This population-based cohort and Norwegian biobank study examines the association of folic acid supplements and maternal folate concentration in pregnancy with the frequency and degree of autistic traits in children exposed to antiepileptic drugs in utero.
Key Points
Question
Is folic acid supplementation associated with reduced risk of autistic traits in children exposed to antiepileptic drugs in utero?
Findings
This population-based cohort and Norwegian biobank study that included 104 946 children found that children exposed to antiepileptic drugs in utero had a significantly lower risk of autistic traits if the mother used periconceptional folic acid supplements. Maternal plasma folate concentration in gestational weeks 17 to 19 was inversely associated with the degree of autistic traits.
Meaning
Risk of autistic traits in children exposed to antiepileptic drugs in utero may be mitigated by periconceptional folic acid supplementation and folate status.
Abstract
Importance
Strategies to prevent autism in children exposed to antiepileptic drugs (AEDs) during pregnancy are important.
Objective
To explore whether folic acid supplementation and folate status in pregnancy are associated with reduced risk of autistic traits owing to in utero AED exposure.
Design, Setting, and Participants
The population-based, prospective Norwegian Mother and Child Cohort Study approached Norwegian-speaking women attending routine ultrasonographic examinations from June 1999 through December 31, 2008 (163 844 of 277 702 women refused). No exclusion criteria were applied beyond language. Questionnaires during and after pregnancy, analysis of blood samples, and linkage to the Medical Birth Registry of Norway were performed. Children aged 18 to 36 months of women with available information on use of AEDs and of folic acid supplementation (n = 104 946) were included in the analysis from March 1, 2016, through June 13, 2017.
Exposures
Maternal folic acid supplementation 4 weeks before to 12 weeks after conception. Plasma folate concentration was analyzed at gestational weeks 17 to 19.
Main Outcomes and Measures
Autistic traits were evaluated using the Modified Checklist for Autism in Toddlers and Social Communication Questionnaire. Odds ratios (ORs) for autistic traits in children by maternal use vs nonuse of folic acid supplements were adjusted for maternal health and socioeconomic factors. Folate concentrations and folic acid doses were associated with the degree of autistic traits.
Results
The overall mean (SD) age of the 104 946 mothers of participating children was 29.8 (4.6) years, with complete information available for analysis in 103 868. Mean (SD) age of women with epilepsy who received AED treatment was 29.4 (4.9); women with epilepsy who did not receive AED treatment, 29.1 (4.9); and without epilepsy, 29.8 (4.6) years. In the 335 children exposed to AEDs, the risk for autistic traits was significantly higher at 18 months of age (adjusted OR [AOR], 5.9; 95% CI, 2.2-15.8) and 36 months of age (AOR, 7.9; 95% CI, 2.5-24.9) when their mothers had not used folic acid supplements compared with children of mothers who had used supplements. Among women without epilepsy, the corresponding risks were lower at 18 months of age (AOR, 1.3; 95% CI, 1.2-1.4) and 36 months of age (AOR, 1.7; 95% CI, 1.5-1.9); among the 389 children of women with untreated epilepsy, the corresponding risks were not significant at 18 months of age (AOR, 1.0; 95% CI, 0.4-3.0) and 36 months of age (AOR, 2.5; 95% CI, 0.4-16.6). Degree of autistic traits was inversely associated with maternal plasma folate concentrations (β = −0.3; P = .03) and folic acid doses (β = −0.5; P < .001). Concentrations of AEDs were not associated with the degree of autistic traits.
Conclusions and Relevance
Risk of autistic traits in children exposed to AEDs in utero may be mitigated by periconceptional folic acid supplementation and folate status. Fertile women using AEDs should take folic acid supplements continuously.
Introduction
Children exposed to antiepileptic drugs (AEDs) during pregnancy have an increased risk of autistic traits.1,2,3,4,5 Autistic traits include impairment of social skills and communication as well as stereotyped or repetitive behavior or interests.6 These traits represent the core symptoms in autism spectrum disorders and often coexist with other neuropsychiatric diseases. Embryotoxic mechanisms and gene-environmental interactions are probably essential causes of autism.7,8
Genetic polymorphisms in folate metabolic pathways are associated with autism.9 In the general population, periconceptional folic acid supplements reduce the risk of autism spectrum disorders.10,11 However, to our knowledge, no previous studies have investigated whether this reduced risk also applies to children of women using AEDs. Several AEDs interfere with folate absorption and metabolism.12,13 Thus, the risk of folate deficiency in pregnancy is higher among women with epilepsy than among healthy women.14 Low-dose folic acid supplements (0.4 mg/d) in the periconceptional period reduce the risk of spina bifida in the general population15 and are recommended for all women.16 High-dose folic acid supplements (5.0 mg/d) are recommended before pregnancy in female AED users,17 but the effect of such treatment is uncertain.12 For women in general, folic acid supplementation with doses greater than 5.0 mg/d had a negative effect on child psychomotor development.18 In mothers using AEDs, use of folic acid supplements has been associated not only with a higher rate of congenital malformations19,20 but also with higher child IQ.21 The aim of this study was to test the a priori hypothesis that folic acid supplements and maternal folate concentration in pregnancy are associated with modified frequency and degree of autistic traits in children exposed to AEDs in utero.
