Key Points
Question
What is the effect of a locally adapted quality improvement tool kit for acute coronary syndrome on major adverse cardiovascular events in Kerala, India?
Findings
In this cluster randomized, stepped-wedge clinical trial that included 63 hospitals and 21 374 participants with acute myocardial infarction, the 30-day composite event rates were 6.4% in the control phase and 5.3% in the intervention phase, a difference that did not reach not statistical significance after adjustment for cluster and temporal trends.
Meaning
Among patients with acute myocardial infarction in Kerala, India, a locally adapted quality improvement tool kit did not reduce the rate of 30-day major adverse cardiovascular events compared with usual care.
Abstract
Importance
Wide heterogeneity exists in acute myocardial infarction treatment and outcomes in India.
Objective
To evaluate the effect of a locally adapted quality improvement tool kit on clinical outcomes and process measures in Kerala, a southern Indian state.
Design, Setting, and Participants
Cluster randomized, stepped-wedge clinical trial conducted between November 10, 2014, and November 9, 2016, in 63 hospitals in Kerala, India, with a last date of follow-up of December 31, 2016. During 5 predefined steps over the study period, hospitals were randomly selected to move in a 1-way crossover from the control group to the intervention group. Consecutively presenting patients with acute myocardial infarction were offered participation.
Interventions
Hospitals provided either usual care (control group; n = 10 066 participants [step 0: n = 2915; step 1: n = 2649; step 2: n = 2251; step 3: n = 1422; step 4; n = 829; step 5: n = 0]) or care using a quality improvement tool kit (intervention group; n = 11 308 participants [step 0: n = 0; step 1: n = 662; step 2: n = 1265; step 3: n = 2432; step 4: n = 3214; step 5: n = 3735]) that consisted of audit and feedback, checklists, patient education materials, and linkage to emergency cardiovascular care and quality improvement training.
Main Outcomes and Measures
The primary outcome was the composite of all-cause death, reinfarction, stroke, or major bleeding using standardized definitions at 30 days. Secondary outcomes included the primary outcome’s individual components, 30-day cardiovascular death, medication use, and tobacco cessation counseling. Mixed-effects logistic regression models were used to account for clustering and temporal trends.
Results
Among 21 374 eligible randomized participants (mean age, 60.6 [SD, 12.0] years; n = 16 183 men [76%] ; n = 13 689 [64%] with ST-segment elevation myocardial infarction), 21 079 (99%) completed the trial. The primary composite outcome was observed in 5.3% of the intervention participants and 6.4% of the control participants. The observed difference in 30-day major adverse cardiovascular event rates between the groups was not statistically significant after adjustment (adjusted risk difference, −0.09% [95% CI, −1.32% to 1.14%]; adjusted odds ratio, 0.98 [95% CI, 0.80-1.21]). The intervention group had a higher rate of medication use including reperfusion but no effect on tobacco cessation counseling. There were no unexpected adverse events reported.
Conclusions and Relevance
Among patients with acute myocardial infarction in Kerala, India, use of a quality improvement intervention compared with usual care did not decrease a composite of 30-day major adverse cardiovascular events. Further research is needed to understand the lack of efficacy.
Trial Registration
clinicaltrials.gov Identifier: NCT02256657
This cluster randomized, stepped-wedge clinical trial compares the effect of a quality improvement tool kit vs usual care on 30-day major adverse cardiovascular events in patients with acute myocardial infarction (AMI) in Kerala, India.
Introduction
In 2015, there were an estimated 7.3 million (95% uncertainty interval, 6.8 million–7.8 million) fatal myocardial infarctions globally, and South Asia was estimated to have the world’s highest age-standardized incident rate of myocardial infarction. High-income countries have developed programs for improving process and outcomes measures for acute myocardial infarction that have been associated with improvements in care and outcomes with concomitant reductions in racial/ethnic disparities in care. Because ischemic heart disease represents the leading cause of global deaths, improving the quality of care and outcomes for patients with acute myocardial infarction, particularly in low- and middle-income countries, is a global health priority.
Previous publications on the presentation, management, and outcomes of 25 748 acute coronary syndrome patients from 125 hospitals in Kerala, India, have demonstrated wide heterogeneity in process and outcome measures across hospitals and identified targets for intervention, including increasing speed and use of guideline-directed medical therapy. Previous acute myocardial infarction quality improvement randomized trials that include physician education, audit and feedback mechanisms, clinical pathways, and checklists have shown favorable effects on process measures in low- and middle-income countries such as Brazil and China. However, these trials were not designed or powered to evaluate the effects of these interventions on clinical outcomes, nor were they designed to account for temporal trends. A 2017 pre/post–intervention study in Tamil Nadu, India, also demonstrated favorable trends with implementation of a hub-and-spoke health system intervention but used a nonrandomized study design.
To determine whether process and outcome measures could be improved for patients with acute myocardial infarction with a locally adapted quality improvement tool kit using a robust study design for better causal inference, we performed a large, pragmatic, cluster randomized, stepped-wedge trial among 63 hospitals in Kerala.
Methods
Study Design
The Acute Coronary Syndrome Quality Improvement in Kerala (ACS QUIK) trial was a pragmatic, cluster randomized, stepped-wedge clinical trial in which hospitals were randomized to receive the quality improvement tool kit intervention at 1 of 5 predefined, 4-month steps over a 24-month period between November 10, 2014, and November 9, 2016, after a period of usual care. The last date of follow-up was December 31, 2016. The methods have been previously published. A stepped-wedge design is useful when evaluating an intervention when clusters have ethical concerns about being randomized to the control group only for the duration of the trial and when it is infeasible to disseminate the intervention simultaneously across a large number of clusters. The study received ethics board approval from local, national, and international bodies and was approved by the Indian Health Ministry Screening Committee. All participants or their proxies provided written informed consent to participate. The study was conducted according to the study protocol (Supplement 1) and analyzed according to the statistical analysis plan (Supplement 2).
Hospitals and Study Participants
We recruited 63 hospitals in Kerala from a sample (n = 125) that had previously participated in the Kerala Acute Coronary Syndrome (ACS) Registry. We also sought government hospitals that had not participated in this registry to include a range of hospital types (ie, government, private, nonprofit/charity). To be eligible, hospitals had to identify 2 individuals who would be willing to serve on a quality improvement team. Patients were eligible to participate if they presented or were transferred for evaluation and management of either non–ST-segment elevation myocardial infarction (NSTEMI) or ST-segment elevation myocardial infarction (STEMI) based on the Third Universal Definition of Myocardial Infarction.
