Key Points
Question
What are the comparative effects of meropenem-vaborbactam vs piperacillin-tazobactam for treatment of complicated urinary tract infection, including acute pyelonephritis?
Findings
In this noninferiority randomized trial that included 550 patients, the difference in the composite outcome of complete resolution or improvement of symptoms along with microbial eradication met the noninferiority margin of 15% when comparing meropenem-vaborbactam vs piperacillin-tazobactam (98.4% vs 94.0%).
Meaning
This study demonstrated noninferiority of meropenem-vaborbactam for the treatment of complicated urinary tract infection.
Abstract
Importance
Meropenem-vaborbactam is a combination carbapenem/beta-lactamase inhibitor and a potential treatment for severe drug-resistant gram-negative infections.
Objective
To evaluate efficacy and adverse events of meropenem-vaborbactam in complicated urinary tract infection (UTI), including acute pyelonephritis.
Design, Setting, and Participants
Phase 3, multicenter, multinational, randomized clinical trial (TANGO I) conducted November 2014 to April 2016 and enrolling patients (≥18 years) with complicated UTI, stratified by infection type and geographic region.
Interventions
Eligible patients were randomized 1:1 to receive meropenem-vaborbactam (2g/2g over 3 hours; n = 274) or piperacillin-tazobactam (4g/0.5g over 30 minutes; n = 276) every 8 hours. After 15 or more doses, patients could be switched to oral levofloxacin if they met prespecified criteria for improvement, to complete 10 days of total treatment.
Main Outcomes and Measures
Primary end point for FDA criteria was overall success (clinical cure or improvement and microbial eradication composite) at end of intravenous treatment in the microbiologic modified intent-to-treat (ITT) population. Primary end point for European Medicines Agency (EMA) criteria was microbial eradication at test-of-cure visit in the microbiologic modified ITT and microbiologic evaluable populations. Prespecified noninferiority margin was −15%. Because the protocol prespecified superiority testing in the event of noninferiority, 2-sided 95% CIs were calculated.
Results
Among 550 patients randomized, 545 received study drug (mean age, 52.8 years; 361 [66.2%] women; 374 [68.6%] in the microbiologic modified ITT population; 347 [63.7%] in the microbiologic evaluable population; 508 [93.2%] completed the trial). For the FDA primary end point, overall success occurred in 189 of 192 (98.4%) with meropenem-vaborbactam vs 171 of 182 (94.0%) with piperacillin-tazobactam (difference, 4.5% [95% CI, 0.7% to 9.1%]; P < .001 for noninferiority). For the EMA primary end point, microbial eradication in the microbiologic modified ITT population occurred in 128 of 192 (66.7%) with meropenem-vaborbactam vs 105 of 182 (57.7%) with piperacillin-tazobactam (difference, 9.0% [95% CI, −0.9% to 18.7%]; P < .001 for noninferiority); microbial eradication in the microbiologic evaluable population occurred in 118 of 178 (66.3%) vs 102 of 169 (60.4%) (difference, 5.9% [95% CI, −4.2% to 16.0%]; P < .001 for noninferiority). Adverse events were reported in 106 of 272 (39.0%) with meropenem-vaborbactam vs 97 of 273 (35.5%) with piperacillin-tazobactam.
Conclusions and Relevance
Among patients with complicated UTI, including acute pyelonephritis and growth of a baseline pathogen, meropenem-vaborbactam vs piperacillin-tazobactam resulted in a composite outcome of complete resolution or improvement of symptoms along with microbial eradication that met the noninferiority criterion. Further research is needed to understand the spectrum of patients in whom meropenem-vaborbactam offers a clinical advantage.
Trial Registration
clinicaltrials.gov Identifier: NCT02166476
This noninferiority randomized trial compares the efficacy and safety of meropenem-vaborbactam vs piperacillin-tazobactam for treatment of adults with complicated urinary tract infection.
Introduction
Spread of beta-lactamase enzymes among gram-negative pathogens threatens the usefulness of many beta-lactam antibiotics and has resulted in greater reliance on carbapenems. Dissemination of carbapenemases (eg, Klebsiella pneumoniae carbapenemase) in Enterobacteriaceae worldwide is identified as an urgent threat by the Centers for Disease Control and Prevention, and the World Health Organization has identified development of new agents as a critical need. Infections due to carbapenem-resistant Enterobacteriaceae are most often urinary tract infections (UTIs), including acute pyelonephritis, and are usually health care–associated. Mortality attributable to carbapenem-resistant Enterobacteriaceae infections is 20% to 54.3% and reflects the need for better treatment options.
Vaborbactam is a cyclic boronic acid–based beta-lactamase inhibitor with broad inhibitory activity against serine beta-lactamases, particularly K pneumoniae carbapenemase. Vaborbactam plus meropenem was developed as a fixed combination product for severe gram-negative infections, including those due to carbapenem-resistant Enterobacteriaceae. Meropenem-vaborbactam has potent in vitro activity against K pneumoniae carbapenemase-producing carbapenem-resistant Enterobacteriaceae and restores activity of meropenem in animal models of infections due to these organisms. Phase 1 studies demonstrated that meropenem and vaborbactam have well-matched plasma and tissue pharmacokinetic properties; exposures in preclinical models were well tolerated. Given these properties, the Targeting Antibiotic Non-Susceptible Gram-Negative Organisms (TANGO I) randomized clinical trial was conducted comparing meropenem-vaborbactam with piperacillin-tazobactam in adults with complicated UTI, including acute pyelonephritis.
Methods
Study Design and Participants
TANGO I was a phase 3, multicenter, randomized, double-blind, double-dummy, active-control trial enrolling adults (≥18 years) with complicated UTI or acute pyelonephritis and conducted from November 2014 to April 2016 in 60 sites in 17 countries: Belarus, Brazil, Bulgaria, Czech Republic, Greece, Hungary, Italy, Peru, Poland, Romania, Slovakia, Slovenia, South Korea, Spain, Taiwan, Ukraine, and the United States.
The study protocol and informed consent form were reviewed and approved by the institutional review board or independent ethics committee at each participating site. The trial was conducted in accordance with ethical principles of Good Clinical Practice, according to the International Conference on Harmonisation Harmonized Tripartite Guideline. Prior to initiation of any study-related procedures, an informed consent form was signed by the patient or by the patient’s guardian or legal representative.
Detailed inclusion and exclusion criteria are reported in Supplement 1. Eligible patients were 18 years or older, 185 kg or less, needed 5 or more days of intravenous antibiotics, and had documented or suspected complicated UTI or acute pyelonephritis (Supplement 1).