Methods
Study Population
The Norwegian Mother and Child Cohort Study (MoBa) is a prospective, population-based, long-term cohort study conducted by the Norwegian Institute of Public Health.22 From June 1999 through December 31, 2008, most pregnant women in Norway received postal invitations, with a participation rate of 41% (95 267 women).22 No exclusion criteria were applied except inability to read Norwegian. Follow-up conducted by regular questionnaires is ongoing. The mothers completed questionnaires during gestational weeks 17 to 19 (Q1) and 30 (Q2) and at child ages 18 months (Q3) (response rate, 72.8%) and 36 months (Q4) (response rate, 56.1%) and an epilepsy-specific follow-up questionnaire in 2013 (Q5). The present study is based on version 8 of the quality-assured data files of the MoBa. The study was approved by the Norwegian Data Inspectorate and the Regional Committee for Medical Research Ethics. Written informed consent was obtained from the mothers.
Supplementary clinical information was obtained through a database linkage to the mandatory Medical Birth Registry of Norway.23 Diagnostic information, including generalized tonic-clonic seizures during pregnancy, AED dose, and folic acid dose, was obtained from Q5. Seizure occurrence during pregnancy reported by the women was confirmed by hospital records in 75% of the cases, and seizure-free status was confirmed in 89%.24
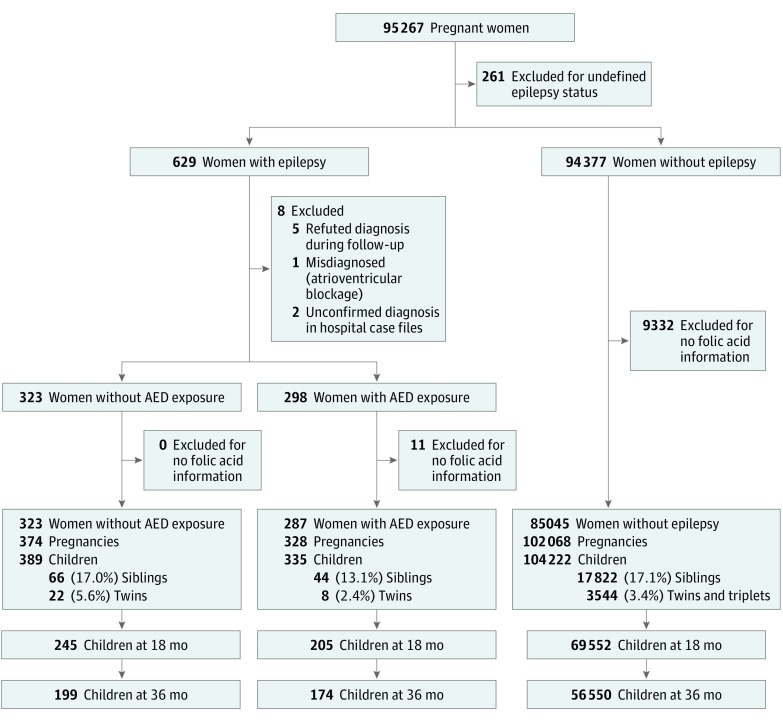
We included children for whom information concerning their mothers’ use of folic acid supplements and/or plasma folate concentration during pregnancy was available (90.2%). Our study group included children of women with reported epilepsy and AED use during pregnancy (n = 335) (Figure 1). The MoBa epilepsy cohort has been validated and described previously.24 With use of the International League Against Epilepsy criteria,25 our hospital case file review in a geographical subsample (7.5%) confirmed the diagnosis in 100% of women with reported epilepsy and AED use. Plasma concentration analyses confirmed AED use as reported by 238 of 256 mothers, yielding a positive predictive value for reported AED use of 93.0%. All children of mothers without reported epilepsy served as a reference group (n = 104 222). Children of women with reported epilepsy but no AED intake during pregnancy served as an internal control group (n = 389) (Figure 1). Blood samples in this group were not analyzed for AEDs, but none of the patients had any AED use registered in the Medical Birth Registry of Norway. In this group, 127 (70.9%) reported inactive epilepsy defined as not using AEDs during the 2 years before conception or no seizures during the previous 5 years.
Figure 1. Flowchart of Included and Excluded Cases.
AED indicates antiepileptic drug.
AED Use
Based on information from the MoBa and the Medical Birth Registry of Norway, we identified 268 children exposed to AED monotherapy (lamotrigine [n = 104], carbamazepine [n = 70], valproate sodium [n = 40], levetiracetam [n = 16], topiramate [n = 10], oxcarbazepine [n = 9], clonazepam [n = 7], phenytoin or phenytoin sodium [n = 4], phenobarbital [n = 4], gabapentin [n = 2], primidone [n = 1], and clobazam [n = 1]) and 65 exposed to polytherapy (19.5%) (eMethods 1 in the Supplement). Valproate was included in 19 combinations (29.2%). In 2 cases included in the analyses, the AED regime was unspecified.
Folic Acid Supplementation
Folic acid supplement use before conception and in gestational weeks 0 to 4, 5 to 8, 9 to 12, and 13 or more was reported in Q1 and at gestational weeks 13 to 16, 17 to 20, 22 to 24, 25 to 28, and 29 or more in Q2. Periconceptional intake was defined as use of a folic acid supplement from 4 weeks before to 12 weeks after conception. Use of high-dose folic acid (>0.4 mg/d) was reported by 84 of 139 mothers with epilepsy taking AEDs (60.4%). In this group, 17 (20.2%) used polytherapy, 4 (4.8%) including valproate (eMethods 2 in the Supplement).
Laboratory Analyses
Blood samples were obtained during gestational weeks 17 to 19 and from the umbilical cord immediately after delivery and sent to the MoBa Biobank (eMethods 3 in the Supplement).26 In women with epilepsy using AEDs (n = 229), the plasma samples from gestational weeks 17 to 19 were analyzed for the biologically active 5-methyltetrahydrofolate (mTHF), the degradation products 4-α-hydroxy-mTHF (hmTHF), p-aminobenzoylglutamate (pABG), p-acetamidobenzoylglutamate (apABG), and unmetabolized folic acid by liquid chromatography tandem mass spectrometry (eMethods 3 in the Supplement).27 The prevailing folate form in plasma is mTHF, which is not stable at room temperature but can be largely recovered as hmTHF.28 Plasma folate concentrations are therefore given as the sum of mTHF and hmTHF levels.