Randomization and Treatment Assignments
The study biostatisticians (D.K. and L.Z.) performed central computer-based randomization of hospitals. The other members of the study team and the selected sites were informed of the 12 or 13 sites that would cross over to the intervention period 2 weeks before each of the predefined steps to maintain allocation concealment while aiding in training logistics. We stratified randomization into 4 groups by projected recruitment (≤200, 201-500, 501-1000, and >1000 participants) based on the Kerala ACS Registry to minimize potential imbalance between the intervention and control periods. By nature of the trial design, neither personnel nor participants were blinded to the intervention.
Interventions
We used formative mixed-methods research and evidence synthesis to adapt previously reported strategies with the goal of improving process of care and outcome measures. We created an audit and feedback reporting mechanism based on key data elements used by the American College of Cardiology and American Heart Association. These reports, which included site-specific measures on performance and which were sent monthly via email to site investigators, compared hospital-level performance with other hospitals in each cohort and with all hospitals in the trial (see eAppendix in Supplement 3 for sample report). We trained sites on the interpretation of these reports and on the use of these reports for informing quality improvement team meetings, for which we provided meeting templates based on the Plan-Do-Study-Act cycle of change. The tool kit also included standardized admission and discharge order sets; translated and culturally adapted patient education materials related to tobacco cessation, dietary advice, and physical activity; and linkage to emergency cardiovascular care training based on the low prevalence of code teams among the participating hospitals (see eAppendix in Supplement 3 for sample checklists and patient education materials). We sought and received feedback from the participating investigators in the development of this tool kit and provided free online health care quality and patient safety training to sites and their teams through the Institute for Healthcare Improvement. The control condition was usual care according to local hospital practice. We performed a concurrent mixed-methods process evaluation for assessing context, implementation fidelity, and mechanisms of impact based on the Medical Research Council’s recommendations; these results will be reported separately.
The study team performed central and on-site training for 90 to 120 minutes at each site including with the site investigator and other members of the quality improvement team and medical staff. We did not include a transition period. Date of admission was used to define allocation of participants to intervention or control.
Outcomes
The primary outcome was the composite end point of 30-day major adverse cardiovascular events, defined as death, reinfarction (defined by the Third Universal Definition of Myocardial Infarction), stroke, and major bleeding (defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries [GUSTO] criteria, which is defined by intracerebral hemorrhage or bleeding resulting in substantial hemodynamic compromise requiring treatment). Outcome data were collected at each site either through in-person visits or by telephone and were reported centrally. If a site was unable to reach a participant after 3 attempts, then that participant was considered lost to follow-up. Although the outcome assessors were not blinded, there did not appear to be a high risk of ascertainment bias to influence the treatment effect for objective outcomes such as these.
Secondary outcomes included 30-day all-cause mortality, 30-day cardiovascular mortality, in-hospital mortality, 30-day myocardial reinfarction, 30-day stroke, 30-day major GUSTO bleeding, optimal in-hospital medication use (composed of aspirin, adenosine diphosphate receptor antagonist [clopidogrel, prasugrel, or ticagrelor], anticoagulant, and β-blocker; in-hospital statin use was additionally predefined but data were not collected), optimal discharge medication use (composed of aspirin, adenosine diphosphate receptor antagonist [clopidogrel, prasugrel, or ticagrelor], statin, and β-blocker), and tobacco cessation advice. We performed post hoc analyses evaluating the effect of the intervention on major adverse cardiovascular events plus in-hospital incident heart failure, cardiogenic shock, and cardiac arrest.
Statistical Analysis
We initiated the trial with a target sample size of 15 750 participants from 63 hospitals with 5 steps to have 80% power to achieve a 2.4% absolute reduction in major adverse cardiovascular events with a 2-sided α = .05. We assumed a rate of loss to follow-up of 5% and an intraclass correlation coefficient of 0.05 based on data from the Kerala ACS Registry (unpublished data) and a previous quality improvement trial in Brazil. This intervention effect estimate was based on the difference between leading and lagging hospitals’ performance and outcomes from previous observational research. Prespecified participant-level results are reported.
Baseline characteristics (unadjusted and adjusted for within-hospital clustering and temporal trends) were summarized for intervention and control groups. For the primary outcome, the overall difference in 30-day major adverse cardiovascular event rates between control and intervention periods was reported. In the primary analysis, 30-day major adverse cardiovascular events were modeled using mixed-effects logistic regression with a random cluster (hospital) effect and a fixed time effect for every 4-month step. As a secondary analysis, the model was also adjusted for age, sex, type of myocardial infarction, systolic blood pressure, heart rate, serum creatinine level, acute heart failure, cardiogenic shock, and resuscitated cardiac arrest, which are covariates included in the Global Registry of Acute Coronary Events (GRACE) risk score. We performed post hoc analyses to evaluate the potential effect of the intervention on an expanded definition of major adverse cardiovascular events to include cardiogenic shock, incident heart failure, and cardiac arrest and the interaction between time spent in the intervention period and the adjusted 30-day major adverse cardiovascular event rate. Results are also reported by prespecified subgroups of participant age, sex, STEMI vs NSTEMI, hospital size, and hospital type, for which we also tested interaction effects.
All results are reported using an intention-to-treat analysis. The interim analysis was performed at 12 months for reporting to the data and safety monitoring board, but only the study biostatisticians (D.K. and L.Z.) were unblinded to the results. To adjust for the interim analysis, the O’Brien-Fleming stopping boundary was set at z>2.797 with a 2-sided significance level of .005 for the interim analysis and z>1.977 with a 2-sided significance level of .048 for the final analysis. We did not make additional a priori adjustments to the significance threshold for secondary outcomes based on multiple testing; therefore, these analyses should be considered exploratory.
Biweekly central statistical monitoring was done using previously published algorithms for risk-based site monitoring. After study completion, 1 site was identified with an improbably high rate of reinfarction, which prompted quality assurance sampling of 10% of cases with corresponding controls among the study sites through blinded site surveys. Two additional sites with systematic error were identified in outcome reporting, which resulted in 13 changes to the primary outcome (1% of overall events).
For statistical analyses, Stata, version 14 (Stata Corp), SAS, version 9.4 (SAS Institute Inc), and R, version 3.3.0 (R Foundation), were used. StatTag, version 3.0, was used for the preparation of results in this article. StatTag facilitates reproducible research by embedding output from statistical programs (Stata, SAS, and R) in Microsoft Word documents. Code files used for generation of results are available at https://github.com/abigailbaldridge/ACS-QUIK.