Complicated UTI was defined, based on US Food and Drug Administration (FDA) guidance, as having at least 2 of the following clinical criteria: (1) chills, rigors, or fever (temperature ≥38.0°C); (2) elevated white blood cell count (>10 000/mm3) or left shift (>15% immature polymorphonuclear leukocytes); (3) nausea or vomiting; (4) dysuria, increased urinary frequency, or urinary urgency; or (5) lower abdominal pain or pelvic pain; AND the presence of pyuria as evidenced by 1 of the following: (1) white blood cell count 10 cells/μL or greater in unspun urine; (2) 10 or more cells per high-power field in urine sediment; or (3) positive leukocyte esterase on urinalysis; AND at least 1 associated risk factor, including indwelling catheter, neurogenic bladder, obstructive uropathy, azotemia due to renal disease, or urinary retention in men due to benign prostatic hypertrophy. Indwelling urinary catheters or devices (including nephrostomy tubes, indwelling stents, or both) were removed or replaced (if feasible) within 12 hours of randomization. Signs or symptoms of acute pyelonephritis could also be defined by flank pain or costovertebral angle tenderness on physical examination.
Race/ethnicity was included as part of required data collected for a registered trial. Patients self-reported race/ethnicity based on fixed categories as per regulatory guidance.
Patients were excluded if they required either an antibiotic in addition to study drug or antifungal therapy; received an antibiotic within 48 hours before randomization (except for single dose of a short-acting oral or intravenous antibiotic); and had estimated creatinine clearance less than 30 mL/min. Patients who received more than 48 hours of an antibiotic could be enrolled if they had clinical evidence of treatment failure or developed complicated UTI or acute pyelonephritis.
Randomization, Stratification, and Blinding
Eligible patients were randomized 1:1 using a computer-generated central randomization code, using a dynamic randomization algorithm and interactive voice/web response system to receive either meropenem-vaborbactam (2g/2g via 3-hour infusion) or piperacillin-tazobactam (4g/0.5g via 30-minute infusion) every 8 hours, for 10 days of total treatment (intravenous ± oral) (Figure 1). Patients also received drug-free saline infusate (30-minute or 3-hour infusion) to maintain blinding. Blinded dose adjustment was made for patients receiving meropenem-vaborbactam and with estimated creatinine clearance less than 50 mL/min (not needed for patients receiving piperacillin-tazobactam).
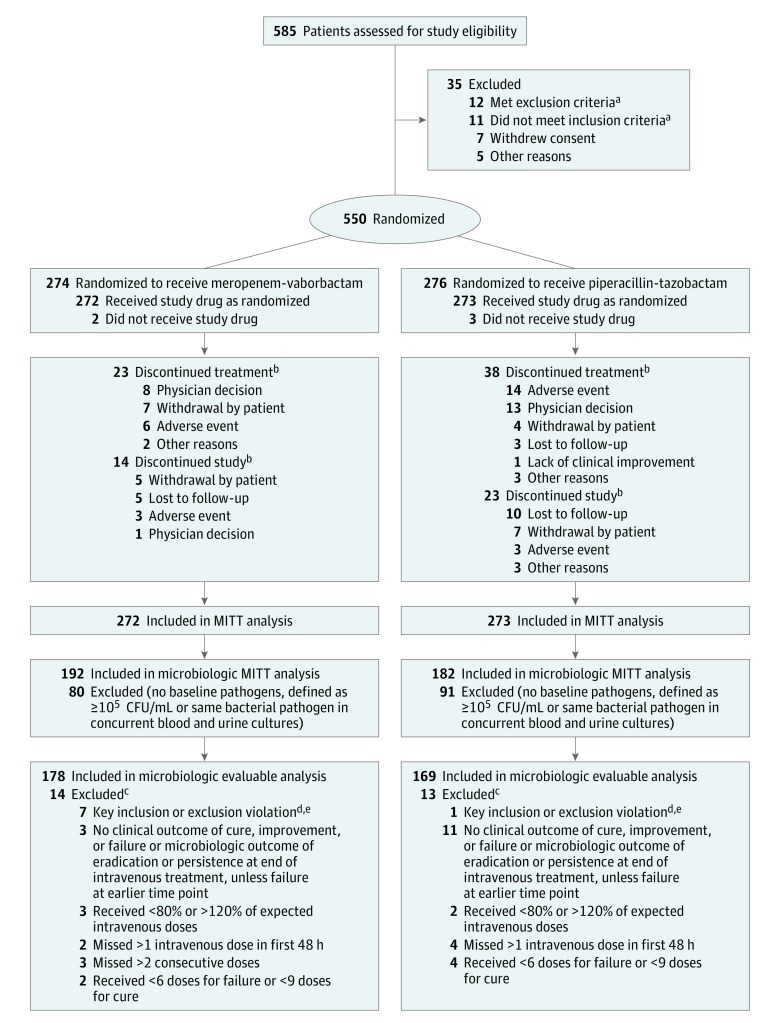
Figure 1. Flow of Patients in TANGO I Randomized Clinical Trial.
MITT indicates modified intent-to-treat.
aSpecific reasons and numbers for patient inclusion and exclusion not collected.
bA patient could discontinue study treatment but remain in the study, discontinue study treatment but discontinue study at a later date, or discontinue study treatment and discontinue study at the same time.
cPatients could have been excluded from microbiologic evaluable population for more than 1 reason.
dKey inclusion criteria include documented or suspected complicated urinary tract infection (UTI) or acute pyelonephritis and removal/replacement of indwelling catheter before or within 12 hours of randomization. Key exclusion criteria included presence of perinephric abscess; renal corticomedullary abscess; uncomplicated UTI; polycystic kidney disease; chronic vesicoureteral reflux; previous or planned renal transplantation; hemodialysis; previous or planned cystectomy or ileal loop surgery, or known candiduria; presence of suspected/confirmed acute bacterial prostatitis, orchitis, epidymitis, or chronic bacterial prostatitis by history/physical examination; urinary tract surgery 7 days prior to or within randomization; estimated creatinine clearance less than 30 mL/min (Cockcroft-Gault); known nonrenal source of infection within 7 days of randomization; signs of severe sepsis; receipt of antibiotic agent with 48 hours of randomization; presence of immunodeficiency, immunocompromised condition, or immunosuppressive therapy; or neutropenia (less than 1000 polymorphonuclear leukocytes/µL). Detailed inclusion and exclusion criteria are reported in Supplement 1.
eIf a patient met multiple criteria, the patient was counted only once when counting the total number of patients excluded from the clinical evaluable and microbiologic evaluable populations.
Randomization was stratified by geographic region (defined as North America, Europe, Asia Pacific, and the rest of the world) and type of infection (acute pyelonephritis, complicated UTI with removable focus, and complicated UTI with nonremovable focus). For a patient to be included in the group with complicated UTI and a removable source of infection, the site care team was instructed to remove or replace the removable focus within 12 hours after enrollment. The specifics and timing of the removal or replacement of the removable source of infection were not captured in the study database.
After 15 or more doses of intravenous therapy, and if they met prespecified criteria for improvement, patients could be switched to oral levofloxacin (500 mg every 24 hours), to complete 10 days of total treatment (intravenous + oral). Criteria for switching to oral therapy are reported in Supplement 1.