The maternal samples from gestational weeks 17 to 19 and the umbilical cord plasma samples (n = 198) were analyzed for AED concentrations and calculated as mean levels, as described elsewhere.24 In short, carbamazepine, carbamazepine-10, 11-epoxide, the oxcarbazepine monohydroxy derivative metabolite, lamotrigine, levetiracetam, and topiramate were analyzed with liquid chromatography mass spectrometry. Valproate was analyzed by a commercially available kit (Cobas Integra 400 Plus System; Roche Diagnostics).
Outcome Measurements
Autistic traits in the children were investigated with the Modified Checklist for Autism in Toddlers (M-CHAT) at 18 months of age and the Social Communication Questionnaire (SCQ) at 36 months of age, as previously reported.5 The instruments are based on parent-reported measures focusing on behavior that deviates from normal social development (eTables 1 and 2 in the Supplement). Children aged 16 to 30 months who missed any 3 of 23 items or 2 of 6 critical items in the M-CHAT are at risk of having autism spectrum disorders.29 For estimating autism spectrum disorders at 10 years of age using the M-CHAT administered in the same age group and with the same criteria as in our study, the sensitivity was 0.52 (95% CI, 0.38-0.65); specificity, 0.84 (95% CI, 0.81-0.87); negative predictive value, 0.96 (95% CI, 0.94-0.97); and positive predictive value, 0.20 (95% CI, 0.14-0.27).30 The SCQ produces a total score of 0 to 39 for verbal children and 0 to 33 for nonverbal children.39 A score of 13 or more was defined as having autistic traits.29 The agreement between the M-CHAT and the SCQ was moderate (Cohen κ coefficient, 0.54; 95% CI, 0.39-0.69).29,43 The estimation-maximization procedure in SPSS Statistics software (version 21; IBM, Inc) was used to impute missing values. Children with more than 4 missing items in SCQ (854 [1.5%]) and M-CHAT (5040 [7.2%]) were excluded.
Covariates
The following covariates in the logistic regression analysis were chosen based on relevance for development of autism spectrum disorders8,31,32 and were reported during pregnancy (Q1): maternal age, parental socioeconomic status (single mother, low educational attainment [≤9 years], low household income [<€42 404/y, or US $49 336]), parity (prior pregnancies past 21 gestational weeks), smoking (any), alcohol use (any), maternal depressive symptoms (mean score on the Hopkins Symptom Checklist, >1.75),33 and AED polytherapy. Linear regression models also included AED plasma concentration and number of generalized tonic-clonic seizures during pregnancy. The plasma concentrations and doses were normalized relative to the ranges observed within each drug group according to the formula 100 × [(observed value − minimum value)/(maximum value − minimum value)].34
Statistical Analysis
Data were analyzed from May 1, 2016, through June 13, 2017. We used SPSS Statistics software for statistical analysis. Two-sided tests were performed, with P < .05 regarded as statistically significant. Group differences for continuous variables were investigated with Mann-Whitney test (2-tailed unpaired t test for normally distributed variables). Categorical variables were compared using the Pearson χ2 test, with the Fisher exact test used if any cross-table cell had an expected count of less than 5. Risk estimates were calculated as odds ratios (ORs) with 95% CIs using logistic multivariable regression analysis. The primary measures of association were the crude OR and the adjusted OR (AOR) for autistic traits in children of women with vs without periconceptional use of folic acid supplements. Calculations were performed separately (stratified) for women with epilepsy and AED use during pregnancy, women with epilepsy and no AED use during pregnancy, and women without epilepsy. A second predefined key outcome was the dose-response association between maternal plasma concentrations of folate and the degree of autistic symptoms analyzed with multivariable linear regression analyses (mean SCQ score) and Spearman rank correlation coefficient (mean M-CHAT score). Residual plots were inspected to ensure that assumptions concerning normal distribution of the residuals were met.
Results
The overall mean (SD) maternal age of the 104 946 mothers of participating children was 29.8 (4.6) years. Complete demographic information was available for analysis in 103 868 mothers. Demographic and clinical information is reported in Table 1.
Table 1. Maternal Clinical Characteristicsa.