Results
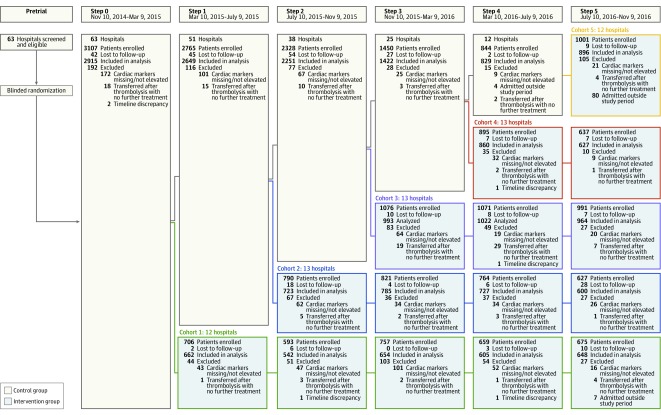
Figure 1 shows the flow of hospitals and participants. We recruited 22 557 participants from 63 hospitals, including 67% private, 14% government affiliated with medical colleges, and 19% nonprofit/charity. Participants were ineligible and excluded if they had cardiac biomarker measurements that were missing or not elevated (for NSTEMI only because some patients with ST elevations and chest pain did not undergo biomarker testing [n = 954]), were transferred after thrombolytic therapy for STEMI without any further therapy (n = 132), were admitted outside the study period (n = 91), or had implausible time discrepancies (n = 6). Follow-up through 30 days was 99%. Among 21 374 eligible participants, 295 (1%) had incomplete outcome data (215 patients were not followed up by the enrolling site and 80 patients could not be reached after 3 contact attempts). Unadjusted baseline characteristics between included participants and participants missing follow-up data were generally similar except for a lower initial troponin level in the former group (1.3 ng/mL vs 4.6 ng/mL; P < .001) (eTable 1 in Supplement 3). The intervention and control groups included 11 308 (53%) and 10 066 (47%) participants, respectively.
Figure 1. Flow of Hospitals and Participants Through the ACS QUIK Trial.
All hospitals approached agreed to participate and were included. Randomization occurred at a single time point prior to patient enrollment. Hospitals were blinded to their assigned cohort until 2 weeks prior to the time point at which their cohort moved from the control stage to intervention stage. Participants lost to follow-up include those who could not be reached after 3 contact attempts and those for whom the site did not perform the follow-up procedure. Patients lost to follow-up were included in the analysis.
Participants
Table 1 shows the baseline characteristics for the intervention and control groups. The mean age of the participants was 60.6 years, 76% were men, 31% had a history of tobacco use, and 44% had a history of diabetes mellitus. Sixty-four percent of participants presented with STEMI, with a median (symptom-to-door time of 240 (interquartile range, 120-840) minutes in the control group compared with 255 (interquartile range, 111-825) minutes in the intervention group.
Table 1. Baseline Characteristics of ACS QUIK Patients and Hospitals by Intervention and Control Group.
| Characteristics | Control (n = 10 066)a | Intervention (n = 11 308)a | Difference (95% CI)b |
|---|---|---|---|
| Patient Characteristics | |||
| Age, mean (SD), y | 60.3 (12.0) | 60.9 (12.1) | 0.6 (0.3 to 1.0) |
| Male, No. (%) | 7654 (76.0) | 8529 (75.4) | −0.6 (−1.8 to 0.5) |
| History of tobacco use, No. (%) | 3772 (37.5) | 2842 (25.1) | −12.3 (−13.6 to −11.1) |
| History of diabetes, No. (%) | 4151 (41.2) | 5333 (47.2) | 5.9 (4.6 to 7.3) |
| Transferred from another facility, No. (%) | 4202 (41.7) | 4199 (37.1) | −4.6 (−5.9 to −3.3) |
| No insurance, No. (%) | 6878 (68.3) | 8664 (76.6) | 8.3 (7.1 to 9.5) |
| ST-elevation myocardial infarction, No. (%) | 6921 (68.8) | 6768 (59.9) | −8.9 (−10.2 to −7.6) |
| Symptom-to-door time, median (IQR), min | 240 (120-840) (n = 9711) | 255 (111-825) (n = 10 849) | 15 (4 to 26) |
| Body weight, mean (SD), kg | 63.6 (9.8) (n = 10 065) | 63.3 (9.6) (n = 11 304) | −0.3 (−0.5 to 0.0) |
| Systolic blood pressure, mean (SD), mm Hg | 138.1 (29.1) (n = 10 061) | 139.0 (28.9) (n = 11 303) | 0.9 (0.1 to 1.7) |
| Heart rate, mean (SD), /min | 80.0 (18.7) (n = 10 065) | 79.9 (19.1) (n = 11 303) | −0.1 (−0.6 to 0.5) |
| Initial troponin, median (IQR), ng/mL | 1.6 (0.4-8.0) (n = 4566) | 1.1 (0.2-4.4) (n = 4483) | −0.5 (−0.7 to −0.4) |
| Low-density lipoprotein cholesterol, mean (SD), mg/dL | 126.2 (40.3) (n = 7288) | 119.1 (41.2) (n = 7542) | −7.1 (−8.4 to −5.8) |
| Triglycerides, median (IQR), mg/dL | 121 (89-167) (n = 7308) | 122 (90-163) (n = 7552) | 1 (−1 to 3) |
| Serum creatinine, median (IQR), mg/dL | 1.0 (0.8-1.2) (n = 4246) | 1.0 (0.9-1.2) (n = 9589) | 0.0 (0.0 to 0.0) |
| Fasting glucose, median (IQR), mg/dL | 124 (98-172) (n = 6658) | 130 (105-180) (n = 6740) | 6 (4 to 8) |
| Hemoglobin, mean (SD), mg/dL | 13.3 (2.0) (n = 9763) | 13.2 (2.0) (n = 11 079) | −0.1 (−0.2 to 0.0) |
| Hospital Characteristics | |||
| Hospital type, No. (%) | |||
| Government (n = 9) | 4097 (40.7) | 3036 (26.8) | −13.9 (−15.1 to −12.6) |
| Nonprofit or charity (n = 12) | 2785 (27.7) | 2964 (26.2) | −1.5 (−2.7 to −0.3) |
| Private (n = 42) | 3184 (31.6) | 5308 (46.9) | 15.3 (14.0 to 16.6) |
| Hospital size by anticipated enrollment, No. (%) | |||
| Extra large (>1000) (n = 5) | 1853 (18.4) | 1707 (15.1) | −3.3 (−4.3 to −2.3) |
| Large (501-1000) (n = 15) | 3561 (35.4) | 4962 (43.9) | 8.5 (7.2 to 9.8) |
| Medium (201-500) (n = 24) | 3847 (38.2) | 3568 (31.6) | −6.7 (−7.9 to −5.4) |
| Small (≤200) (n = 19) | 805 (8.0) | 1071 (9.5) | 1.5 (0.7 to 2.2) |
| Catheterization laboratory, No. (%) | |||
| Installed during study (n = 3) | 171 (1.7) | 325 (2.9) | 1.2 (0.8 to 1.6) |
| No (n = 17) | 1998 (19.8) | 1554 (13.7) | −6.1 (−7.1 to −5.1) |
| Yes (n = 43) | 7897 (78.5) | 9429 (83.4) | 4.9 (3.9 to 6.0) |
Abbreviation: IQR, interquartile range.