Analysis Populations
The modified intent-to-treat (ITT) population was the population to assess adverse events and comprised all patients who received 1 or more doses of study drug. The microbiologic modified ITT population included all patients in the modified ITT population with 1 or more bacterial pathogens of 105 colony-forming units (CFU)/mL or more in baseline urine culture or the same bacterial pathogen present in concurrent blood and urine cultures. The microbiologic evaluable population included all patients who met criteria for the microbiologic modified ITT population and who had a clinical outcome and microbiologic outcome at end of intravenous treatment; received 80% or more and 120% or less of expected intravenous doses for completed treatment duration; missed 1 intravenous dose, or no doses, in the first 48 hours and missed 2 or fewer consecutive intravenous doses overall; received 6 or more doses of study drug if classified as experiencing treatment failure on overall outcome; or received 9 or more doses if classified as experiencing cure on overall outcome.
Procedures
Study procedures included assessment of study outcomes, collection of urine for urinalysis and cultures, collection of blood for culture, and pharmacokinetic analyses. Assessments of adverse events, clinical laboratory evaluations, vital signs, physical examinations, and electrocardiograms were also conducted.
Clinical Assessments
Clinical outcome assessment was performed on day 3 of study treatment, at end of intravenous treatment, on the last day of total therapy (end of treatment), at the test-of-cure visit (end of treatment +7 days), and at the late follow-up visit (end of treatment +14 days) (eFigure in Supplement 2). Visit assessments were combined at end of intravenous treatment and end of treatment for patients who did not switch to oral therapy. Investigators made clinical outcome assessments at each visit based on improvement, no change, or worsening of the baseline symptoms and signs.
Microbiologic Assessments
Urine and blood cultures were sent to a local laboratory for culture and susceptibility testing. Up to 2 isolated pathogens were allowed per urine culture (at ≥105 CFU/mL urine). Urine cultures with 3 or more bacterial organisms were considered contaminated. If an organism grew concurrently in urine and blood, it was not considered a contaminant. All isolates cultured at the local laboratory and designated as pathogens were sent to the reference laboratory (JMI Laboratories, North Liberty, Iowa) for confirmation of identification and susceptibility testing results. Susceptibility testing was completed using Clinical and Laboratory Standards Institute methods.
For microbiologic outcome, eradication was defined as baseline bacterial pathogens reduced to less than 104 CFU/mL on urine culture (FDA criteria) or less than 103 CFU/mL (European Medicines Agency [EMA] criteria) AND negative blood culture for an organism identified as a uropathogen (if repeated after positive finding at baseline blood culture).
Primary and Secondary End Points
The primary efficacy end point was assessed using different criteria for the FDA and the EMA. For the FDA criteria, the primary end point was overall success, a composite outcome of clinical cure (complete resolution or significant improvement of baseline signs and symptoms of complicated UTI or acute pyelonephritis) and microbial eradication (baseline pathogens reduced to <104 CFU/mL urine) at the end of intravenous treatment visit for the microbiologic modified ITT population. For the EMA criteria, the primary end point was microbial eradication (baseline pathogens reduced to <103 CFU/mL urine) at the test-of-cure visit for the microbiologic modified ITT and microbiologic evaluable populations.
Secondary end points reported here include proportion of patients with overall success at end of intravenous treatment and at test-of-cure visits (by infection type) in the microbiologic modified ITT population; clinical cure at end of intravenous treatment and at test-of-cure visits in the microbiologic modified ITT population; microbial eradication to less than 104 CFU/mL of urine for the FDA criteria and less than 103 CFU/mL of urine for the EMA criteria at end of intravenous treatment and at test-of-cure visits in the microbiologic modified ITT and microbiologic evaluable populations; per-pathogen outcomes (overall success, clinical cure, and microbial eradication) in the microbiologic modified ITT population; and adverse event and tolerability profile of meropenem-vaborbactam as assessed by vital signs, clinical laboratory tests, electrocardiograms, and physical examinations.
Secondary end points not reported here include pharmacokinetic characterization of plasma exposure of meropenem and vaborbactam; assessments in the clinical evaluable population; and assessments on day 3 and the last follow-up visit.
Statistical Analysis
A sample size of approximately 500 patients with 60% in the microbiologic modified ITT population, an overall success rate at end of intravenous treatment of 80% in both treatment groups, and a noninferiority margin of 15% provided 90% power to demonstrate noninferiority of meropenem-vaborbactam to piperacillin-tazobactam in the microbiologic modified ITT population. A sample size of 500 patients with 50% in the microbiologic evaluable population provided 84% power to demonstrate noninferiority of meropenem-vaborbactam to piperacillin-tazobactam in the microbiologic evaluable population.
For primary end point analyses, the proportion of patients with overall success and the proportion of patients with microbial eradication were summarized by treatment group. Two-sided 95% CIs were presented for between-group differences (meropenem-vaborbactam − piperacillin-tazobactam), based on the method of Miettinen and Nurminen. The prespecified noninferiority margin was a difference of 15%. Noninferiority was concluded if the lower limit of the 2-sided 95% CI for the treatment difference for overall success at end of intravenous treatment was greater than −15%. For the EMA criteria, meropenem-vaborbactam was noninferior only if noninferiority was demonstrated for microbial eradication at test of cure in both the microbiologic modified ITT and microbiologic evaluable populations. If noninferiority was demonstrated in FDA or EMA primary end points, the protocol and statistical analysis plan included an assessment of superiority using the CI to determine whether the lower bound of the 2-sided 95% CI was greater than 0. A sensitivity analysis of the FDA primary end point assessing only those who were cured, as opposed to improved, was also conducted. The study was not powered to demonstrate noninferiority for secondary end points.
In designing this study to support regulatory registration, we considered noninferiority margins that would be clinically acceptable as well as recognize the activity of meropenem-vaborbactam against carbapenem-resistant Enterobacteriaceae, a pathogen family that the US Centers for Disease Control and Prevention considers to be an urgent antimicrobial resistance threat. In developing agents to address these threats and provide therapy for patients who do not have other treatment options to thus address this unmet need, it is recognized that clinicians and regulators are willing to accept a lower level of certainty about differences in efficacy between an established comparator and a test agent that can provide activity against drug-resistant pathogens.
As stated in the FDA Guidance Antibacterial Therapies for Patients with an Unmet Medical Need for the Treatment of Serious Bacterial Infections, “the guidance for industry Complicated Urinary Tract Infections: Developing Drugs for Treatment recommends a noninferiority margin of 10 percent for a trial evaluating a new antibacterial drug based on reliable and reproducible evidence of an antibacterial drug treatment effect of approximately 30 percent (the effect of the antibacterial drug over placebo). An active-controlled trial showing noninferiority based on the selection of a noninferiority margin greater than 10 percent but less than the treatment effect of 30 percent (eg, a noninferiority margin of 15 percent) still establishes effectiveness for the treatment of complicated UTI but allows for a greater potential loss of efficacy. Such a finding of noninferiority could be acceptable for patients who have limited or no alternative treatment options for their serious infectious disease.” Accordingly, a value of −15% for the lower bound of the 95% CI of the difference between meropenem-vaborbactam vs piperacillin-tazobactam was assumed in designing and powering this study.
The Breslow-Day test was used to assess the homogeneity of the odds ratios across regions. In a post hoc analysis, the same test was used to assess the homogeneity of the odds ratios among all centers. If no significant center × treatment interaction was observed, a mixed-effects logistic regression was conducted.