| Characteristics | Maternal Epilepsy With AED
Exposure (n = 328) |
Maternal Epilepsy Without
AED Exposure (n = 389) |
No Maternal
Epilepsy (n = 103 151) |
|||
|---|---|---|---|---|---|---|
| No
FAS (n = 68) |
FAS (n = 260) |
No FAS (n = 100) |
FAS (n = 289) |
No FAS (n = 25 222) |
FAS (n = 77 929) |
|
| Age, mean (SD), y | 29.4 (5.3) | 29.3 (4.8) | 28.6 (5.4) | 29.3 (4.8) | 29.4 (5.1) | 29.9 (4.4) |
| Parity, mean (SD), No.b | 1.9 (0.9) | 1.7 (0.9) | 1.9 (0.9) | 1.6 (0.9) | 1.9 (1.0) | 1.7 (0.8) |
| Smoking during pregnancyc | 11 (16.2) | 24 (9.3) | 20 (20.0) | 12 (4.2) | 3380 (13.4) | 4116 (5.3) |
| Alcohol use during pregnancyc | 6 (8.8) | 6 (2.3) | 4 (4.0) | 5 (1.7) | 804 (3.2) | 1867 (2.4) |
| No partner | 5 (7.4) | 10 (3.8) | 9 (9.0) | 8 (2.8) | 1002 (4.0) | 1421 (1.8) |
| Low educational attainmentd | 4 (5.9) | 8 (3.1) | 9 (9.0) | 12 (4.2) | 1476 (5.9) | 1352 (1.7) |
| Low incomee | 8 (13.0) | 27 (11.0) | 17 (19.1) | 18 (6.4) | 2342 (9.8) | 4126 (5.4) |
| Unplanned pregnancy | 23 (33.8) | 53 (20.6) | 33 (33.7) | 57 (19.7) | 6760 (26.8) | 12 974 (16.8) |
| Depression in pregnancyf | 11 (17.2) | 52 (21.0) | 17 (17.9) | 39 (13.7) | 3080 (12.7) | 7803 (10.2) |
| Polytherapy | 20 (29.4) | 45 (17.3) | NA | NA | NA | NA |
| Valproate sodium | 14 (20.6) | 42 (16.2) | NA | NA | NA | NA |
| Carbamazepine | 22 (32.4) | 68 (26.2) | NA | NA | NA | NA |
| Lamotrigine | 27 (39.7) | 108 (41.5) | NA | NA | NA | NA |
| Levetiracetam | 5 (7.4) | 30 (11.5) | NA | NA | NA | NA |
| Topiramate | 3 (4.4) | 16 (6.2) | NA | NA | NA | NA |
| Oxcarbazepine | 7 (10.3) | 17 (6.5) | NA | NA | NA | NA |
| Clonazepam | 3 (4.4) | 13 (5.0) | NA | NA | NA | NA |
| Focal epilepsy | 10 (33.3) | 27 (20.9) | 8 (18.2) | 23 (17.0) | NA | NA |
| Primary generalized epilepsy | 4 (13.3) | 24 (18.6) | 2 (4.5) | 7 (5.2) | NA | NA |
| GTC in pregnancy | 3 (10.0) | 17 (13.2) | 2 (5) | 3 (2) | NA | NA |
| AED dose in pregnancyg | 33.4 (27.0) | 36.2 (31.2) | NA | NA | NA | NA |
| AED concentration in second trimesterg | 48.8 (41.0) | 52.2 (44.2) | NA | NA | NA | NA |
Abbreviations: AED, antiepileptic drug; FAS, periconceptional folic acid supplementation; GTC, generalized tonic-clonic seizure; NA, not applicable.
Data are expressed as number (percentage) of women unless otherwise indicated. Denominators may vary within the groups owing to missing data.
Indicates number of prior pregnancies in the past 21 gestational weeks.
Indicates any.
Indicates 9 years or less.
Indicates €42 404/y or less or 400 000 kr/y or less (≤$49 336/y).
Indicates a mean score of greater than 1.75 on the Hopkins Symptom Checklist (range, 1-4; higher scores indicate depression).33
Indicates mean of standardized data.
Folic Acid Supplementation and Risk of Autistic Traits in the Offspring
Among children aged 18 months and exposed to AEDs, the AOR for autistic traits if the mother did not use folic acid supplements was 5.9 (95% CI, 2.2-15.8) compared with use of supplements (Table 2). The corresponding AOR for children aged 36 months was 7.9 (95% CI, 2.5-24.9). In women without epilepsy, the corresponding AORS were 1.3 (95% CI, 1.2-1.4) at 18 months of age and 1.7 (95% CI, 1.5-1.9) at 36 months of age, and the CIs did not overlap those in the AED group (Table 2). In children of mothers with untreated epilepsy not taking folic acid supplements compared with children of mothers who took supplements, no significant risk of autistic traits was found at 18 months of age (AOR, 1.0; 95% CI, 0.4-3.0) and 36 months of age (AOR, 2.5; 95% CI, 0.4-16.6).
Table 2. Risk of Autistic Traits in Association With FAS Stratified by Maternal AED Treatment.
| Trait or FAS Status | Maternal Epilepsy With AED Exposure | Maternal Epilepsy Without AED Exposure | No Maternal Epilepsy |
|---|---|---|---|
| Autistic traits, No. (%) without/with FAS | |||
| 18 moa | 11 (32.4)/15 (8.8) | 5 (8.9)/18 (9.5) | 1294 (8.9)/3723 (6.8) |
| 36 mob | 9 (25.7)/8 (5.8) | 2 (4.9)/4 (2.5) | 725 (6.2)/1665 (4.2) |
| OR (95% CI) for FAS vs no FAS by age | |||
| 18 mo | |||
| Crude | 5.0 (2.0-12.2) | 0.9 (0.3-2.6) | 1.4 (1.3-1.4) |
| Adjusted model 1c | 5.0 (2.0-12.3) | 0.9 (0.3-2.7) | 1.3 (1.2-1.4) |
| Adjusted model 2d | 6.2 (2.4-16.1) | 1.0 (0.4-3.0) | 1.3 (1.2-1.4) |
| Adjusted model 3e | 5.9 (2.2-15.8) | NA | NA |
| 36 mo | |||
| Crude | 5.7 (2.0-16.1) | 2.0 (0.4-11.2) | 1.7 (1.6-1.9) |
| Adjusted model 1c | 7.1 (2.4-21.2) | 2.0 (0.3-12.6) | 1.7 (1.6 - 1.9) |
| Adjusted model 2d | 7.6 (2.5-23.5) | 2.5 (0.4-16.6) | 1.7 (1.5-1.9) |
| Adjusted model 3e | 7.9 (2.5-24.9) | NA | NA |
Abbreviations: AED, antiepileptic drug; FAS, periconceptional folic acid supplementation; NA, not applicable; OR, odds ratio.