SI conversions: To convert low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; serum creatinine to μmol/L, multiply by 88.4; and glucose to mmol/L, multiply by 0.0555.
Total numbers are shown for variables for which data were not completely reported.
Crude difference = intervention minus control.
Table 2 shows the association of process of care and time-based characteristics with the intervention, both unadjusted and adjusted for within-hospital clustering and temporal trends. Compared with the control group, the intervention group had a higher rate of prehospital aspirin use (18.6% vs 16.9%; adjusted risk difference, 3.80% [95% CI, 2.08%-5.52%]; adjusted odds ratio [OR], 1.40 [95% CI, 1.21-1.62]) but similarly low rates of prehospital thrombolysis (<1%). Among eligible individuals without contraindications, rates of in-hospital aspirin use and a second antiplatelet medication (clopidogrel, prasugrel, or ticagrelor) were high (>95%) and similar in both groups. Compared with the control group, the intervention group had a higher rate of in-hospital β-blocker use (43% vs 37%; adjusted risk difference, 6.25% [95% CI, 4.10%-8.10%]; adjusted OR, 1.46 [95% CI, 1.29-1.65]) and anticoagulant use (86% vs 86%; adjusted risk difference, 2.60% [95% CI, 0.87%-4.33%]; adjusted OR, 1.27 [95% CI, 1.09-1.49]).
Table 2. Characteristics and Association of Process of Care and Time-Based Outcomes by Treatment Group, Adjusted for Within-Hospital Clustering and Temporal Trends.
| Measures of Care | No./Total No. (%)a | Difference, % (95% CI)b | Odds Ratio or β-Coefficient (95% CI)b | |
|---|---|---|---|---|
| Control (n = 10 066) | Intervention (n = 11 308) | |||
| Medications | ||||
| Prehospital aspirin | 1696/10 052 (16.9) | 2100/11 300 (18.6) | 3.80 (2.08 to 5.52) | 1.40 (1.21-1.62) |
| Prehospital thrombolysis (STEMI) | 25/6921 (0.4) | 37/6768 (0.5) | −0.04 (−0.76 to 0.68) | 0.95 (0.40-2.28) |
| In-hospital aspirin | 9858/10 042 (98.2) | 11 027/11 286 (97.7) | −0.05 (−0.95 to 0.84) | 0.98 (0.69-1.39) |
| In-hospital second antiplatelet | 9861/10 050 (98.1) | 11 112/11 297 (98.4) | −0.05 (−0.89 to 0.79) | 0.98 (0.67-1.43) |
| In-hospital β-blocker | 3676/9874 (37.2) | 4638/10 885 (42.6) | 6.25 (4.10 to 8.40) | 1.46 (1.29-1.65) |
| In-hospital anticoagulant | 8602/10 051 (85.6) | 9654/11 281 (85.6) | 2.60 (0.87 to 4.33) | 1.27 (1.09-1.49) |
| Studies and procedures | ||||
| Echocardiography | 9209/10 066 (91.5) | 10 516/11 308 (93.0) | 5.62 (3.35 to 7.89) | 2.50 (1.95-3.21) |
| Diagnostic angiography | 6181/10 066 (61.4) | 6500/11 308 (57.5) | Nonestimable | Nonestimable |
| PCI | 5282/10 066 (52.5) | 5271/11 308 (46.6) | −1.66 (−3.27 to −0.04) | 0.87 (0.77-0.99) |
| Primary PCI (STEMI) | 3476/6921 (50.2) | 3234/6768 (47.8) | −1.87 (−3.85 to 0.11) | 0.86 (0.73-1.01) |
| Door-to-balloon time (STEMI), median (IQR), min | 65 (53-105) (n=4022) | 77 (55-118) (n=3639) | 6.18 (−4.78 to 17.14) | 13.00 (3.64-22.36)c |
| Thrombolysis (STEMI) | 1596/6921 (23.1) | 1571/6768 (23.2) | 5.56 (3.21 to 7.91) | 1.59 (1.33-1.92) |
| Door-to-needle time (STEMI), median (IQR), min | 44 (30-67) (n=1455) | 45 (27-75) (n=1433) | 0.40 (−5.23 to 6.03) | 5.00 (−5.20-15.20)c |
| Any reperfusion (STEMI) | 5067/6921 (73.2) | 4805/6768 (71.0) | 3.43 (0.87 to 6.00) | 1.24 (1.06-1.46) |
| Rescue PCI (STEMI) | 644/6891 (9.3) | 1031/6767 (15.2) | 2.45 (0.51 to 4.38) | 1.39 (1.08-1.79) |
| Discharge treatment and counseling | ||||
| Discharge aspirin | 8777/8998 (97.5) | 10 360/10 559 (98.1) | 1.36 (0.23 to 2.49) | 1.65 (1.15-2.37) |
| Discharge second antiplateletd | 8824/9011 (97.9) | 10 377/10 580 (98.1) | 0.41 (−0.31 to 1.13) | 1.24 (0.86-1.79) |
| Discharge β-blocker | 5808/8894 (65.3) | 6799/10 178 (66.8) | 6.69 (4.43 to 8.95) | 1.48 (1.30-1.68) |
| Discharge statin | 8700/9006 (96.6) | 10 289/10 579 (97.3) | 1.21 (0.07 to 2.35) | 1.42 (1.04-1.92) |
| Discharge ACE inhibitor or ARB | 534/1029 (51.9) | 643/1495 (43.0) | 6.57 (0.58 to 12.57) | 1.45 (1.03-2.04) |
| Cardiac rehabilitation referral | 2362/9228 (25.6) | 3322/10 791 (30.8) | Nonestimable | Nonestimable |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; IQR, interquartile range; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as No./total No. of participants unless otherwise indicated. Totals include eligible participants for whom process measures were reported. Patients with contraindications to medications were excluded from relevant process measures. Discharge measures were assessed only among patients discharged. Discharge ACE inhibitors or ARBs were assessed only among discharged patients with ejection fractions of 40% or lower.
Odds ratios and β-coefficients represent effect of intervention compared with control and are calculated as the difference in marginal effects (intervention group minus control group) in a mixed-effects logistic regression or quantile regression model including a random-effects term to account for within-hospital clustering and temporal trends.
β-Coefficient.
Second antiplatelet includes clopidogrel, prasugrel, or ticagrelor.