Results
Patient Disposition
A total of 550 patients at 60 sites in 17 countries were randomized between November 2014 and April 2016: 274 to meropenem-vaborbactam and 276 to piperacillin-tazobactam. The distribution of patients by sites is reported in eTable 1 in Supplement 2. Of the 550 patients, 545 received 1 or more doses of study drug (modified ITT population): 272 received meropenem-vaborbactam and 273 piperacillin-tazobactam (Figure 1). Of the 545 patients who received study drug, 374 (68.6%) had bacterial pathogens of 105 CFU/mL or greater in baseline urine culture or the same bacterial pathogen present in concurrent blood and urine cultures; these 374 comprised the microbiologic modified ITT population (192 meropenem-vaborbactam; 182 piperacillin-tazobactam).
Baseline characteristics for the 3 study populations (modified ITT, microbiologic modified ITT, microbiologic evaluable) are reported in Table 1 and in eTable 2 in Supplement 2. The meropenem-vaborbactam and piperacillin-tazobactam groups included numerically similar percentages of patients with acute pyelonephritis (59.2% and 59.0%, respectively), complicated UTI with removable source of infection (19.5% and 18.7%), and complicated UTI with nonremovable source of infection (21.3% and 22.3%).
Table 1. Baseline Characteristics, Pathogens, and Drug Resistance.
| Baseline Characteristics (MITT Population)a | No. (%) | |
|---|---|---|
| Meropenem-Vaborbactam (n = 272) | Piperacillin-Tazobactam (n = 273) | |
| Acute pyelonephritis | 161 (59.2) | 161 (59.0) |
| Complicated urinary tract infection | 111 (40.8) | 112 (41.0) |
| With removable source of infectionb | 53 (19.5) | 51 (18.7) |
| With nonremovable source of infection | 58 (21.3) | 61 (22.3) |
| Age, mean (SD), y | 53.0 (19.4) | 52.6 (20.9) |
| ≥65 | 87 (32.0) | 103 (37.7) |
| Female sex | 181 (66.5) | 180 (65.9) |
| White race | 254 (93.4) | 252 (92.3) |
| Creatinine clearance, mean (SD), mL/min/1.73 m2 | 93.5 (34.4) | 89.2 (36.4) |
| ≤50 | 31 (11.4) | 37 (13.5) |
| Diabetes mellitus | 42 (15.4) | 44 (16.1) |
| Systemic inflammatory response syndromec | 77 (28.3) | 90 (33.0) |
| Charlson Comorbidity Index score ≥3d | 143 (52.6) | 147 (53.8) |
| Common baseline pathogens (microbiologic MITT population)e,f | n = 192 | n = 182 |
| Escherichia coli | 125 (65.1) | 117 (64.3) |
| Klebsiella pneumoniae | 30 (15.6) | 28 (15.4) |
| Enterococcus faecalis | 13 (6.8) | 14 (7.7) |
| Proteus mirabilis | 6 (3.1) | 12 (6.6) |
| Enterobacter cloacae species complex | 10 (5.2) | 5 (2.7) |
| Drug resistance (FDA/CLSI criteria) in the most common baseline urinary gram-negative pathogens (microbiologic MITT population)a,g,h | ||
| E coli | n = 124 | n = 115 |
| Meropenem | 0 | 0 |
| Piperacillin-tazobactam | 7 (5.6) | 6 (5.2) |
| K pneumoniae | n = 30 | n = 27 |
| Meropenem | 1 (3.3) | 2 (7.4) |
| Piperacillin-tazobactam | 15 (50) | 9 (33.3) |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; FDA, US Food and Drug Administration; MITT, modified intent-to-treat.
Population comprised all patients who received 1 or more doses of study drug.
Includes urinary catheter or removable kidney stones.
Defined as the occurrence of ≥2 of fever (temperature >38°C [100.4°F]) or temperature <36°C (96.8°F); heart rate >90/min; respiratory rate >20/min or arterial carbon dioxide tension <32 mm Hg; abnormal white blood cell count (>12 000/μL or <4000/μL or >10% immature [band] forms).
Index used to categorize comorbidities based on ICD diagnosis codes. Each category has an associated weight (range, 1-6), based on adjusted risk of mortality or resource use. The sum of weights results in a comorbidity score; higher score indicates higher likelihood that the predicted outcome will result in mortality or higher resource use.
Organisms observed in ≥15 patients at baseline. Some patients had >1 pathogen at baseline. Overall, in the microbiologic MITT population, 12 of 192 patients (6.3%) in the meropenem-vaborbactam group and 20 of 182 (11.0%) in the piperacillin-tazobactam group had >1 baseline pathogen.
The microbiologic MITT population included all patients in the MITT population with bacterial pathogens of ≥105 CFU/mL in baseline urine culture or the same bacterial pathogen present in concurrent blood and urine cultures.
The FDA/CLSI breakpoint for piperacillin-tazobactam resistance of Enterobacteriaceae is >64 μg/mL. The FDA/CLSI breakpoint for meropenem resistance of Enterobacteriaceae is ≥4 μg/mL.
Only pathogens with a frequency of at least 10 patients total are included. Only data from urinary isolates are included. If the same pathogen was isolated from the same source (urine or blood), only the result for the isolate with the highest minimum inhibitory concentration was included.
Patients presented with a mean of 3.5 symptoms (range, 2-6) at baseline in both the modified ITT and microbiologic modified ITT populations. Baseline urinary pathogens were numerically similar across the meropenem-vaborbactam and piperacillin-tazobactam groups of the microbiologic modified ITT population, and Escherichia coli (65.1% and 64.3%, respectively) and K pneumoniae (15.6% and 15.4%) were the most commonly isolated urinary pathogens. Overall, nearly all baseline urinary isolates were susceptible to meropenem and approximately 12% were resistant to piperacillin-tazobactam by FDA criteria. Resistance to the most common pathogens using FDA criteria is reported in Table 1 and using European Committee on Antimicrobial Susceptibility Testing criteria is reported in eTable 3 in Supplement 2.
Most patients in both groups of the modified ITT population (91.5% meropenem-vaborbactam; 86.1% piperacillin-tazobactam) completed study treatment (intravenous + oral). Of those not completing treatment, most prematurely discontinued intravenous therapy (8.1% meropenem-vaborbactam group; 12.8% piperacillin-tazobactam). Treatment discontinuations because of an adverse event (2.2% meropenem-vaborbactam; 5.1% piperacillin-tazobactam) and physician decision (2.9% meropenem-vaborbactam; 4.8% piperacillin-tazobactam) were the primary reasons for the higher percentages of intravenous treatment discontinuations in the piperacillin-tazobactam group.
The majority of patients in both treatment groups (94.9% meropenem-vaborbactam group; 91.6% piperacillin-tazobactam) completed the study. Mean study duration was approximately 25 days (maximum, 31 days). The primary reasons for not completing the study in the meropenem-vaborbactam and piperacillin-tazobactam groups were loss to follow-up (1.8% and 3.7%, respectively), patient withdrawal (1.8% and 2.6%), and an adverse event (1.1% and 1.1%).