Evaluated using the Modified Checklist for Autism in Toddlers as failing any 3 of 23 items or any 2 of 6 critical items.
Evaluated using the Social Communication Questionnaire as a score of at least 13 (range, 0-39 for verbal children and 0-33 for nonverbal children).
Adjusted for maternal age, parental socioeconomic status (single mother, low educational attainment [≤9 y], low household income [≤€42 404/y or ≤399 999 kr/y; ≤$49 336/y]), parity (prior pregnancies in past 21 gestational weeks), smoking (any), alcohol use (number of units per month from conception to week 19).
Adjusted for all covariates in model 1 plus depressive symptoms (mean score >1.75 on the Hopkins Symptom Checklist; range, 1-5; higher scores indicate depression).33
Adjusted for all covariates in model 2 plus AED polytherapy.
Plasma Folate and Degree of Autistic Traits
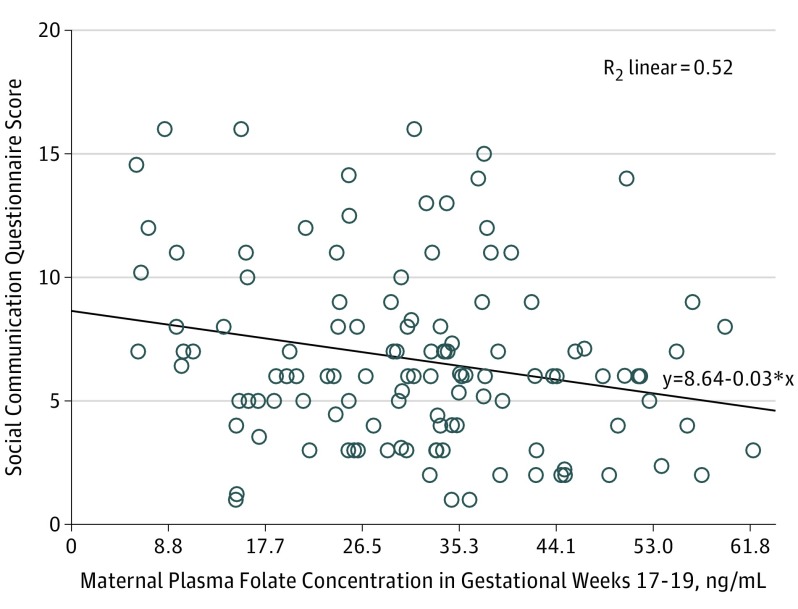
In AED-exposed children, an inverse association existed between mean SCQ score and maternal plasma folate concentration during gestational weeks 17 to 19 (Figure 2 and eTable 3 in the Supplement). The quartile with the lowest folate concentrations had a higher SCQ score than the quartile with the highest folate concentrations (eTable 4 in the Supplement). Ad hoc analyses adjusted for confounding factors similarly showed an inverse linear association between mean SCQ score and folic acid supplement dose (discrete variable according to intake as registered by the women: 0.4, 1.0, 2.0, or ≥4.0 mg/d) at all times during pregnancy. The association was most pronounced for first trimester folic acid supplementation (β = −0.45; P < .001) (eFigure 1 in the Supplement). Total M-CHAT score was associated with the folic acid supplement dose before pregnancy (ρ = −0.2; P = .01) and in the first trimester (ρ = −0.3; P = .004), but not with plasma folate concentration (eFigure 2 in the Supplement). No difference in median M-CHAT scores across the quartiles of folate concentrations was apparent. Antiepileptic drug concentration (eTable 5 and eFigure 3 in the Supplement), AED dose, number of generalized tonic-clonic seizures, unmetabolized folic acid concentration, and the concentration of the folate degradation products pABG and apABG were not associated with degree of autistic traits.
Figure 2. Scatterplot of the Association Between Maternal Plasma Folate Concentration and Child Outcome.
Data are shown for the Social Communication Questionnaire score (range, 0-39 for verbal children and 0-33 for nonverbal children, with a score of ≥13 defined as having autistic traits) of the child at 36 months of age vs the maternal plasma folate concentration during gestational weeks 17 to 19 among women who used antiepileptic drugs in pregnancy. To convert folate concentrations to nanomoles per liter, multiply by 2.266. Diagonal line indicates linear regression analysis.
Timing of Folic Acid Intake
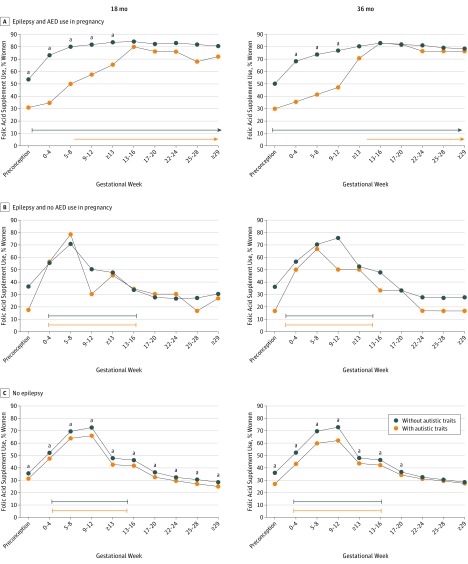
In the AED-exposed group, mothers of children with autistic traits were less likely to have used folic acid supplements in pregnancy weeks 0 to 12 than mothers of children without autistic traits (Figure 3). Frequency of mothers using folic acid supplements after week 12 was similar between groups. Median time for starting supplementation was gestational week 6.5 for mothers of children with autistic traits at 18 months of age and week 12.5 for those with autistic traits at 36 months of age. In contrast, mothers of children without autistic traits more frequently started folic acid supplementation before pregnancy (median, preconceptional week 1) compared with mothers of children with autistic traits at 18 months of age (median, 6.5 weeks; P = .007) and at 36 months of age (median, 12.5 weeks; P = .01). Median supplementation start time did not differ between mothers of children with and without autistic traits among those not using AEDs (Figure 3).
