Abstract
Objective
To identify and save parathyroid glands during thyroidectomy by displaying their autofluorescence.
Methods
Autofluorescence imaging was carried out during thyroidectomy with and without central lymph node dissection. After visual recognition by the surgeon, the parathyroid glands and the surrounding tissue were exposed to near-infrared light with a wavelength of 690–770 nm using a modified Karl Storz near infrared/indocyanine green endoscopic system. Parathyroid tissue was expected to show near infrared autofluorescence at 820 nm, captured in the blue channel of the camera.
Results
We investigated 41 parathyroid glands from 20 patients; 37 glands were identified correctly based on near-infrared autofluorescence. Neither lymph nodes nor thyroid revealed substantial autofluorescence and nor did adipose tissue.
Conclusions
Parathyroid tissue is characterised by showing autofluorescence in the near-infrared spectrum. This effect can be used to identify and preserve parathyroid glands during thyroidectomy.
Keywords: Parathyroid glands, Autofluorescence, Near infrared light, Thyroidectomy
Introduction
Hypoparathyroidism caused by devascularisation or accidental removal of parathyroid glands remains a major postoperative complication of total thyroidectomy.1–3 The resulting hypocalcaemia may cause severe symptoms and anxiety in affected patients. While transient hypocalcaemia generally responds well to a replacement therapy with calcium and vitamin D, permanent hypocalcaemia represents a disastrous situation with lifelong morbidity.2,4 The underlying problem is the difficulty in accurately localising the parathyroid glands at an early stage in the surgery. They are small, vary widely in their location and are often difficult to distinguish from surrounding lymph nodes or adipose tissue.5,6 Their vascularisation is fragile and can easily be compromised during the operation.7,8 It would therefore be desirable to possess a simple and reliable technique for identifying parathyroid glands intraoperatively. Initially, methylene blue or 5-aminolevulinic acid (5-ALA) were tested to improve the visualisation of parathyroid tissue.9–13 Although these methods have been successful in initial trials, they have not become generally accepted in clinical practice. Another recent concept involves indocyanine green angiography to localise parathyroid glands and to assess their perfusion.14–17 In 2011, biomedical engineers at Vanderbilt University, Nashville, Tennessee, first described the autofluorescent properties of parathyroid tissue in the near infrared spectrum.18 Several studies have shown that autofluorescence spectroscopy represents an effective technique to visualise parathyroid glands intraoperatively.19–21 In a preliminary study, we investigated the autofluorescence mainly of parathyroid adenomas using a slightly modified commercially available indocyanine green imaging system.22 In the present study, we have focused on the identification of normal parathyroid tissue during total thyroidectomy.
Patients and methods
Patients
Patients presenting with primary thyroid disease and undergoing thyroidectomy were recruited for this study. Informed consent was obtained from all participants. The study was approved by the Ethical Review Board of the Medical Faculty of the University of Munich.
Imaging system
Parathyroid autofluorescence was detected using a commercially available near infrared/indocyanine green endoscopic system (Karl Storz, Tuttlingen, Germany). The system comprises a high-end full high definition camera system (H3-Z 3-Chip Full HD camera, Karl Storz; Fig 1) connected to a 10-mm 0-degree indocyanine green laparoscope (Hopkins™ II, Karl Storz). The laparoscope is equipped with a specific filter for optimal detection of white light and near infrared fluorescence. The systems xenon light source (D-Light P, Karl Storz) provides both visible and near infrared excitation light. The surgeon can use a footswitch to change from white light to near infrared. The additional costs involved for a near infrared/indocyanine green system are approximately 4,000 euros (around £3,390) compared with a standard laparoscopy tower.
Figure 1.
