, suggesting that TE+IMP effectively prevents the development of clinically significant depressive symptoms in this population. Importantly, these results were significant despite statistically adjusting for change in vasomotor symptom bother, suggesting that TE+IMP has direct prophylactic mood benefits that are independent from its beneficial effects on menopausal symptoms, as previously observed in women with major depressive disorder treated with TE+IMP.
It is noteworthy that more women receiving TE+IMP compared with those taking placebo experienced heavy (37% vs 13%) or prolonged (15% vs 1%) vaginal bleeding, which may be an important factor when considering TE+IMP as a treatment option. Nonetheless, despite early concerns that hormone therapy increases the risk of breast cancer and cardiovascular events, sparked by the initial findings of the Women’s Health Initiative, research has since demonstrated that hormone therapy, particularly TE+IMP, is safe for perimenopausal and early postmenopausal women when given at the lowest dose for the shortest amount of time to treat menopausal symptoms. The North American Menopause Society, the American Society for Reproductive Medicine, and the Endocrine Society agree that hormone therapy is indicated for the treatment of menopausal symptoms in younger women who are within 10 years of menopause. If confirmed in a larger sample of early perimenopausal women, the findings of this study, in combination with the few small trials finding estradiol therapy to be an effective treatment for perimenopausal depression, suggest that hormone therapy may also be indicated for the prevention and/or treatment of depressive symptoms appearing in the early menopause transition, regardless of whether menopausal symptoms are present.
To our knowledge, this study is also the first to assess baseline predictors of TE+IMP’s prophylactic mood benefits. The findings suggest that TE+IMP may be particularly beneficial for women in the early menopause transition and in women reporting more baseline stressful life events. Baseline vasomotor symptoms, a positive history of major depression, and a history of physical or sexual abuse were not associated with a beneficial effect of TE+IMP, suggesting that women may benefit from TE+IMP regardless of these baseline characteristics. Given that a history of depression is a strong risk factor for the development of perimenopausal depressive symptoms, it is somewhat surprising that it was not found to moderate the effects of TE+IMP. However, this is consistent with the findings of a small pilot study observing that neither vasomotor symptoms nor depression history moderated the beneficial effects of TE on perimenopausal depression. While the reasons that depression history did not predict a beneficial effect of TE+IMP are unknown, this negative finding is likely, in part, owing to TE+IMP’s efficacy in those without a history of depression. The fact that depression history was associated with greater mean levels of depressive symptoms across both treatment groups also suggests that women with a history of depression are susceptible to perimenopausal depressive symptoms through mechanisms, whether neurobiological or psychosocial, that are unaffected by TE+IMP.
These findings have important clinical implications. First, our findings confirm previous studies suggesting that the incidence of clinically significant depressive symptoms is not uncommon in perimenopausal and early postmenopausal women, particularly in the early perimenopausal period and among women experiencing stressful life events. Second, our findings suggest that a brief clinical interview assessing menstrual bleeding patterns and the presence of stressful life events may be best suited to identifying the patients for whom TE+IMP will be most beneficial during the menopause transition. In this way, these findings, if replicated, have relevance for the individualization of the therapeutic approach for women at this reproductive stage.
Our novel findings that reproductive stage and recent stressful life events predict the prophylactic mood benefit of TE+IMP begs the question as to why that might be the case. Owing to multiple simultaneous changes in the reproductive axis, triggered by advancing age, the menopause transition is characterized by increased estradiol fluctuation owing to higher estradiol peaks and lower estradiol troughs than the mean menstrual cycle; in contrast, the late postmenopausal period, when depression risk declines, is characterized by low but stable estradiol levels. Prior work has observed that women with a history of perimenopausal depression exhibit a rapid increase in depressive symptoms following an abrupt experimental withdrawal from estradiol that is not observed in women without such a history, supporting the notion that the changing hormonal milieu of the menopause transition contributes to the increased risk for perimenopausal depression. By raising trough estradiol levels, TE+IMP may minimize the magnitude of estradiol withdrawal experienced in the menopause transition. Based on 1 report, TE may also minimize instances of extreme estradiol fluctuation by reducing the incidence of ovulation in perimenopausal women; however, it likely does not uniformly do so. The observation that number of stressful life events moderates the effect of treatment is consistent with our 2015 report suggesting that perimenopausal women experiencing greater estradiol variability are more emotionally sensitive to psychosocial stress in the laboratory and also more prone to depressive symptoms in the context of stressful life events. A 2015 conceptual review posited a neurobiological mechanism by which perimenopausal estradiol variability may trigger increased sensitivity to stress involving GABAergic influences on the hypothalamic-pituitary-adrenal axis, resulting in an increased susceptibility to depressed mood in the context of psychosocial stressors. A reduction in estradiol variability (via increasing trough levels or preventing ovulation) may be a mechanism contributing to TE+IMP’s prophylactic mood benefits among women with a greater number of stressful life events, although this study was not designed to examine TE+IMP’s effects on estradiol variability.
Limitations and Strengths
This study has several limitations. First, more frequent assessments of estradiol levels before and during treatment would have allowed us to more directly test the hypothesis that reducing the magnitude of fluctuation in estradiol levels represents a mechanism by which TE+IMP benefits mood. Relatedly, prerandomization estradiol measurements would have been best taken at standard times in relation to women’s menstrual cycles (for women who were still menstruating) such as during the early follicular phase. Second, the active and placebo patches were not identical. However, our findings remain significant when adjusting for participants’ beliefs about their treatment condition. This study also had many notable strengths. First, to our knowledge, it is the first to assess whether TE+IMP can prevent the emergence of clinically significant depressive symptoms in initially euthymic perimenopausal and early postmenopausal women. Second, its comprehensive assessment of multiple psychosocial variables allowed for a unique investigation of predictors of treatment response. Third, its 12-month duration and bimonthly assessment of symptoms allowed us to examine the progression of symptoms over time.