Figure 3. Gestational Timing of Folic Acid Supplement Intake by Child Outcome.
Percentages of folic acid supplement use in pregnancy in mothers giving birth to a child with and without autistic traits at 18 and 36 months of age. Median start and withdrawal times for supplementation are shown as lines under the graphs. Among mothers of antiepileptic drug (AED)–exposed children, mothers of children with autistic traits started folic acid supplementation significantly later (range, 16-31 weeks) than mothers of children without autistic features at 18 months of age (P = .007) and 36 months of age (P = .01).
aP < .05.
In a post hoc analysis, we subdivided the AED-exposed cohort into groups with autistic traits at 18 and 36 months of age (n = 4 [group 1]), autistic traits at one age but not the other (n = 35 [group 2]), or no autistic traits at either age (n = 184 [group 3]). In the AED-exposed group, the rate of maternal periconceptional folic acid use was 0 in group 1, 37.1% (13 mothers) in group 2, and 53.3% (98 mothers) in group 3; the median start of folic acid supplementation was gestational week 15 in group 1, gestational week 7 in group 2, and before conception in group 3 (eTable 6 in the Supplement).
Type of AED
The association of periconceptional folic acid supplement use with the risk of autistic traits in AED-exposed children appeared to exist across all AEDs (eFigure 4 in the Supplement). In the valproate subgroup (n = 38), 4 (12.9%) had autistic traits at 18 and/or 36 months if the mother used folic acid supplements, compared with 2 (28.6%) in the group with mothers who did not use supplements (P = .19). A post hoc analysis investigating the association of the interaction between AEDs and folic acid supplement intake with the number of autistic traits showed that this interaction was highly significant (B = −3.1; SE = 1.1; β = −0.42; P = .004) (eFigure 5 in the Supplement).
Discussion
Mothers using an AED during pregnancy had a 5 to 8 times increased risk of having a child with autistic traits if they did not use folic acid in the periconceptional period. Without periconceptional folic acid supplementation, 1 in 3 children exposed to AEDs had autistic traits at 18 and 36 months of age. Moreover, the maternal plasma concentration of folate during gestational weeks 17 to 18 was inversely associated with the degree of autistic traits at 3 years of age. For children who were not exposed to AEDs during pregnancy, the risk for autistic traits was only slightly increased if the mother did not use folic acid supplements in the periconceptional period. The association was present regardless of AED type.
The main finding of our study is in line with the results of an uncontrolled study of 105 children exposed to AEDs in pregnancy2 in whom lack of folic acid supplementation in the first trimester seemed to be associated with autistic traits. Our data demonstrate the critical importance of an early start of folic acid supplement intake. In the group of mothers with children developing autistic traits, fewer women had used folic acid supplements before and until week 13 of pregnancy compared with those with children without autistic traits. The proportion using folic acid later in pregnancy was similar between the 2 groups. The critical importance of folic acid supplementation early in pregnancy is supported by studies of autism spectrum disorders in the general population.11 Folic acid intake before pregnancy and until gestational weeks 4 to 8 was associated with a reduced risk of autism in the child.10,35 Supplementation later in pregnancy did not have a protective effect.10 These findings match the 20- to 40-day postconception time frame hypothesized to be critical for the development of autism spectrum disorders during pregnancy, at days 20 to 40 after conception.7,36
We found an inverse association between maternal plasma folate concentration at gestational weeks 17 to 19 and the degree of autistic traits at 3 years of age. This association should reflect folate status also at the critical point 20 to 40 days after conception, because folate has a half-life of approximately 100 days.37 We also demonstrated an inverse association between the degree of autistic traits and folic acid dose, especially during the first trimester.
Supplementation timing represents a clinical challenge, because pregnancies may not be recognized until after the period when folate status is critical. In patients with epilepsy, 24% to 79% of pregnancies are unintended.34,38 We therefore recommend that folic acid supplements be used continuously by all women taking AEDs who could become pregnant.
Strengths and Limitations
This prospective, population-based, controlled, but observational study was derived from a single cohort. Studies from other cohorts are needed. In particular, the effect of folic acid supplements on autism in the child should be studied in different AED treatment groups with sufficient statistical power to evaluate specific monotherapy and polytherapy cohorts. The validity of the self-reported exposure variables was confirmed by biological measurements. We controlled for relevant confounding factors. Parental rating of autistic traits may represent a limitation compared with a clinically based diagnostic evaluation. However, questionnaires completed by parents provide information about children that reflect a wider range of dysfunction after AED exposure.3 The stability of the results with different rating instruments (SCQ and M-CHAT) and across different child ages (18 and 36 months) strengthens our findings. The positive predictive value for autistic disorders from a screening tool for autism ranges from 10% to 60% for young children at low risk recruited from the community.30,39 However, the specificity is high, and most children with positive screening results will have autistic traits in the frame of a developmental disorder even if they do not fulfill all the criteria for an autism disorder.30,39,40,41,42 In our study, we have therefore focused on autistic traits, not autistic disorders.