Compared with the control group, the intervention group had a higher rate of echocardiography (93% vs 92%; adjusted risk difference, 5.62% [95% CI, 3.35%-7.89%]; adjusted OR, 2.50 [95% CI, 1.95-3.21]), and among patients with STEMI, higher rates of thrombolysis (23% vs 23%; adjusted risk difference, 5.56% [95% CI, 3.21%-7.91%]; adjusted OR, 1.59 [95% CI, 1.33-1.92]), reperfusion (71% vs 73%; adjusted risk difference, 3.43% [95% CI, 0.87%-6.00%]; adjusted OR, 1.24 [95% CI, 1.06-1.46]), and rescue percutaneous coronary intervention (15% vs 9%; adjusted risk difference, 2.45% [95% CI, 0.51%-4.38%]; adjusted OR, 1.39 [95% CI, 1.08-1.79]) but lower rates of diagnostic coronary angiography (58% vs 61%) and percutaneous coronary intervention (47% vs 53%; adjusted risk difference, −1.66% [95% CI, −3.27% to −0.04%]; adjusted OR, 0.87 [95% CI, 0.77-0.99]).
The intervention group had a higher rate of discharge aspirin use (98% vs 98%; adjusted risk difference, 1.36% [95% CI, 0.23%-2.49%]; adjusted OR, 1.65 [95% CI, 1.15-2.37]), β-blocker use (67% vs 65%; adjusted risk difference, 6.63% [95% CI, 4.43%-8.95%]; adjusted OR, 1.47 [95% CI, 1.30-1.68]), statin use (97% vs 97%; adjusted risk difference, 1.21% [95% CI, 0.07%-2.35%]; adjusted OR, 1.42 [95% CI, 1.04-1.92]), angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use among patients with ejection fractions of 40% or lower (43% vs 52%; adjusted risk difference, 6.57% [95% CI, 0.58%-12.57%]; adjusted OR, 1.45 [95% CI, 1.03-2.04]), and referral for cardiac rehabilitation (31% vs 26%). Rates of discharge second antiplatelet use and tobacco cessation counseling were high (>95%) and similar between groups.
Primary Analysis
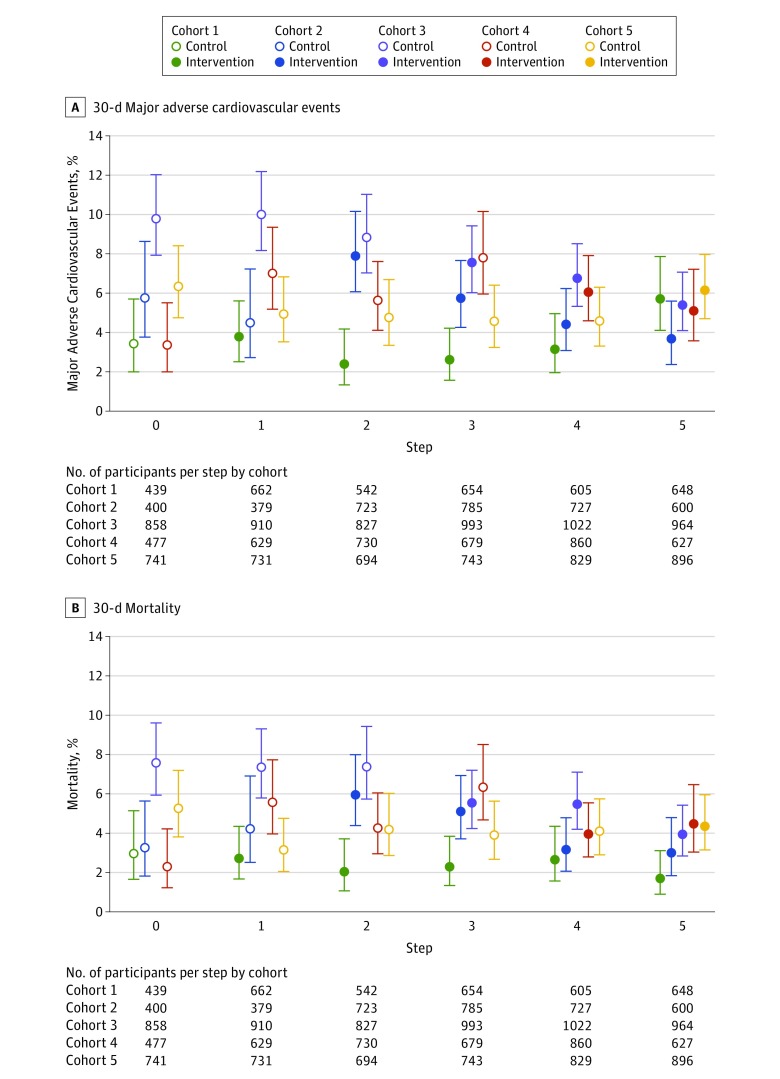
Table 3 shows the cluster-adjusted and cluster- and temporal-adjusted primary and secondary trial outcome event rates using mixed-effects logistic regression models that account for within-hospital clustering and temporal trends. The unadjusted rate of 30-day major adverse cardiovascular events was 5.3% in the intervention group compared with 6.4% in the control group. Figure 2A shows the temporal trends for 30-day major adverse cardiovascular events by group and step. The cluster-adjusted OR for 30-day major adverse cardiovascular events was 0.92 (95% CI, 0.81-1.04), which was not statistically significant after further adjustment for temporal trends (adjusted risk difference, −0.09% [95% CI, −1.32% to 1.14%]; adjusted OR, 0.98 [95% CI, 0.80-1.21]). Results were similar after multiple imputation of data from individuals who had missing follow-up data or were lost to follow-up. After sensitivity analyses of hypothetical scenarios to account for the potential effects of missing follow-up events and using multiple imputation, the range of results varied more broadly, but overall plausible results were consistent with the reported primary analysis.
Table 3. Unadjusted and Adjusted Primary and Secondary Trial Outcomes Using Mixed-Effects Logistic Regression Models That Account for Within-Hospital Clustering and Clustering and Temporal Trends.