Duration of Treatment
Treatment duration was similar across both groups. Mean duration of intravenous and oral step-down therapy in the modified ITT population was approximately 10 days in the meropenem-vaborbactam group (10.1 days [range, 1-17]) and piperacillin-tazobactam group (9.9 days [range, 2-15]). Most patients in both groups received 5 to 11 days of intravenous therapy; the mean duration was 8.0 days in both groups (1-15 days for meropenem-vaborbactam; 2-15 days for piperacillin-tazobactam).
Levofloxacin was used as oral step-down therapy in 146 patients (93.6%) receiving meropenem-vaborbactam and 137 (95.1%) receiving piperacillin-tazobactam. A percentage of patients in both groups were switched to oral step-down therapy with levofloxacin despite having a levofloxacin-resistant pathogen at baseline by FDA criteria (9.9% meropenem-vaborbactam; 8.2% piperacillin-tazobactam) and European Committee on Antimicrobial Susceptibility Testing criteria (9.9% in each group).
Efficacy Evaluation
Primary End Points
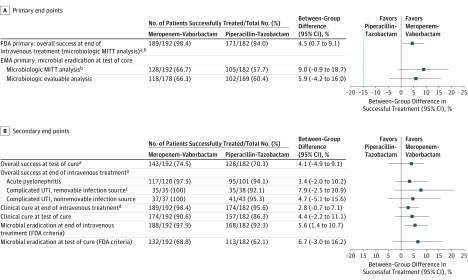
Noninferiority was met for the FDA primary end point of overall success in the microbiologic modified ITT population at end of intravenous treatment and for the EMA primary end point of microbiologic outcome of eradication at test of cure in the microbiologic modified ITT and microbiologic evaluable populations (Figure 2A).
Figure 2. Primary and Secondary Study End Points.
For microbiologic outcome, eradication was defined as baseline bacterial pathogens reduced to less than 104 colony-forming units (CFU)/mL on urine culture (US Food and Drug Administration [FDA] criteria) or less than 103 CFU/mL (European Medicines Agency [EMA] criteria) AND negative blood culture for an organism that was identified as a uropathogen (if repeated after positive at baseline blood culture). In panel A, blue dashed line at x = −15 indicates the noninferiority margin. MITT indicates modified intent-to-treat; SIRS, systemic inflammatory response syndrome; UTI, urinary tract infection.
aAt end of intravenous treatment, overall success represents patients with clinical cure or improvement and microbial eradication. At test of cure, overall success represents patients with clinical cure and microbial eradication.
bThe microbiologic MITT population included all patients in the MITT population with bacterial pathogens of 105 CFU/mL or greater in the baseline urine culture or the same bacterial pathogen present in concurrent blood and urine cultures.
cIncludes urinary catheter or removable kidney stones.
dAt end of intravenous treatment, clinical cure represents patients with complete resolution or significant improvement of baseline signs and symptoms of complicated UTI or acute pyelonephritis.
At end of intravenous treatment, the overall success rate in the microbiologic modified ITT population was 98.4% with meropenem-vaborbactam vs 94.0% with piperacillin-tazobactam (difference, 4.5% [95% CI, 0.7% to 9.1%]; P < .001 for noninferiority). The lower limit of the 95% CI (0.7%) was greater than the prespecified noninferiority margin of −15%, demonstrating that meropenem-vaborbactam was noninferior to piperacillin-tazobactam for the FDA primary end point (Figure 2A). Additionally, according to the prespecified statistical plan, superiority of meropenem-vaborbactam over piperacillin-tazobactam was concluded for this end point since the lower limit of the 95% CI (0.7%) exceeded 0 (P = .01).
Overall success for the primary end point at the end of intravenous treatment was clinical cure or improvement and microbial eradication. Results of a sensitivity analysis assessing only patients who were cured, as opposed to improved, demonstrated rates of overall success of 81.3% for meropenem-vaborbactam vs 78.6% for piperacillin-tazobactam (difference, 2.7% [95% CI, −5.5% to 10.9%]).
In the microbiologic modified ITT population, at end of intravenous treatment, overall success was 97.5% for meropenem-vaborbactam and 94.1% for piperacillin-tazobactam (difference, 3.4% [95% CI, −2.0% to 10.2%]) for patients with acute pyelonephritis; 100% and 92.1% (difference, 7.9% [95% CI, −2.5% to 20.9%]) for patients with complicated UTI and a removable source of infection; and 100% and 95.3% (difference, 4.7% [95% CI, −5.1% to 15.6%]) for patients with complicated UTI and a nonremovable source of infection (Figure 2B).
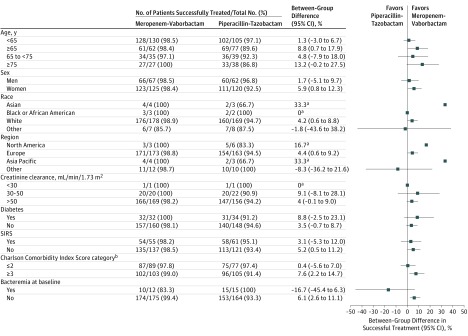
The differences and 95% CIs between treatment groups for overall success at end of intravenous treatment for subgroups based on age, sex, renal function, diabetes status, systemic inflammatory response syndrome status, Charlson Comorbidity Index score, and geographic region are presented in Figure 3. In patients with bacteremia, follow-up cultures were negative in all patients; the overall success for complicated UTI, including acute pyelonephritis, at end of intravenous treatment was 83.3% (10/12) for meropenem-vaborbactam and 100% (15/15) for piperacillin-tazobactam (difference, −16.7% [95% CI, −45.4% to 6.3%]). The 2 patients in the meropenem-vaborbactam group who did not achieve overall treatment success for their UTI were deemed to have experienced treatment failure because study drug was prematurely discontinued owing to an adverse event.
Figure 3. Overall Success at End of Intravenous Treatment, by Subgroup (Microbiologic Modified Intent-to-Treat Analysis).
SI conversion factor: To convert creatinine clearance values to mL/s/m2, multiply by 0.0167.
aNumbers of patients too small to permit calculation of 95% CIs.
bUsed to categorize comorbidities of patients based on the International Classification of Diseases diagnosis codes. Each comorbidity category has an associated weight ranging from 1 to 6, based on the adjusted risk of mortality or resource use. The sum of all the weights results in a comorbidity score. A higher score indicates a higher likelihood that the predicted outcome will result in mortality or higher resource use.
For the Europe region, overall success at the end of intravenous treatment was 98.8% (171/173) for meropenem-vaborbactam and 94.5% (154/163) for piperacillin-tazobactam. The number of patients enrolled outside Europe was relatively small, but overall success was similar: 94.7% (18/19) for meropenem-vaborbactam and 89.5% (17/19) for piperacillin-tazobactam. Of these 19 patients outside Europe in each group, overall success for the patients in the US region was 100% (3/3) for meropenem-vaborbactam and 83.3% (5/6) for piperacillin-tazobactam. There were no differences in the primary end point by prespecified geographic region. The P value to test for homogeneity of the odds ratio across regions was .27 (Breslow-Day test), indicating no significant region difference in the odds ratio.