We did not reveal significant associations between AED plasma concentrations or doses and autistic traits. Data on concentrations of AEDs during pregnancy in association with child outcome are sparse. The neutral association in our study is in line with a population-based study that found no difference in risk regarding autism spectrum disorders in children of mothers using a high dose (>750 mg/d) vs a low dose (≤750 mg/d) of valproate.1 Another study found a dose effect of valproate on risk for autistic traits.2 The mean daily valproate sodium dose used in the MoBa epilepsy cohort was 754 mg/d (range, 200-1200 mg/d),24 which is lower than reported by clinic-based studies.2,21 For some of the AEDs, statistical power was low. Thus, the neutral association between AED load and autistic traits must be interpreted with caution.
Conclusions
Folic acid supplementation in early pregnancy is associated with a reduced risk of autistic traits in children of mothers with epilepsy using AEDs. The benefit of folic acid supplements was not restricted to children exposed to valproate. Folic acid intake was critical during the first 12 gestational weeks. We detected a dose-effect association for the degree of autistic traits. Unplanned pregnancies are common in the epilepsy cohort, and folic acid supplements should be taken continuously by all women taking AEDs if they could become pregnant.
eMethods 1. Polytherapy Combinations
eMethods 2. Antiepileptic Drug Types Used by Mothers on High-Dose Folic Acid Supplements
eMethods 3. Biobank Analysis of Blood Samples and Folate
eTable 1. Modified Checklist for Autism in Toddlers (M-CHAT)—Child Age 18 Months
eTable 2. Social Communication Questionnaire (SCQ)—Child Age 36 Months
eTable 3. Multivariable Linear Regression Analysis Adjusted for Confounding Factors
eTable 4. Autistic traits (Social Communication Questionnaire Score) in Relation to Quartiles of Plasma Folate
eTable 5. Autistic Traits (Social Communication Questionnaire Score) in Relation to Plasma Concentration Quartiles of Antiepileptic Drugs
eTable 6. Characteristics in Mothers of Children Without and With Autistic Traits at 18 and 36 Months or at Both Measurement Times
eFigure 1. Degree of Autistic Traits at 18 and 36 Months of Age According to Folic Acid Dose Used Before and During Pregnancy
eFigure 2. Scatterplot Between Total M-CHAT Score in the Child and Maternal Plasma Folate Concentration in Gestational Week 17-19
eFigure 3. Degree of Autistic Traits According to Antiepileptic Drug Concentrations in GESTATIONAL WEEK 17-19 and in the Umbilical Cord After Delivery
eFigure 4. Effect of Folic Acid Supplement in Antiepileptic Drug Subgroups
eFigure 5. The Antiepileptic Drug and Folic Acid Supplement Interaction
References
- 1.Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood AG, Nadebaum C, Anderson V, et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia. 2015;56(7):1047-1055. [DOI] [PubMed] [Google Scholar]
- 3.Bromley RL, Mawer GE, Briggs M, et al. ; Liverpool and Manchester Neurodevelopment Group . The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasalam AD, Hailey H, Williams JH, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47(8):551-555. [DOI] [PubMed] [Google Scholar]
- 5.Veiby G, Daltveit AK, Schjølberg S, et al. Exposure to antiepileptic drugs in utero and child development: a prospective population-based study. Epilepsia. 2013;54(8):1462-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 7.Heyer DB, Meredith RM. Environmental toxicology: sensitive periods of development and neurodevelopmental disorders. Neurotoxicology. 2017;58:23-41. [DOI] [PubMed] [Google Scholar]
- 8.Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaik Mohammad N, Sai Shruti P, Bharathi V, et al. Clinical utility of folate pathway genetic polymorphisms in the diagnosis of autism spectrum disorders. Psychiatr Genet. 2016;26(6):281-286. [DOI] [PubMed] [Google Scholar]
- 10.Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309(6):570-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Sheng C, Xie RH, et al. New Perspective on impact of folic acid supplementation during pregnancy on neurodevelopment/autism in the offspring children: a systematic review. PLoS One. 2016;11(11):e0165626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol. 2009;28(1):1-10. [DOI] [PubMed] [Google Scholar]
- 13.Apeland T, Mansoor MA, Strandjord RE, Kristensen O. Homocysteine concentrations and methionine loading in patients on antiepileptic drugs. Acta Neurol Scand. 2000;101(4):217-223. [DOI] [PubMed] [Google Scholar]
- 14.Dansky LV, Andermann E, Rosenblatt D, Sherwin AL, Andermann F. Anticonvulsants, folate levels, and pregnancy outcome: a prospective study. Ann Neurol. 1987;21(2):176-182. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) Spina bifida and anencephaly before and after folic acid mandate—United States, 1995-1996 and 1999-2000. MMWR Morb Mortal Wkly Rep. 2004;53(17):362-365. [PubMed] [Google Scholar]
- 16.National Institute for Health Care Excellence. Maternal and child nutrition. https://www.nice.org.uk/guidance/PH11/chapter/4-Recommendations#folic-acid-2. Updated November 2014. Accessed June 14, 2017.