| Outcomes | No. (%) | Cluster-Adjusted Difference, % (95% CI)a | Cluster-Adjusted Odds Ratio (95% CI)a | Primary Analysis Difference, % (95% CI)a | Primary Analysis Odds Ratio (95% CI)a | ICC | |
|---|---|---|---|---|---|---|---|
| Control (n = 10 066) | Intervention (n = 11 308) | ||||||
| Primary outcome | |||||||
| 30-d MACE | 645 (6.4) | 602 (5.3) | −0.51 (−1.28 to 0.26) | 0.92 (0.81-1.04) | −0.09 (−1.32 to 1.14) | 0.98 (0.80-1.21) | 0.15 |
| Secondary outcomes | |||||||
| 30-d mortality | 509 (5.1) | 445 (3.9) | −0.65 (−1.34 to 0.03) | 0.87 (0.75-1.00) | −0.28 (−1.35 to 0.80) | 0.94 (0.74-1.19) | 0.18 |
| 30-d cardiovascular mortality | 494 (4.9) | 434 (3.8) | −0.58 (−1.24 to 0.09) | 0.88 (0.76-1.02) | −0.26 (−1.31 to 0.80) | 0.94 (0.74-1.20) | 0.19 |
| In-hospital mortality | 331 (3.3) | 321 (2.8) | −0.05 (−0.58 to 0.47) | 0.98 (0.82-1.17) | −0.23 (−1.07 to 0.60) | 0.93 (0.70-1.22) | 0.18 |
| 30-d reinfarction | 121 (1.2) | 135 (1.2) | 0.12 (−0.31 to 0.55) | 1.08 (0.82-1.42) | 0.50 (−0.24 to 1.24) | 1.39 (0.87-2.22) | 0.33 |
| 30-d stroke | 60 (0.6) | 90 (0.8) | 0.20 (−0.05 to 0.45) | 1.34 (0.94-1.93) | 0.14 (−0.23 to 0.52) | 1.24 (0.71-2.15) | 0.14 |
| 30-d major GUSTO bleedingb | 19 (0.2) | 30 (0.3) | 0.13 (−0.05 to 0.30) | 1.56 (0.84-2.88) | 0.23 (−0.05 to 0.52) | 2.34 (0.93-5.89) | 0.20 |
| Optimal in-hospital medicationc | 3122 (31.7) | 3878 (35.8) | 8.61 (6.98 to 10.23) | 1.70 (1.57-1.85) | 6.00 (3.90 to 8.11) | 1.45 (1.28-1.64) | 0.40 |
| Optimal discharge medicationd | 5454 (61.8) | 6483 (64.0) | 9.97 (8.32 to 11.61) | 1.73 (1.59-1.87) | 8.66 (6.30 to 11.03) | 1.61 (1.42-1.82) | 0.28 |
| Tobacco cessation advicee | 3526 (96.0) | 2618 (94.7) | 0.30 (−1.20 to 1.80) | 1.06 (0.80-1.39) | 0.30 (−2.15 to 2.76) | 1.06 (0.67-1.67) | 0.37 |
Abbreviations: ICC, intracluster correlation; MACE; major adverse cardiovascular events, defined as death, reinfarction, stroke, and major GUSTO bleeding.
Odds ratios represent effect of intervention compared with control and are calculated as the difference in marginal effects (intervention group minus control group) in a mixed-effects logistic regression model including a random-effects term to account for within-hospital clustering. Primary analysis additionally accounted for temporal trends.
Major bleeding is defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) criteria, which is defined by intracerebral hemorrhage or bleeding resulting in substantial hemodynamic compromise requiring treatment.
Composed of aspirin, adenosine diphosphate receptor antagonist (clopidogrel, prasugrel, or ticagrelor), anticoagulant, and β-blocker among patients eligible to receive all medications.
Composed of aspirin, adenosine diphosphate receptor antagonist (clopidogrel, prasugrel, or ticagrelor), statin, and β-blocker among discharged patients eligible to receive all medications.
Among discharged patients who reported smoking at baseline.
Figure 2. Unadjusted Temporal Trends in 30-Day Major Adverse Cardiovascular Event Rates and Mortality Rates.
Open circles indicate rates during the control period; solid circles represent rates during the intervention period. Error bars indicate 95% CIs.
Secondary and Post Hoc Analyses
The rate of 30-day death was 3.9% in the intervention group compared with 5.1% in the control group. Figure 2B shows the temporal trends for 30-day death by group and step. The cluster-adjusted OR for 30-day death was 0.87 (95% CI, 0.75-1.00), an effect that was not statistically significant after adjusting for temporal trends (adjusted risk difference, −0.28% [95% CI, −1.35% to 0.80%]; adjusted OR, 0.94 [95% CI, 0.74-1.19]). These results did not materially change after adjustment for GRACE score covariates (eTable 5 in Supplement 3). In-hospital outcomes demonstrated a similar pattern overall. We also tested post hoc for a potential interaction for length of time exposed to the intervention to evaluate whether the intervention might have been more effective if given over a longer period, and the overall effects were similar (eTable 6 in Supplement 3).
In a post hoc analysis, we evaluated the effect of the intervention on the expanded outcome of 30-day major adverse cardiovascular event plus incident in-hospital heart failure, cardiogenic shock, or cardiac arrest (eTable 7 in Supplement 3). The unadjusted rate was 7.0% in the intervention group and 9.1% in the control group. The cluster-adjusted OR for this outcome was 0.89 (95% CI, 0.80-1.00), which was similar after temporal adjustment (adjusted risk difference, −1.34% [95% CI, −2.72% to 0.04%]; adjusted OR, 0.84 [95% CI, 0.70-1.00]).
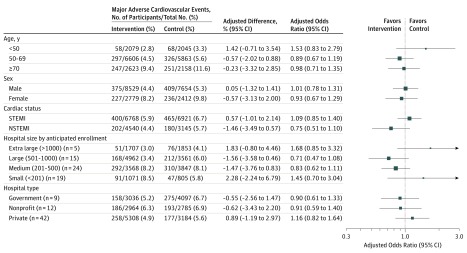
Figure 3 shows the effect of the intervention on the rate of 30-day major adverse cardiovascular events by prespecified subgroups, including age, sex, STEMI vs NSTEMI, hospital size, and hospital type. We found a similar pattern of effect across subgroups. eFigure 1A in Supplement 3 shows the heterogeneity of the primary outcome by hospital through a residual plot, which evaluates the event rate by site compared with the mean event rate throughout the entire trial. eFigure 1B in Supplement 3 shows the unadjusted within-hospital difference in the rate of 30-day composite major adverse cardiovascular events between the intervention and control groups. eFigures 2A and 2B in Supplement 3 show temporal changes in the primary outcome and 30-day mortality rates within and among hospitals.
Figure 3. Effect of Quality Improvement Tool Kit Intervention on 30-Day Major Adverse Cardiovascular Events by Prespecified Subgroups After Adjustment for Within-Hospital Clustering and Temporal Trends.
NSTEMI indicates non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. Size of the data markers indicates precision of the point estimates.
Discussion
The use of a locally adapted quality improvement tool kit did not reduce the primary outcome of death, reinfarction, stroke, or major bleeding at 30 days among patients presenting with acute myocardial infarction to Kerala hospitals. Major adverse cardiovascular event rates were lower than previously estimated from Kerala, and in-hospital and discharge treatment rates were higher, which may have influenced the results. Symptom-to-door times were also shorter than in previous reports from India. Hospitals’ previous participation in the Kerala ACS Registry or contamination between intervention and control groups may have also influenced these results through improved care throughout the state over the study period. However, the intervention may not have been effective because of insufficient training, implementation or adoption of the intervention, or period of exposure to sufficiently change hospital practice, including among process measures such as speed of reperfusion therapy. Although the absolute event rate was lower in the intervention group, the stepped-wedge trial design demonstrates the importance of accounting for temporal trends for this type of intervention.