In addition, as a post hoc analysis, a test for homogeneity of the odds ratios among all centers was conducted using the Breslow-Day test. The P value for this test was .37. Since no significant center × treatment interaction was observed, a mixed-effects logistic regression was conducted. In this model, the primary end point (overall success at end of intravenous treatment) was used as the dependent variable, with treatment as fixed effect (meropenem-vaborbactam vs piperacillin-tazobactam) and center as random effect. The odds ratio obtained from the model was 4.23 (95% CI, 1.14 to 15.69). The result demonstrates that the superiority of the overall success rate in the meropenem-vaborbactam group over the piperacillin-tazobactam group was maintained when center effect was accounted for in the model. These results were consistent with the findings in the prespecified primary analysis (including all centers) using risk difference as the test statistic.
For the EMA primary end point in the microbiologic modified ITT population, microbial eradication occurred in 66.7% of patients (128/192) in the meropenem-vaborbactam group vs 57.7% (105/182) in the piperacillin-tazobactam group at test of cure (difference, 9.0% [95% CI, −0.9% to 18.7%]; P < .001 for noninferiority). For the EMA primary end point in the microbiologic evaluable population, microbial eradication occurred in 66.3% of patients (118/178) in the meropenem-vaborbactam group vs 60.4% (102/169) in the piperacillin-tazobactam group at test of cure (difference, 5.9% [95% CI, −4.2% to 16.0%]; P < .001 for noninferiority) (Figure 2A). Based on these data, meropenem-vaborbactam was noninferior to piperacillin-tazobactam because the lower limit of the 95% CI for the differences between treatment groups in both populations was greater than the prespecified noninferiority margin of −15%.
Key Secondary End Points
Overall Success
Overall success in the meropenem-vaborbactam group was noninferior to that in the piperacillin-tazobactam group at test of cure (74.5% and 70.3%, respectively; difference, 4.1% [95% CI, −4.9% to 9.1%]) (Figure 2B). At test of cure, overall success was 82.5% for meropenem-vaborbactam and 75.2% for piperacillin-tazobactam (difference, 7.3% [95% CI, −3.5% to 18.3%]) in patients with acute pyelonephritis, 60.0% and 60.5% (difference, −0.5% [95% CI, −22.7% to 21.6%]) in patients with complicated UTI and a removable source of infection, and 62.2% and 67.4% (difference, −5.3% [95% CI, −26.0% to 15.5%]) in patients with complicated UTI and a nonremovable source of infection. Overall success by infection type at end of intravenous treatment is shown in Figure 2B.
Clinical Cure
In the microbiologic modified ITT population, clinical cure at end of intravenous treatment (clinical cure or improvement) was 98.4% in the meropenem-vaborbactam group and 95.6% in the piperacillin-tazobactam group (difference, 2.8% [95% CI, −0.7% to 7.1%]) and at test of cure was 90.6% and 86.3% (difference, 4.4% [95% CI, −2.2% to 11.1%]) (Figure 2B).
Microbial Eradication
In the microbiologic modified ITT population, eradication at test of cure was 74.2% in the meropenem-vaborbactam group and 63.4% in the piperacillin-tazobactam group (difference, 10.8% [95% CI, −1.4% to 23.0%]) in patients with acute pyelonephritis; 60.0% and 52.6% (difference, 7.4% [95% CI, −15.4% to 29.3%]) in patients with complicated UTI and a removable source of infection; and 48.6% and 48.8% (difference, −0.2% [95% CI, −21.7% to 21.4%]) in patients with complicated UTI and a nonremovable source of infection.
In the microbiologic evaluable population, eradication at test of cure was 74.8% in the meropenem-vaborbactam group and 67.4% in the piperacillin-tazobactam group (difference, 7.4% [95% CI, −5.1% to 20.0%]) in patients with acute pyelonephritis; 58.8% and 55.9% (difference, 2.9% [95% CI, −20.3% to 25.9%]) in patients with complicated UTI and a removable source of infection; and 45.5% and 48.8% (difference, −3.4% [95% CI, −25.3% to 19.0%]) in patients with complicated UTI and a nonremovable source of infection.
Outcomes by Pathogen
Of the 374 patients in the microbiologic modified ITT population, 85.9% (165/192) of patients in the meropenem-vaborbactam group and 84.6% (154/182) in the piperacillin-tazobactam group had Enterobacteriaceae as a baseline pathogen; the majority had a single pathogen.
Outcomes at end of intravenous treatment in patients in the microbiologic modified ITT group with Enterobacteriaceae by baseline minimum inhibitory concentration to meropenem-vaborbactam and piperacillin-tazobactam are reported in Table 2. Despite 11.0% (17/154) of baseline Enterobacteriaceae isolates in the piperacillin-tazobactam group being resistant to piperacillin-tazobactam, all 17 patients achieved clinical cure, and 16 of 17 achieved microbial eradication at the primary end point.
Table 2. Cure, Eradication, and Overall Success Rates at End of Intravenous Treatment and Test-of-Cure Visits in Patients With Enterobacteriaceae by Baseline MIC (Microbiologic MITT Population)a.
| Meropenem-Vaborbactam (n = 192) | Piperacillin-Tazobactam (n = 182)b | ||||||
|---|---|---|---|---|---|---|---|
| MIC, μg/mL | No./Total (%) | MIC, μg/mL | No./Total (%) | ||||
| Clinical Curec | Microbial Eradication | Overall Successd | Clinical Curec | Microbial Eradication | Overall Successd | ||
| End of Intravenous Treatment | |||||||
| ≤0.06 | 146/149 (98.0) | 146/149 (98.0) | 146/149 (98.0) | ≤0.05 | 10/10 (100) | 10/10 (100) | 10/10 (100) |
| 0.12 | 12/12 (100) | 11/12 (91.7) | 12/12 (100.0) | 1 | 14/15 (93.3) | 14/15 (93.3) | 14/15 (93.3) |
| 0.25 | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2 | 57/60 (95.0) | 56/60 (93.3) | 57/60 (95.0) |
| 0.5 | 1/1 (100) | 1/1 (100) | 1/1 (100) | 4 | 21/22 (95.5) | 20/22 (90.9) | 20/22 (90.9) |
| 32b | 1/1 (100) | 1/1 (100) | 1/1 (100) | 8 | 6/6 (100) | 6/6 (100) | 6/6 (100.0) |
| 16 | 10/11 (90.9) | 10/11 (90.9) | 10/11 (90.9) | ||||
| 32 | 10/11 (90.9) | 8/11 (72.7) | 10/11 (90.9) | ||||
| 64 | 2/2 (100) | 2/2 (100) | 2/2 (100) | ||||
| >64 | 17/17 (100) | 16/17 (94.1) | 17/17 (100) | ||||
| Total | 162/165 (98.0) | 161/165 (98.0) | 162/165 (98.2) | 147/154 (95.0) | 142/154 (92.0) | 146/154 (94.8) | |
| Test-of-Cure Visit | |||||||
| ≤0.06 | 135/149 (90.6) | 105/149 (70.5) | 113/149 (75.8) | ≤0.05 | 9/10 (90.0) | 6/10 (60.0) | 9/10 (90.0) |
| 0.12 | 10/12 (83.3) | 7/12 (58.3) | 7/12 (58.3) | 1 | 14/15 (93.3) | 13/15 (86.7) | 14/15 (93.3) |
| 0.25 | 2/2 (100.0) | 1/2 (50.0) | 1/2 (50.0) | 2 | 52/60 (86.7) | 40/60 (66.7) | 46/60 (76.7) |
| 0.5 | 1/1 (100.0) | 1/1 (100) | 1/1 (100) | 4 | 19/22 (86.4) | 12/22 (54.5) | 15/22 (68.2) |
| 32e | 1/1 (100.0) | 1/1 (100) | 1/1 (100) | 8 | 6/6 (100) | 5/6 (83.3) | 5/6 (83.3) |
| 16 | 7/11 (63.6) | 4/11 (36.4) | 4/11 (36.4) | ||||
| 32 | 8/11 (72.7) | 5/11 (45.5) | 6/11 (54.5) | ||||
| 64 | 2/2 (100) | 1/2 (50.0) | 1/2 (50.0) | ||||
| >64 | 17/17 (100) | 9/17 (52.9) | 10/17 (58.8) | ||||
| Total | 149/165 (90.3) | 115/165 (69.7) | 123/165 (74.5) | 110/154 (71.4) | 95/154 (61.7) | 110/154 (71.4) | |
Abbreviations: MIC, minimum inhibitory concentration; MITT, modified intent-to-treat.
