Key Points
Question
Can retinal morphology using spectral-domain optical coherence tomography be a potential biomarker in eyes with diabetic macular edema?
Findings
In a cross-sectional observational case series including 102 eyes from 80 individuals, disorganization of the inner retinal layers was strongly associated with disruption of outer retinal layers. Disorganization of the inner retinal layers was more frequently observed in eyes with increasing severity of diabetic retinopathy and was associated with poorer visual acuity.
Meaning
Disorganization of the inner retinal layer may be associated with morphological changes in the outer retina and worse levels of diabetic retinopathy in eyes with diabetic macular edema, although the cross-sectional design precludes determining cause-and-effect relationships.
This cross-sectional observational case series examines possible association of disorganization of the inner retinal layers with morphological changes in the outer retina and severity of diabetic retinopathy in eyes with diabetic macular edema.
Abstract
Importance
In diabetic macular edema (DME), identification of baseline markers on spectral-domain optical coherence tomography (SD-OCT) and their association with severity of diabetic retinopathy (DR) might aid in disease management and the design of future trials.
Objective
To examine associations between DR severity, retinal morphology on SD-OCT, and visual acuity in participants with DME.
Design, Setting, and Participants
This cross-sectional observational case series was conducted at a single tertiary care referral center. Demographics, visual acuity, SD-OCT, and color fundus photographs of 80 individuals with DME (102 eyes) seen between December 28, 2013, and April 30, 2014, were analyzed between May 1 and July 31, 2016.
Main Outcomes and Measures
Features captured on SD-OCT and thickness metrics. On SD-OCT we graded type and shape of DME, shape and presence of septae within the intraretinal cystoid abnormalities, presence of hyperreflective dots and foci, integrity of the external limiting membrane and ellipsoid zone, presence and extent of disorganization of the inner retinal layers (DRIL), and the status of the vitreomacular interface and epiretinal membrane. We measured retinal thickness at the fovea and at the site of maximum pathology, choroidal thickness at the fovea, and 1000 μm temporal and nasal to the fovea. Color photographs were graded to derive a DR severity stage.
Results
The mean (SD) age was 63 (11) years, and 30 participants (37.5%) were women. The odds of having DRIL were greater in eyes with disrupted external limiting membrane (odds ratio [OR], 4.4; 95% CI, 1.6-12.0; P = .003), disrupted ellipsoid zone (OR, 2.7; 95% CI, 1.0-7.2; P = .03), presence of epiretinal membrane (OR, 2.8; 95% CI, 1.0-7.4; P = .03), and increase in retinal thickness at the fovea (OR, 1.6; 95% CI, 1.1-2.2; P < .001). Occurrence of DRIL was more likely in eyes with proliferative DR (OR, 7.3; 95% CI, 1.7-31.4; P = .007). Mean visual acuity decreased by approximately 4.7 letters for each 100-μm increase in the average global DRIL (95% CI, −7.9 to 1.4; P = .006).
Conclusions and Relevance
An association was found between DRIL and disruption of the outer retina and increasing DR severity. Further longitudinal studies seem warranted to determine whether DRIL is a clinically relevant noninvasive morphological marker in eyes with DME.
Introduction
Diabetic retinopathy (DR) represents a spectrum of pathological changes that occur in the microvasculature of the eye in patients with diabetes mellitus. Diabetic macular edema (DME) is a characteristic feature of DR and an important cause of vision loss in people with diabetes. In patients with DME, fluid accumulates within the macular tissue layers as a consequence of failure of the blood-retinal barrier. Typically DME causes blurring and distortion of vision, which is reflected in a reduction in visual acuity (VA). The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) found that 20% of patients with type 1 diabetes and 25% of those with type 2 diabetes will develop DME after 10 years of follow-up.
Although DME is detectable as increases in macular thickness based on biomicroscopic examination and through stereoscopic assessments of fundus color images, the introduction of time-domain optical coherence tomography (OCT) enabled a more precise quantification of overall retinal thickness; however, this measure correlates only modestly with VA. The subsequent availability of spectral-domain OCT (SD-OCT), which has superior resolution and reproducibility, has resulted in improved ability to detect early DME and also permits characterization of pathology on a retinal layer-by-layer basis. Features such as hyperreflective foci can be localized to individual retinal layers or within the walls of microaneurysms. Studies have established correlations between VA and certain features of retinal morphology, such as the intactness of the ellipsoid zone (EZ) and external limiting membrane (ELM). A recent report from Sun et al described an OCT feature termed disorganization of the retinal inner layers (DRIL); improvement in DRIL was predictive of better VA outcomes. Notably, DRIL was found to be associated with VA after resolution of center-involving DME. Although associations of DRIL and VA have been explored, we know of no data on the associations of DRIL and other OCT features of DME or with severity of DR. The present study represents a systematic examination of the associations of DRIL and SD-OCT features of DME, severity of DR, and VA in a large series of eyes with DME.