The intervention led to lower 30-day major adverse cardiovascular event and mortality rates in the first step compared with other steps. Potential reasons for this observation include (1) smaller sample size and instability of the estimate; (2) lack of contamination; (3) baseline participant- or hospital-level characteristics; or (4) chance. The intervention led to a higher rate of in-hospital use of reperfusion, thrombolytic therapy, and anticoagulation and a higher rate of discharge aspirin, β-blocker, statin, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker prescriptions. A post hoc analysis demonstrated that the intervention led to a lower rate of expanded major adverse cardiovascular events, which also included in-hospital heart failure, cardiogenic shock, and cardiac arrest, but other secondary clinical outcomes were not different between groups. It is possible that these post hoc outcome findings were driven by the improvements in medication use, including reperfusion, but they may also be due to recruitment or detection bias or chance and should be considered only hypothesis generating.
The primary outcome results were consistent across prespecified individual- and hospital-level subgroups despite heterogeneity of effect among hospitals. This heterogeneity may have been driven by site-level personnel, intervention fidelity, recruitment or detection bias, or chance but requires future research.
Previous cluster randomized trials of acute coronary syndrome quality improvement in low- and middle-income countries, including the Brazilian Intervention to Increase Evidence Usage in Acute Coronary Syndromes (BRIDGE-ACS), and Clinical Pathways for Acute Coronary Syndrome-Phase 2 (CPACS-2), demonstrated improvements in composite medication use and reperfusion among patients with STEMI. These smaller trials did not demonstrate statistically significant improvements in clinical outcomes but were also not powered to detect such differences. However, these trials also demonstrated lower intraclass correlation coefficients than in the present study and did not use a stepped-wedge design to account for temporal trends. The recently completed Clinical Pathways for Acute Coronary Syndrome–Phase 3 (CPACS-3) trial enrolled more than 29 000 Chinese participants using a cluster randomized, stepped-wedge design may be helpful for understanding the effects of quality improvement interventions in low- and middle-income country settings. Results from the present trial contrast with the favorable temporal trends in clinical outcomes that have been demonstrated in nonrandomized quality improvement and health system intervention studies, including a 2017 nonrandomized trial designed to increase primary percutaneous coronary intervention use in Tamil Nadu. These differences may be related to study design, intervention targets, components, training and implementation, comparator group outcomes, or a combination thereof. Event rates in this trial were similar to outcomes reported from the American College of Cardiology/American Heart Association Acute Coronary Treatment and Intervention Outcomes Network–Get With the Guidelines (ACTION-GWTG) program in 2014 (in-hospital mortality, 6.4% for STEMI and 3.4% for NSTEMI). However, there are important gaps in guideline-directed care that remain in acute myocardial infarction care in Kerala, including rate and speed of reperfusion, and likely gaps in other acute cardiovascular conditions (eg, stroke, heart failure), other states in India, and other low- and middle-income countries that warrant further study.
This study has several strengths. First, this trial built on previous observational data from the Kerala Acute Coronary Syndrome Registry and collaborated with the Cardiological Society of India–Kerala Chapter to execute the largest randomized cardiovascular intervention trial in India to date. Second, this study used a trial design with appropriate statistical methods to improve internal validity of the results. Third, this trial used advanced yet low-cost trial monitoring procedures, including central statistical, risk-based monitoring, to capture high-quality data from many sites that had not previously participated in clinical trials.
Limitations
This study has several limitations. First, the complex intervention included several evidence-based components (eg, audit and feedback to improve process measures, checklists to reduce errors) that may not have been fully implemented at all sites or for a sufficient duration to improve clinical outcomes. Understanding the external and internal conditions that were associated with improved intervention implementation and outcomes requires further research. Second, the trial was susceptible to recruitment bias because, while randomization occurred at the cluster level, informed consent was required from individual participants for 30-day follow-up. However, baseline differences in key covariates associated with the primary outcome were limited. Third, these results demonstrated higher-than-anticipated intraclass correlation coefficients than previous trials and pretrial estimates, which reduced statistical power. However, it seems unlikely that this would have materially changed the primary outcome estimate. Fourth, quality of clinical care was higher and event rates were lower than anticipated in the trial. It is uncertain whether such an intervention would be effective in an environment where the quality of care is lower or the event rates are higher. Fifth, secondary analyses were not adjusted for multiple comparisons, which increases the possibility of type I error.
Conclusions
Among patients with acute myocardial infarction in Kerala, India, use of a quality improvement intervention compared with usual care did not decrease a composite of 30-day major adverse cardiovascular events. Further research is needed to understand the lack of efficacy.
Trial Protocol
Statistical Analysis Plan
eAppendix. ACS QUIK Toolkit: Sample Audit and Feedback Report and Admission and Discharge Checklists
eTable 1. Baseline Characteristics by Included Participants and Participants Missing Follow-up
eTable 2. Baseline Characteristics by Intervention and Control Group, Adjusted for Within-Hospital Clustering and Temporal Trends With 95% Confidence Intervals
eTable 3. Baseline Characteristics in ACS QUIK Participants by Step and Intervention and Control Group
eTable 4. Baseline Characteristics in ACS QUIK Participants by Step
eTable 5. Primary and Secondary Outcomes Adjusted for Clustering and Temporal Trends as well as for GRACE Score Covariates, Transfer Status, Insurance Status, or Use of Pre-hospital Aspirin
eTable 6. Primary and Secondary Outcomes Adjusted for Clustering and Temporal Trends and Interaction Effect for Time Exposed to the Intervention
eTable 7. Exploratory Analyses Evaluating the Effect of the Intervention on Major Adverse Cardiovascular Events Plus In-Hospital Incident Heart Failure, Cardiogenic Shock, and Cardiac Arrest Using Mixed Effect Logistic Regression Models That Account for Within-Hospital Clustering and Clustering and Temporal Trends
eFigure 1A. Residual of 30-Day Major Adverse Cardiovascular Event Rate (Expected – Observed) by Individual Hospitals
eFigure 1B. Waterfall Plot Showing Unadjusted Within-Hospital Differences in the Rate of 30-Day Composite Major Adverse Cardiovascular Events Between the Intervention and Control Groups
eFigure 2A. Unadjusted Temporal Trends in 30-Day Major Adverse Cardiovascular Event Rates With 95% Confidence Intervals by Hospital, Cohort, Intervention and Control Group, and Step During the Trial