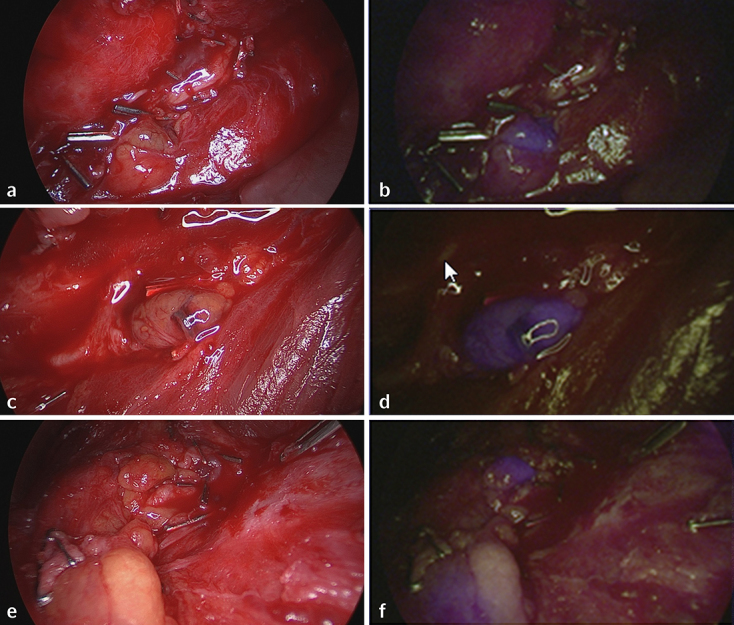
Autofluorescence of parathyroid glands. Parathyroid glands exposed to normal white light (a, c, e) and near infrared light (b, d, f). Parathyroid tissue displays a well-recognisable bluish violet colour representing autofluorescence; the surrounding structures remain nonfluorescent. Images (e) and (f) display two parathyroid glands.
After previous investigations showed a considerable amount of light being backscattered and recorded in the blue channel, the light source was modified by interposing an additional long pass filter. Further, a bandpass filter was added to reduce the light in the green and red spectral region.
Intraoperative fluorescence imaging
Fluorescence imaging was carried out during thyroid surgery for benign and malignant disease. Special care was taken to visualise the parathyroid glands and to preserve their vascularisation. However, we did not search for them when they were not apparent during initial thyroid dissection. Images were collected only after definite identification of a parathyroid gland. In most cases, the thyroid had been laterally mobilised but was still in place. The tip of the laparoscope was held stationary approximately 5cm above the tissue. First, white light images were collected. Second, with all operating room lights switched off, the parathyroid gland and the surrounding tissue were exposed to near infrared light. As near-infrared light was detected and displayed as a blue signal by the camera system, the parathyroid gland was expected to be displayed in the blue colour channel.
In the near infrared-mode, the light source emitted little green and red light to provide visualisation of the surrounding tissue. Therefore, the bluish violet coloured parathyroid glands could be seen in their exact anatomical position (Fig 1). To be able to compare parathyroid autofluorescence with other tissue entities we also investigated thyroid tissue, adipose tissue and lymph nodes.
Results
Twenty patients were included in this initial study. We examined 41 parathyroid glands, 17 lymph nodes and in each patient adipose and thyroid tissue; 37 parathyroid glands showed the typical bluish violet colour of near infrared autofluorescence, displayed in the blue colour channel of the camera. It was easily possible to discriminate them from the surrounding tissue. None of the other tissue entities examined revealed autofluorescence. In two patients (four parathyroid glands) we could not visualise parathyroid autofluorescence and we are unable to specify the reasons. In two cases, autofluorescence imaging was helpful in identifying an inferior parathyroid gland during central lymph node dissection and to preserve it for autotransplantation (Fig 2). The sensitivity of identifying a parathyroid gland by autofluorescence was 90%. We required approximately 3 minutes to prepare the imaging system for use and 3 minutes to visualise both parathyroid glands on one side, including photodocumentation. All in all, we calculated an extra 10 minutes of operating time for the procedure.
Figure 2.
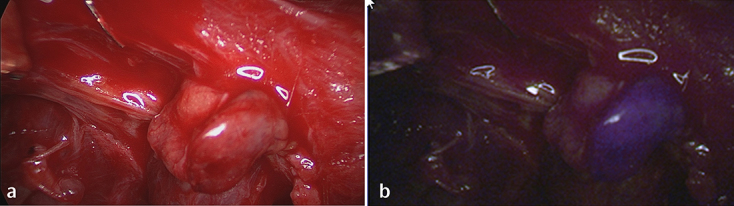
An inferior parathyroid gland identified during central lymph node dissection and stuck to a lymph node. In the near infrared mode (b) the parathyroid tissue displays a well-recognisable bluish violet colour; (a) shows the same parathyroid gland exposed to white light.