Methods
This was a cross-sectional retrospective observational case series at a single clinical center. The study adhered to the tenets of the Declaration of Helsinki and received full ethical approval from the Research Ethics Committee for Northern Ireland. Patient data was deidentified, so informed consent was not required. Details of patients attending this clinic recorded in the electronic medical records were reviewed.
A standardized protocol using trained examiners was used to test and record VA, which was measured on the Early Treatment Diabetic Retinopathy Study chart. Tomographic images were captured (Spectralis; Heidelberg Engineering) on both eyes at all visits and consisted of a single horizontal and vertical line scan through the fovea followed by a 6 × 6-mm macular raster scan (37 raster lines, spacing of 120 μm, 20° × 15°). Color photographs (2 fields, macula centered and optic disc centered) were captured on a Visucam 500 (Carl Zeiss Meditec) after pupillary dilation.
Medical records were queried for patients with a diagnosis of DME who attended a retina service between December 28, 2013, and April 30, 2014. The data analysis was performed from May 1, 2016 to July 31, 2016.
From the 94 unique cases, we selected the records of 80 patients with a confirmed diagnosis of type 1 or 2 diabetes, OCT features of DME, color fundus photography, and a VA record corresponding to the same visit. Patients without diabetes, OCT, or fundus photographs and those younger than 18 years were excluded.
We extracted information on age, sex, duration of diabetes, history of hypertension, hyperlipidemia, and cigarette smoking from the medical record for each case.
Image Analysis
Grading was performed by 2 of us (R.D. and G.S.) after training by the senior clinician (U.C.), who checked 20% of the grading outputs. Intergrader variability was assessed by κ statistics. The images were scrutinized on the proprietary software provided by the manufacturer of the OCT acquisition system. All of the B-scans of the 6 × 6 macular raster were examined, and we recorded the type and shape of DME, shape of the intraretinal cystoid abnormalities and septae if seen within the cystoid abnormalities, presence or absence of hyperreflective dots and foci, and the status of the vitreomacular interface and presence of an epiretinal membrane (ERM). We identified the foveal B-scan (defined as the scan passing through the maximal foveal depression) and measured the horizontal extent of DRIL and disruption of the ELM and EZ. In the data set, fewer than 10% of eyes were classified as not showing a foveal dip. In these cases where the foveal scan was not clearly identifiable, we used the scan offset by 5° from the horizontal as the scan of choice. We also measured the retinal thickness at the fovea (RTF) and at the site of maximum pathology (maximum retinal thickness; MRT). We measured choroidal thickness (CT) at the fovea and 1000 μm temporal and nasal to fovea. The grading form is shown in eTable 1 in the Supplement.
We defined DRIL as the presence of a region on the B-scan where the boundaries between the ganglion cell and inner plexiform layer complex, inner nuclear layer, and outer plexiform layer could not be separately identified. The method used to determine the horizontal extent of DRIL in each of 7 B-scans was identical to that described by Sun et al in that we only measured the diameter up to a maximum of 1000 μm. The B-scans that were selected were the foveal scan and the 3 immediately superior and inferior to the foveal scan. The measurements from these 7 scans were summed to derive an average global DRIL measure for each eye.
We graded the color photographs according to the Early Treatment Diabetic Retinopathy Study grading protocol to derive a DR severity stage.
Statistical Analysis
We analyzed the data using SPSS version 22 statistical software (IBM). We used κ statistics to test the intergrader variability. We examined associations between VA, age, and duration of diabetes using linear regression. We performed 1-way analysis of variance for associations between VA and hypertension, hyperlipidemia, and smoking. We also used analysis of variance to test associations between VA and the various categorical variables (retinal morphological features observed on OCT and DR severity staging). We used the Pearson coefficient to test for correlations between VA and OCT linear variables (diameter of DRIL, disrupted ELM and EZ, height of RTF, MRT, and CT). With VA as the dependent variable, those variables that reached significance in the preceding steps were then tested using general linear regression modeling after controlling for RTF, which had been previously identified as a confounding factor by Sun et al. As DRIL had been identified as a key determinant of VA outcome, we performed binary logistic regression to seek associations of presence or absence of DRIL with SD-OCT features and DR severity. We tested pairs of variables and did not create a multivariable model, as the modest sample size could have led to invalidation of the independence of the observations on the variable of interest. The interclass correlation of eyes with the same person was 0.08, so we did not deem it necessary to adjust for correlations between 2 eyes. We considered a P value of .05 or less as statistically significant in these analyses.