The microbiologic MITT population included all patients in the MITT population with 1 or more bacterial 1 pathogens of 105 CFU/mL or greater in baseline urine culture or the same bacterial pathogen present in concurrent blood and urine cultures.
US Food and Drug Administration/Clinical and Laboratory Standards Institute breakpoint for piperacillin-tazobactam susceptibility of Enterobacteriaceae is 16 μg/mL or less; resistance is greater than 64 μg/mL..
At end of intravenous treatment, clinical cure represents patients with complete resolution or significant improvement of baseline signs and symptoms of complicated urinary tract infection, including acute pyelonephritis.
At end of intravenous treatment, overall success represents patients with clinical cure or improvement and microbial eradication. At test of cure, overall success represents patients with clinical cure and microbial eradication.
For an organism containing an OXA-48 enzyme.
Evaluation of Adverse Events
In the meropenem-vaborbactam and piperacillin-tazobactam groups, respectively, the proportions of patients who experienced an adverse event (39.0% and 35.5%), study drug-related adverse event (15.1% and 12.8%), severe adverse event (2.6% and 4.8%), or life-threatening adverse event (congestive cardiac failure, septic shock secondary to salpingo-oophoritis, and an infusion-related reaction) (1.1% and 0%) were similar; the number of patients who died (0.7% in each group), experienced a serious adverse event (4.0% and 4.4%) or an adverse event leading to study drug discontinuation (2.6% and 5.1%), or study discontinuation (1.1% in each group) was low and similar across treatment groups (eTable 4 in Supplement 2).
Adverse events occurring in 1.5% or more of patients in either treatment group are reported in Table 3. Headache, all instances of which were mild or moderate in severity, was the most common adverse event reported with meropenem-vaborbactam treatment, and no patients discontinued study treatment because of headache. The proportion of patients with a potentially clinically significant elevation in a laboratory test value or vital sign was low and numerically similar in the treatment groups.
Table 3. Treatment-Emergent Adverse Events Occurring in 1.5% or More of Patients (Either Group; Modified Intent-to-Treat Population)a.
| Adverse Event | No. (%)b | ||
|---|---|---|---|
| Meropenem-Vaborbactam (n = 272) | Piperacillin-Tazobactam (n = 273) | Total (n = 545) | |
| Headache | 24 (8.8) | 12 (4.4) | 36 (6.6) |
| Diarrhea | 9 (3.3) | 12 (4.4) | 21 (3.9) |
| Nausea | 5 (1.8) | 4 (1.5) | 9 (1.7) |
| Asymptomatic bacteriuria | 4 (1.5) | 4 (1.5) | 8 (1.5) |
| Catheter site phlebitisc | 5 (1.8) | 3 (1.1) | 8 (1.5) |
| Infusion site phlebitis | 6 (2.2) | 2 (0.7) | 8 (1.5) |
| Urinary tract infection | 4 (1.5) | 4 (1.5) | 8 (1.5) |
| Hypokalemia | 3 (1.1) | 4 (1.5) | 7 (1.3) |
| Vaginal infection | 1 (0.4) | 6 (2.2) | 7 (1.3) |
| Alanine aminotransferase increased | 5 (1.8) | 1 (0.4) | 6 (1.1) |
| Anemia | 2 (0.7) | 4 (1.5) | 6 (1.1) |
| Aspartate aminotransferase increased | 4 (1.5) | 2 (0.7) | 6 (1.1) |
| Pyrexia | 4 (1.5) | 2 (0.7) | 6 (1.1) |
| Dyspnea | 0 | 5 (1.8) | 5 (0.9) |
Treatment-emergent adverse events are adverse events with a start date and time on or after the first dose of study drug.
Frequency data are for numbers of participants experiencing an event, not for numbers of adverse events. Percentages calculated using the number of patients in the column heading as the denominator.
Phlebitis associated with catheter sites, not with intravenous infusion of study drug.
Discussion
Noninferiority was demonstrated for meropenem-vaborbactam compared with piperacillin-tazobactam for the FDA primary end point of overall success at the end of intravenous treatment in the microbiologic modified ITT population. In addition, superiority was observed. This finding is notable, given the observed high efficacy of piperacillin-tazobactam (overall success, 94.0%). Noninferiority was also observed for the EMA primary end point of eradication at the test-of-cure visit in the coprimary populations (microbiologic modified ITT and microbiologic evaluable).
The overall success at the test-of-cure visit (74.5% in the meropenem-vaborbactam group, 70.3% in the piperacillin-tazobactam group) was lower for both treatment groups than at the end of intravenous treatment (98.4% vs 94.0%, respectively). These differences were driven primarily by lower rates of microbial eradication at the test-of-cure visit (meropenem-vaborbactam, 68.8%; piperacillin-tazobactam, 62.1%). The lower outcomes at test of cure may have been affected by the approximately 10% of patients who received levofloxacin as oral therapy despite having a levofloxacin-resistant organism; however, these patients were distributed equally in the 2 groups. Clinical cure rates remained high at the test-of-cure visit (90.6% in the meropenem-vaborbactam group vs 86.3% in the piperacillin-tazobactam group), indicating that most of the patients with microbiologic recurrence or persistence had asymptomatic bacteriuria.
Piperacillin-tazobactam continues to be highly active against Enterobacteriaceae isolated from patients with UTIs, including extended-spectrum beta-lactamase producers, with 94.2% of isolates from patients at US medical centers with UTIs in a 2012 to 2014 survey shown to be susceptible. Piperacillin-tazobactam is widely used to treat serious gram-negative infections, including UTIs. Although approximately 12% of Enterobacteriaceae were resistant to piperacillin-tazobactam at baseline, there was no apparent relationship between piperacillin-tazobactam minimum inhibitory concentrations and overall success, clinical cure, or microbial eradication by FDA or EMA criteria. This result may reflect that resistance is defined according to plasma exposures and that high urinary concentrations of piperacillin-tazobactam are achieved.