Discussion
Parathyroid glands are characterised by a unique autofluorescence when exposed to near infrared light. This effect can be used intraoperatively to distinguish them from thyroid tissue, lymph nodes and adipose tissue. The fluorescent signature with an excitation wavelength of 785 nm and a peak emission at 822 nm was first described by Paras et al. in 2011.18 Subsequent clinical investigations confirmed the utility of autofluorescence spectroscopy for intraoperative parathyroid detection. The system applied consisted of a 785 nm diode laser, a fibreoptic probe and a spectrometer. McWade et al. investigated nearly 150 patients undergoing cervical operations and reported a sensitivity of 97%.19–21 De Leeuw et al. validated autofluorescence imaging in 2016 by using a commercially available indocyanine green fluorescence imaging device (Fluobeam® 800, Fluoptics) which excites tissue with a wavelength of 750 nm and collects near infrared light with wavelengths over 800 nm. The system provides real time images of fluorescent structures. The authors reported a sensitivity of 94% and specificity of 80%.23
Similar to De Leeuw et al., we intended to detect parathyroid autofluorescence by using a commercially available near infrared endoscopic system. However, in contrast to the two other research groups, who applied a diode laser to generate near infrared light, our system comprises a xenon light source which is able to switch between white and near infrared light. The charge coupled device camera used is sensitive for near infrared light in the blue channel. In the near infrared mode, the light source emits no blue light and very little green and red light to provide visualisation of the surrounding tissue. This is a major advantage compared with the intraoperative images generated by McWade et al. and De Leeuw et al.19–22 McWade et al. aimed to characterise the distribution and biochemical properties of this unique parathyroid fluorophore.24 Biochemical and structural analyses showed that it was mainly present in cytoplasm organelles but not in nuclei, cell membranes or extracellular connective tissue. The fluorophore had a molecular weight of approximately 15 kDa and was relatively resistant to heat and proteinase activity.
Identification of a parathyroid adenoma does not usually represent a major problem. Enlarged parathyroid glands can be localised preoperatively in most cases and are resected using a minimally invasive approach. Parathyroid detection becomes more important in extensive thyroid surgery such as total thyroidectomy or central lymph node dissection. According to a 2014 meta-analysis by Edafe et al.,1 the estimated prevalence of postoperative transient and permanent hypoparathyroidism following thyroid surgery varies from 19% to 38% and from 0% and 3%, respectively. To prevent this complication, it is of utmost importance to carry out a careful capsular dissection and to assess the vascular pedicles to the parathyroid glands. Every effort should be made to localise the parathyroid glands at an early stage of the operation. Autofluorescence imaging may be helpful in achieving this goal. If a central lymph node dissection becomes necessary, inferior parathyroid glands localised within the thyrothymic ligament are particularly at risk of being resected accidentally. Here, we see another strong argument for using an intraoperative localisation technique. In the present series we identified several inferior parathyroid glands by autofluorescence which otherwise would have been lost for autotransplantation.
Although the spectral features of indocyanine green and the parathyroid fluorochrome are largely similar, the near infrared/indocyanine green system used in this study has not yet been fully optimised for detecting the considerably weaker parathyroid autofluorescence. The described technique cannot therefore be applied as a real screening tool and does not replace meticulous dissection by an experienced endocrine surgeon. Parathyroid glands are often covered by a sheath of adipose tissue which absorbs near infrared light and needs to be partially removed to display the parathyroid capsule. However, the technique is ideal to verify the presence of parathyroid tissue.
Overall, this initial study gives a first impression of the utility of autofluorescence imaging to identify parathyroid glands during thyroidectomy. It is premature to assume that this new concept will assert itself in the future but it has so far been shown to be reliable. It will have to compete with other techniques such as indocyanine green-enhanced fluorescence imaging, which currently has several proponents. In our opinion, it would be worth directly comparing these two different approaches.