eFigure 2B. Unadjusted Temporal Trends in 30-Day Death Rates With 95% Confidence Intervals by Hospital, Cohort, Intervention and Control Group, and Step During the Trial
References
- 1.Roth GA, Johnson C, Abajobir A, et al. . Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MG, Fonarow GC, Peterson ED, et al. . Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines-Coronary Artery Disease program. Circulation. 2010;121(21):2294-2301. [DOI] [PubMed] [Google Scholar]
- 3.Buckley GJ, Pittluck RE; Board on Global Health, Institute of Medicine, National Academies of Sciences, Engineering, and Medicine Improving Quality of Care in Low- and Middle-Income Countries. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 4.Mohanan PP, Mathew R, Harikrishnan S, et al. ; Kerala ACS Registry Investigators . Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34(2):121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huffman MD, Prabhakaran D, Abraham AK, Krishnan MN, Nambiar AC, Mohanan PP; Kerala Acute Coronary Syndrome Registry Investigators . Optimal in-hospital and discharge medical therapy in acute coronary syndromes in Kerala: results from the Kerala Acute Coronary Syndrome Registry. Circ Cardiovasc Qual Outcomes. 2013;6(4):436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivers N, Jamtvedt G, Flottorp S, et al. . Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotter T, Kinsman L, James E, et al. . Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):CD006632. [DOI] [PubMed] [Google Scholar]
- 8.Ko HC, Turner TJ, Finnigan MA. Systematic review of safety checklists for use by medical care teams in acute hospital settings—limited evidence of effectiveness. BMC Health Serv Res. 2011;11(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berwanger O, Guimarães HP, Laranjeira LN, et al. ; BRIDGE-ACS . A multifaceted intervention to narrow the evidence-based gap in the treatment of acute coronary syndromes: rationale and design of the Brazilian Intervention to Increase Evidence Usage in Acute Coronary Syndromes (BRIDGE-ACS) cluster-randomized trial. Am Heart J. 2012;163(3):323-329. [DOI] [PubMed] [Google Scholar]
- 10.Du X, Gao R, Turnbull F, et al. ; CPACS Investigators . Hospital quality improvement initiative for patients with acute coronary syndromes in China: a cluster randomized, controlled trial. Circ Cardiovasc Qual Outcomes. 2014;7(2):217-226. [DOI] [PubMed] [Google Scholar]
- 11.Alexander T, Mullasari AS, Joseph G, et al. . A system of care for patients with ST-segment elevation myocardial infarction in India: the Tamil Nadu-ST-Segment Elevation Myocardial Infarction Program. JAMA Cardiol. 2017;2(5):498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huffman MD, Mohanan PP, Devarajan R, et al. . Acute coronary syndrome quality improvement in Kerala (ACS QUIK): rationale and design for a cluster-randomized stepped-wedge trial. Am Heart J. 2017;185:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Brindis RG, Chaitman BR, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards; American College of Emergency Physicians; Emergency Nurses Association; National Association of Emergency Medical Technicians; National Association of EMS Physicians; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Cardiovascular Patient Care; Society of Thoracic Surgeons . 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards). Circulation. 2013;127(9):1052-1089. [DOI] [PubMed] [Google Scholar]
- 15.Moore GF, Audrey S, Barker M, et al. . Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GUSTO Investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673-682. [DOI] [PubMed] [Google Scholar]
- 17.Wood L, Egger M, Gluud LL, et al. . Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkwood AA, Cox T, Hackshaw A. Application of methods for central statistical monitoring in clinical trials. Clin Trials. 2013;10(5):783-806. [DOI] [PubMed] [Google Scholar]
- 19.Berwanger O, Guimarães HP, Laranjeira LN, et al. ; Bridge-ACS Investigators . Effect of a multifaceted intervention on use of evidence-based therapies in patients with acute coronary syndromes in Brazil: the BRIDGE-ACS randomized trial. JAMA. 2012;307(19):2041-2049. [DOI] [PubMed] [Google Scholar]
- 20.Peterson ED, Roe MT, Mulgund J, et al. . Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295(16):1912-1920. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, Goldberg RJ, Dabbous O, et al. ; Global Registry of Acute Coronary Events Investigators . Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163(19):2345-2353. [DOI] [PubMed] [Google Scholar]
- 22.Hemming K, Girling A. A menu-driven facility for power and detectable-difference calculations in stepped-wedge cluster-randomized trials. Stata J. 2014;14(2):363-380. [Google Scholar]
- 23.Welty LJ, Rasmussen LV, Baldridge AS, et al. . StatTag Chicago, IL: Galter Health Sciences Library; 2016. doi: 10.18131/G36K76 [DOI]
- 24.Xavier D, Pais P, Devereaux PJ, et al. ; CREATE Registry Investigators . Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371(9622):1435-1442. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Wu Y, Du X, et al. ; CPACS-3 Investigators . Rational and design of a stepped-wedge cluster randomized trial evaluating quality improvement initiative for reducing cardiovascular events among patients with acute coronary syndromes in resource-constrained hospitals in China. Am Heart J. 2015;169(3):349-355. [DOI] [PubMed] [Google Scholar]
- 26.Masoudi FA, Ponirakis A, de Lemos JA, et al. . Trends in US cardiovascular care: 2016 report from 4 ACC national cardiovascular data registries. J Am Coll Cardiol. 2017;69(11):1427-1450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix. ACS QUIK Toolkit: Sample Audit and Feedback Report and Admission and Discharge Checklists
eTable 1. Baseline Characteristics by Included Participants and Participants Missing Follow-up
eTable 2. Baseline Characteristics by Intervention and Control Group, Adjusted for Within-Hospital Clustering and Temporal Trends With 95% Confidence Intervals
eTable 3. Baseline Characteristics in ACS QUIK Participants by Step and Intervention and Control Group
eTable 4. Baseline Characteristics in ACS QUIK Participants by Step
eTable 5. Primary and Secondary Outcomes Adjusted for Clustering and Temporal Trends as well as for GRACE Score Covariates, Transfer Status, Insurance Status, or Use of Pre-hospital Aspirin
eTable 6. Primary and Secondary Outcomes Adjusted for Clustering and Temporal Trends and Interaction Effect for Time Exposed to the Intervention
eTable 7. Exploratory Analyses Evaluating the Effect of the Intervention on Major Adverse Cardiovascular Events Plus In-Hospital Incident Heart Failure, Cardiogenic Shock, and Cardiac Arrest Using Mixed Effect Logistic Regression Models That Account for Within-Hospital Clustering and Clustering and Temporal Trends
eFigure 1A. Residual of 30-Day Major Adverse Cardiovascular Event Rate (Expected – Observed) by Individual Hospitals
eFigure 1B. Waterfall Plot Showing Unadjusted Within-Hospital Differences in the Rate of 30-Day Composite Major Adverse Cardiovascular Events Between the Intervention and Control Groups
eFigure 2A. Unadjusted Temporal Trends in 30-Day Major Adverse Cardiovascular Event Rates With 95% Confidence Intervals by Hospital, Cohort, Intervention and Control Group, and Step During the Trial
eFigure 2B. Unadjusted Temporal Trends in 30-Day Death Rates With 95% Confidence Intervals by Hospital, Cohort, Intervention and Control Group, and Step During the Trial