Conclusions
The findings of this study confirm that perimenopausal and early postmenopausal women are at high risk for developing clinically significant depressive symptoms. To our knowledge, we are the first to report that TE+IMP administration prevents this transition-related increase in risk for depressive mood. Health care professionals should be alert to the high risk for clinically significant depressive symptoms in this population. If our results are confirmed in a larger sample, clinicians may consider using TE+IMP as a prophylactic treatment in the prevention of clinically significant depressive symptoms in medically eligible perimenopausal and early postmenopausal women.
Trial Protocol.
eMethods.
eResults.
eFigure. Model-based Estimates of Treatment Effect on CES-D Score Over Time
References
- 1.Bromberger JT, Kravitz HM, Chang Y-F, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromberger JT, Matthews KA, Schott LL, et al. . Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN). J Affect Disord. 2007;103(1-3):267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62-70. [DOI] [PubMed] [Google Scholar]
- 4.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15(2):223-232. [DOI] [PubMed] [Google Scholar]
- 5.Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS. Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition. Menopause. 2016;23(3):257-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt PJ, Ben Dor R, Martinez PE, et al. . Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares CN, Arsenio H, Joffe H, et al. . Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause. 2006;13(5):780-786. [DOI] [PubMed] [Google Scholar]
- 8.Gleason CE, Dowling NM, Wharton W, et al. . Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12(6):e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joffe H, Petrillo LF, Koukopoulos A, et al. . Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011;96(7):E1044-E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt PJ, Nieman L, Danaceau MA, et al. . Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183(2):414-420. [DOI] [PubMed] [Google Scholar]
- 11.Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression: results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4(3):214-220. [DOI] [PubMed] [Google Scholar]
- 12.Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, Girdler SS. Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology. 2016;67:142-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26(2):201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390. [DOI] [PubMed] [Google Scholar]
- 15.Harlow SD, Gass M, Hall JE, et al. ; STRAW+10 Collaborative Group . Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 2012;15(2):105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. [Google Scholar]
- 17.Greene JG. Constructing a standard climacteric scale. Maturitas. 2008;61(1-2):78-84. [DOI] [PubMed] [Google Scholar]
- 18.Sarason IG, Johnson JH. The Life Experiences Survey: Preliminary Findings. DTIC Document; 1976. [Google Scholar]
- 19.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46(5):932-946. [DOI] [PubMed] [Google Scholar]
- 20.Leserman J, Petitto JM, Gu H, et al. . Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32(6):1059-1073. [DOI] [PubMed] [Google Scholar]
- 21.Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-infected men: a 2-year follow-up study. Arch Gen Psychiatry. 1997;54(3):279-285. [DOI] [PubMed] [Google Scholar]
- 22.Leserman J, Ironson G, O’Cleirigh C, Fordiani JM, Balbin E. Stressful life events and adherence in HIV. AIDS Patient Care STDS. 2008;22(5):403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leserman J, Drossman DA, Li Z. The reliability and validity of a sexual and physical abuse history questionnaire in female patients with gastrointestinal disorders. Behav Med. 1995;21(3):141-150. [DOI] [PubMed] [Google Scholar]
- 24.Leserman J, Drossman DA, Li Z, Toomey TC, Nachman G, Glogau L. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med. 1996;58(1):4-15. [DOI] [PubMed] [Google Scholar]
- 25.Leserman J, Li Z, Drossman DA, Toomey TC, Nachman G, Glogau L. Impact of sexual and physical abuse dimensions on health status: development of an abuse severity measure. Psychosom Med. 1997;59(2):152-160. [DOI] [PubMed] [Google Scholar]
- 26.Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample: understanding the discrepancies between depression symptom and diagnostic scales. Arch Gen Psychiatry. 1982;39(10):1195-1200. [DOI] [PubMed] [Google Scholar]
- 27.Thomas JL, Jones GN, Scarinci IC, Mehan DJ, Brantley PJ; The Center for Epidemiologic Studies-Depression . The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. Int J Psychiatry Med. 2001;31(1):25-40. [DOI] [PubMed] [Google Scholar]
- 28.Rossouw JE, Anderson GL, Prentice RL, et al. ; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333. [DOI] [PubMed] [Google Scholar]
- 29.Rubinow DR, Girdler SS. Hormones, heart disease, and health: individualized medicine versus throwing the baby out with the bathwater. Depress Anxiety. 2011;28(6):E1-E15. [DOI] [PubMed] [Google Scholar]
- 30.Hale GE, Shufelt CL. Hormone therapy in menopause: an update on cardiovascular disease considerations. Trends Cardiovasc Med. 2015;25(6):540-549. [DOI] [PubMed] [Google Scholar]
- 31.Stuenkel CA, Gass ML, Manson JE, et al. . A decade after the Women’s Health Initiative: the experts do agree. Fertil Steril. 2012;98(2):313-314. [DOI] [PubMed] [Google Scholar]
- 32.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13(6):559-565. [DOI] [PubMed] [Google Scholar]
- 33.Gordon JL, Girdler SS, Meltzer-Brody SE, et al. . Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172(3):227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hale GE, Burger HG. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23(1):7-23. [DOI] [PubMed] [Google Scholar]
- 35.Santoro N, Randolph JF Jr. Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am. 2011;38(3):455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson NR, Studd JW, Riddle AF, Savvas M. Suppression of ovulation by transdermal oestradiol patches. BMJ. 1988;297(6653):900-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eMethods.
eResults.
eFigure. Model-based Estimates of Treatment Effect on CES-D Score Over Time