- 17.National Institute for Health Care Excellence. Epilepsies: diagnosis and management. https://www.nice.org.uk/guidance/cg137/chapter/1-Guidance#women-and-girls-with-epilepsy. Updated February 2016. Accessed November 12, 2017. [Google Scholar]
- 18.Valera-Gran D, García de la Hera M, Navarrete-Muñoz EM, et al. ; Infancia y Medio Ambiente (INMA) Project . Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014;168(11):e142611. [DOI] [PubMed] [Google Scholar]
- 19.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP study group . Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10(7):609-617. [DOI] [PubMed] [Google Scholar]
- 20.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014;261(3):579-588. [DOI] [PubMed] [Google Scholar]
- 21.Meador KJ, Baker GA, Browning N, et al. ; NEAD Study Group . Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45(2):382-388. [DOI] [PubMed] [Google Scholar]
- 23.Irgens LM. The Medical Birth Registry of Norway: epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79(6):435-439. [PubMed] [Google Scholar]
- 24.Bjørk MB. Veiby G, Spigset O, Gilhus NE. Using the Norwegian Mother and Child Cohort Study to determine risk factors for delayed development and neuropsychiatric symptoms in the offspring of parents with epilepsy. Norsk Epidemiol. 2014;24(1-2):79-89. [Google Scholar]
- 25.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30(4):389-399. [DOI] [PubMed] [Google Scholar]
- 26.Paltiel L, Haugan A, Skjerden T, et al. . The biobank of the Norwegian Mother and Child Cohort Study: present status. Norsk Epidemiol. 2014;24(1-2):29-35. [Google Scholar]
- 27.Hannisdal R, Ueland PM, Svardal A. Liquid chromatography-tandem mass spectrometry analysis of folate and folate catabolites in human serum. Clin Chem. 2009;55(6):1147-1154. [DOI] [PubMed] [Google Scholar]
- 28.Hannisdal R, Ueland PM, Eussen SJ, Svardal A, Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J Nutr. 2009;139(7):1415-1418. [DOI] [PubMed] [Google Scholar]
- 29.Snow AV, Lecavalier L. Sensitivity and specificity of the Modified Checklist for Autism in Toddlers and the Social Communication Questionnaire in preschoolers suspected of having pervasive developmental disorders. Autism. 2008;12(6):627-644. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Joseph RM, Frazier JA, et al. ; Extremely Low Gestational Age Newborn (ELGAN) Study Investigators . Predictive validity of the Modified Checklist for Autism in Toddlers (M-CHAT) born very preterm. J Pediatr. 2016;178:101-107.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wozniak RH, Leezenbaum NB, Northrup JB, West KL, Iverson JM. The development of autism spectrum disorders: variability and causal complexity [published online December 1, 2016]. Wiley Interdiscip Rev Cogn Sci. doi: 10.1002/wcs.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker M, Weinberger T, Chandy A, Schmukler S. Depression during pregnancy and postpartum. Curr Psychiatry Rep. 2017;18(3):32. [DOI] [PubMed] [Google Scholar]
- 33.Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry. 2003;57(2):113-118. [DOI] [PubMed] [Google Scholar]
- 34.Bjørk MH, Veiby G, Reiter SC, et al. Depression and anxiety in women with epilepsy during pregnancy and after delivery: a prospective population-based cohort study on frequency, risk factors, medication, and prognosis. Epilepsia. 2015;56(1):28-39. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (Childhood Autism Risks From Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96(1):80-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ploeger A, Raijmakers ME, van der Maas HL, Galis F. The association between autism and errors in early embryogenesis: what is the causal mechanism? Biol Psychiatry. 2010;67(7):602-607. [DOI] [PubMed] [Google Scholar]
- 37.Shane B. Folate in Health and Disease. 2nd ed Boca Raton: Taylor & Francis Group; 2010. [Google Scholar]
- 38.Herzog AG, Mandle HB, Cahill KE, Fowler KM, Hauser WA. Predictors of unintended pregnancy in women with epilepsy. Neurology. 2017;88(8):728-733. [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends, and outcomes. Stud Fam Plann. 2010;41(4):241-250. [DOI] [PubMed] [Google Scholar]
- 40.Pandey J, Verbalis A, Robins DL, et al. Screening for autism in older and younger toddlers with the Modified Checklist for Autism in Toddlers. Autism. 2008;12(5):513-535. [DOI] [PubMed] [Google Scholar]
- 41.Baird G, Charman T, Baron-Cohen S, et al. . A screening instrument for autism at 18 months of age: a 6-year follow-up study. J Am Acad Child Adolesc Psychiatry. 2000;39(6):694-702. [DOI] [PubMed] [Google Scholar]
- 42.Chlebowski C, Robins DL, Barton ML, Fein D. Large-scale use of the Modified Checklist for Autism in low-risk toddlers. Pediatrics. 2013;131(4):e1121-e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandler S, Charman T, Baird G, et al. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1324-1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Polytherapy Combinations
eMethods 2. Antiepileptic Drug Types Used by Mothers on High-Dose Folic Acid Supplements
eMethods 3. Biobank Analysis of Blood Samples and Folate
eTable 1. Modified Checklist for Autism in Toddlers (M-CHAT)—Child Age 18 Months
eTable 2. Social Communication Questionnaire (SCQ)—Child Age 36 Months
eTable 3. Multivariable Linear Regression Analysis Adjusted for Confounding Factors
eTable 4. Autistic traits (Social Communication Questionnaire Score) in Relation to Quartiles of Plasma Folate
eTable 5. Autistic Traits (Social Communication Questionnaire Score) in Relation to Plasma Concentration Quartiles of Antiepileptic Drugs
eTable 6. Characteristics in Mothers of Children Without and With Autistic Traits at 18 and 36 Months or at Both Measurement Times
eFigure 1. Degree of Autistic Traits at 18 and 36 Months of Age According to Folic Acid Dose Used Before and During Pregnancy
eFigure 2. Scatterplot Between Total M-CHAT Score in the Child and Maternal Plasma Folate Concentration in Gestational Week 17-19
eFigure 3. Degree of Autistic Traits According to Antiepileptic Drug Concentrations in GESTATIONAL WEEK 17-19 and in the Umbilical Cord After Delivery
eFigure 4. Effect of Folic Acid Supplement in Antiepileptic Drug Subgroups
eFigure 5. The Antiepileptic Drug and Folic Acid Supplement Interaction