Compliance with Ethical Standards
Karl Storz GmbH & Co. KG supported the study by providing an endoscopic near infrared/indocyanine green system free of charge and assisted in modifying the system. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
References
- 1.Edafe O, Antakia R, Laskar N et al. . Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014; : 307–320. [DOI] [PubMed] [Google Scholar]
- 2.Lorente-Poch L, Sancho JJ, Munoz-Nova JL et al. . Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015; : 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorente-Poch L, Sancho JJ, Ruiz S et al. . Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015; : 359–367. [DOI] [PubMed] [Google Scholar]
- 4.Stack BC Jr, Bimston DN, Bodenner DL et al. . American Association of Clinical Endocrinologists and American College of Endocrinology. Disease state clinical review: postoperative hypoparathyroidism- definitions and management. Endocr Pract 2015; (6): 674–685. [DOI] [PubMed] [Google Scholar]
- 5.Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery 1984; (1): 14–21. [PubMed] [Google Scholar]
- 6.Thompson NW, Eckhauser FE, Harness JK. The anatomy of primary hyperparathyroidism. Surgery 1982, (5): 814–821. [PubMed] [Google Scholar]
- 7.Bergenfelz AO, Jansson SK, Wallin GK et al. . Impact of modern techniques on short-term outcome after surgery for primary hyperparathyroidism: a multicenter study comprising 2,708 patients. Langenbecks Arch Surg 2009; (5): 851–860. [DOI] [PubMed] [Google Scholar]
- 8.Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg 2002; : 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han N, Bumpous JM, Goldstein RE et al. . Intra-operative parathyroid identification using methylene blue in parathyroid surgery. Am Surg 2007, (8):820–823. [PubMed] [Google Scholar]
- 10.Prosst RL, Weiss J, Hupp L et al. . Fluorescence-guided minimally invasive parathyroidectomy: clinical experience with a novel intraoperative detection technique for parathyroid glands. World J Surg 2010; (9):2 217–2,222. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Numata T, Shibuya M. Intraoperative photodynamic detection of normal parathyroid glands using 5-aminolevulinic acid. Laryngoscope 2011; (7): 1,462–1,466. [DOI] [PubMed] [Google Scholar]
- 12.Patel HP, Chadwick DR, Harrison BJ et al. . Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg 2012; (10): 1,345–1,351. [DOI] [PubMed] [Google Scholar]
- 13.van der Vorst JR, Schaafsma BE, Verbeek FP et al. . Intraoperative near-infrared fluorescence imaging of parathyroid adenomas with use of low-dose methylene blue. Head Neck 2014; (6): 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavazza M, Liu X, Wu C et al. . Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg 2016; (5): 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidal Fortuny J, Belfontali V, Sadowski SM et al. . Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 2016; (5): 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaidi N, Bucak E, Yazici P et al. . The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016; (7): 775–778. [DOI] [PubMed] [Google Scholar]
- 17.Lang BH, Wong CK, Hung HT et al. . Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 2017; (1): 87–95. [DOI] [PubMed] [Google Scholar]
- 18.Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. Near-infrared auto-fluorescence for the detection of parathyroid glands. J Biomed Optics 2011; : 067012. [DOI] [PubMed] [Google Scholar]
- 19.McWade MA, Paras C, White LM et al. . A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013; (6): 1,371–1,317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McWade MA, Paras C, White LM et al. . Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 2014; (12): 4,574–4,580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McWade MA, Sanders ME, Broome JT et al. . Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016; (1): 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladurner R, Sommerey S, Arabi NA et al. . Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc. 2016. November 14. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.De Leeuw F, Breuskin I, wAbbaci M et al. . Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: a feasibility study. World J Surg 2016; (9): 2,131–2,138. [DOI] [PubMed] [Google Scholar]
- 24.Thomas G, McWade MA, Sanders ME et al. . Identifying the novel endogenous near-infrared fluorophore within parathyroid and other endocrine tissues. Biomedical Optics 2016, OSA Technical Digest (online) (Optical Society of America, 2016), paper PTu3A.5. [Google Scholar]