Meropenem-vaborbactam was well tolerated, with 2.6% of patients discontinuing study treatment because of an adverse event compared with 5.1% discontinuing piperacillin-tazobactam. Overall incidence of adverse events and serious adverse events was numerically similar between treatment groups. The adverse event profile of meropenem-vaborbactam was comparable to that of piperacillin-tazobactam in this trial and to that published for meropenem alone.
Limitations
This study has several limitations. First, the majority of patients were enrolled outside of the United States and, although all were symptomatic, had pyuria, had urine cultures with pathogen growth greater than 105 CFU/mL, and had significant comorbidities, less than one-third met the criteria for systemic inflammatory response syndrome, and it is unclear whether all would have met criteria for hospitalization in the United States.
Second, 31% of patients enrolled did not have a baseline pathogen present at 105 CFU/mL or greater, despite meeting the clinical and pyuria criteria, and therefore were not included in the primary analysis population (ie, the microbiologic modified ITT population). However, in 2 recent registration programs for complicated UTI including more than 1000 patients each for ceftazidime-avibactam and ceftolozane-tazobactam, using the same inclusion criteria resulted in the exclusion of 20.6% and 25.1% of patients, respectively, from the microbiologic modified ITT population. In these trials, as in the current one, the percentage of patients enrolled who meet the symptom/sign inclusion criteria and have a baseline pathogens of 105 CFU/mL or greater ranges from 68% to 78%, suggesting that the complicated UTI or acute pyelonephritis criteria required for enrollment based on regulatory guidance may not be reflective of clinical practice. The results of the noninferiority and superiority analyses are restricted to the 68% of patients who grew a pathogen at baseline.
Third, an extended infusion of piperacillin-tazobactam was not used to match that of meropenem-vaborbactam; although extended infusions of beta-lactam agents are being increasingly used in practice, it is not standard for complicated UTI, and the option was not acceptable to regulatory authorities for this study.
Fourth, this study was not designed to evaluate the use of meropenem-vaborbactam for the treatment of carbapenem-resistant pathogens, because enrolling such patients into a trial with piperacillin-tazobactam as a comparator would be unethical.
Conclusions
Among patients with complicated UTI and growth of a baseline pathogen, meropenem-vaborbactam vs piperacillin-tazobactam resulted in a composite outcome of complete resolution or improvement of symptoms along with microbial eradication that met the noninferiority criterion. Further research is needed to understand the spectrum of patients in whom meropenem-vaborbactam offers a clinical advantage.
Study Protocol
eFigure 1. Study Schema
eTable 1. Distribution of Patients by Site
eTable 2. Baseline Characteristics
eTable 3. Drug Resistance by EUCAST Criteria in Baseline Urinary Gram-Negative Pathogens (m-MITT Population)
eTable 4. Overview of Adverse Events (MITT Population)
eReferences
References
- 1.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58(2):654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamma PD, Avdic E, Keenan JF, et al. What is the more effective antibiotic stewardship intervention: preprescription authorization or postprescription review with feedback? Clin Infect Dis. 2017;64(5):537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Antibiotic resistance threats in the United States, 2013. CDC website. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed April 30, 2017.
- 4.World Health Organization (WHO) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO website. http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed April 30, 2017.
- 5.Lee C-R, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander EL, Loutit J, Tumbarello M, et al. Carbapenem-resistant Enterobacteriaceae infections: results from a retrospective series and implications for the design of prospective clinical trials. Open Forum Infect Dis. 2017;4(2):ofx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levison ME, Kaye D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr Infect Dis Rep. 2013;15(2):109-115. [DOI] [PubMed] [Google Scholar]
- 9.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943-950. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2014;20(9):862-872. [DOI] [PubMed] [Google Scholar]
- 12.Tarazi Z, Sabet M, Rubio-Aparicio D, et al. Efficacy of simulated human exposures of Carbavance (meropenem-RPX7009) against carbapenem-resistant Enterobacteriaceae in an in vitro hollow fiber model. Presented at: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 5-9, 2014; Washington, DC. [Google Scholar]
- 13.Tarazi Z, Sabet M, Rubio-Aparicio D, et al. Efficacy of simulated human exposures of meropenem compared to Carbavance (meropenem-RPX7009) against Pseudomonas aeruginosa in an in vitro hollow fiber model. Presented at: 25th European Congress of Clinical Microbriology and Infectious Diseases; April 25-28, 2015; Copenhagen, Denmark. [Google Scholar]
- 14.Wenzler E, Gotfried MH, Loutit JS, et al. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother. 2015;59(12):7232-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith DC, Rubino CM, Loutit JS, et al. A phase 1 study of the safety, tolerability, and pharmacokinetics of the beta-lactamase inhibitor RPX7009 alone, meropenem alone, and both in combination TID for 7 days in healthy adult subjects (poster 401). Presented at: IDWeek; October 8-12, 2014; Philadelphia, PA. [Google Scholar]
- 16.US Food and Drug Administration (FDA) Complicated urinary tract infections: developing drugs for treatment: guidance for industry. FDA website. https://www.fda.gov/downloads/Drugs/Guidances/ucm070981.pdf. February 2015. Accessed November 15, 2017.
- 17.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Seventh Informational Supplement, M100-S27. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard: M07-A10. 10th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 19.European Medicines Agency Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/11/WC500153953.pdf. October 24, 2013. Accessed November 15, 2017.
- 20.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213-226. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration (FDA) Guidance for Industry. Antibacterial therapies for patients with an unmet medical need for the treatment of serious bacterial diseases: guidance for industry. FDA website. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM359184.pdf. August 2017. Accessed December 7, 2017.
- 22.European Committee on Antimicrobial Susceptibility Testing (EUCAST) EUCAST criteria. EUCAST website. http://www.eucast.org/. Accessed November 17, 2017.
- 23.Sader HS, Castanheira M, Flamm RK, Jones RN. Antimicrobial activities of ceftazidime-avibactam and comparator agents against gram-negative organisms isolated from patients with urinary tract infections in U.S. medical centers, 2012 to 2014. Antimicrob Agents Chemother. 2016;60(7):4355-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magill SS, Edwards JR, Beldavs ZG, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden P. Safety profile of meropenem: an updated review of over 6,000 patients treated with meropenem. Drug Saf. 2007;30(8):657-668. [DOI] [PubMed] [Google Scholar]
- 26.Mohr JF., III Update on the efficacy and tolerability of meropenem in the treatment of serious bacterial infections. Clin Infect Dis. 2008;47(suppl 1):S41-S51. [DOI] [PubMed] [Google Scholar]
- 27.Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63(6):754-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet. 2015;385(9981):1949-1956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eFigure 1. Study Schema
eTable 1. Distribution of Patients by Site
eTable 2. Baseline Characteristics
eTable 3. Drug Resistance by EUCAST Criteria in Baseline Urinary Gram-Negative Pathogens (m-MITT Population)
eTable 4. Overview of Adverse Events (MITT Population)
eReferences