Results
Descriptive characteristics of the study cohort are shown in Table 1. One hundred two eyes of 80 individuals were analyzed. Because of poor-quality OCT images, 1 eye was not graded for OCT morphology and metrics. The mean (SD) age was 63 (11) years, and 30 participants (37.5%) were female, with the majority of the sample (63 [78.8%]) having type 2 diabetes (Table 1). The frequency of the DR severity stages in the 100 eyes (2 eyes were not graded because of poor-quality color images) is shown in Table 1. Less than half of the included eyes had been previously treated with macular laser, and none had received an anti–vascular endothelial growth factor treatment. The intergrader variability represented by the κ statistic ranged from 0.6 to 1 for the variables tested. Linear regression of VA against age and duration of diabetes showed no significant associations. Analysis of variance also confirmed the absence of associations with key systemic features of hypertension, hyperlipidemia, and smoking (eTable 2 in the Supplement). Therefore, these variables were not included in any of the subsequent analyses.
Table 1. Descriptive Characteristics of the Study Cohort.
| Characteristic | Value |
|---|---|
| Participants (n = 80) | |
| Age, mean (SD), y | 63 (11) |
| Female, No. (%) | 30 (37.5) |
| DM duration, mean (SD), y | 16.74 (10.84) |
| DM type, No. (%) | |
| Type 1 | 17 (21.3) |
| Type 2 | 63 (78.8) |
| Hypertension, No. (%) | 51 (63.7) |
| Hyperlipidemia, No. (%) | 54 (67.5) |
| Smoking, No. (%) | 33 (41.3) |
| Ocular characteristic (n = 102) | |
| Diabetic retinopathy stage, No. (%)a | |
| Mild NPDR | 53 (52) |
| Moderate NPDR | 37 (35.3) |
| Severe NPDR | 0 (0) |
| PDR | 10 (10.8) |
| Previous laser surgery, No. (%) | |
| None | 27 (26.5) |
| Macular laser | 44 (43.1) |
| PRP | 14 (13.7) |
| Both macular laser and PRP | 17 (16.7) |
| Visual acuity, mean (SD), letters | 54.37 (16.13) |
Abbreviations: DM, diabetes mellitus; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation.
Sample size was 100 eyes as 2 eyes were ungradable.
Associations between VA and retinal morphological features identified specific variables that reached statistical significance in the analysis of variance . These were shape of DME, presence of septae in intraretinal cystoid abnormalities, presence of DRIL and disruption of ELM and EZ, and DR severity stage. Other variables that were tested (type and symmetry of intraretinal fluid, presence of cystoid abnormalities and ERM) did not reach significance (eTable 3 in the Supplement). Pearson correlation coefficients (eTable 4 in the Supplement) showed significant correlations between VA and horizontal extent of DRIL, ELM and EZ, RTF, and MRT.
The results from general linear regression modeling adjusted for RTF are shown in Table 2. Variables that lost significance in the model were shape of DME and presence of septae in cystoid abnormalities (Table 2), while all others remained significant. The parameter estimates show that better VA is associated with absence of DRIL, intact ELM and EZ, and less severe stages of DR (Table 2). Presence of intact ELM is associated with a 14-letter-better mean VA (95% CI, 7.9-19.8; P < .001) compared with eyes with disrupted ELM. Similar differences in VA were also found when the EZ was intact (10.5 letters; 95% CI, 4.7-16.4; P = .001) and when DRIL was absent (9.8 letters; 95% CI, 3-16.6; P < .001).
Table 2. Associations Between VA and Retinal Morphological Features of DME and DRa.
| Variable | VA, Mean (SD), Letters |
B (95% CI) | P Value |
|---|---|---|---|
| Shape of DME | |||
| Dome | 54.7 (14.4) | −1.7 (−9.1 to 5.5) | .62 |
| Fusiform | 56.9 (15.3) | 1 [Reference] | |
| Septae in cystoid abnormalities | |||
| Yes | 57.1 (13.5) | 1 [Reference] | .19 |
| No | 49.9 (15.0) | −4.7 (−11.9 to 2.4) | |
| DRIL | |||
| Yes | 46.1 (14.9) | 1 [Reference] | <.001b |
| No | 58.1 (13.5) | 9.8 (3.0 to 16.6) | |
| ELM disrupted | |||
| Yes | 46.1 (14.8) | 1 [Reference] | <.001b |
| No | 61.0 (11.5) | 13.9 (7.9 to 19.8) | |
| EZ disrupted | |||
| Yes | 48.2 (14.8) | 1 [Reference] | .001b |
| No | 60.6 (12.4) | 10.5 (4.7 to 16.4) | |
| DR severity stage | |||
| Mild NPDR | 59.9 (14.0) | 11.6 (2.0 to 21.1) | .01b |
| Moderate NPDR | 55.0 (14.6) | 8.8 (−1.0 to 18.7) | .07 |
| PDR | 45.1 (14.2) | 1 [Reference] |
Abbreviations: ELM, external limiting membrane; EZ, ellipsoid zone; DME, diabetic macula edema; DR, diabetic retinopathy; DRIL, disorganization of inner retinal layers; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; VA, visual acuity.
Results are reported from general linear regression model adjusted for retinal thickness at fovea.
Statistically significant (P < .05).
The parameter estimates indicate that for each 100-μm increase in the average global DRIL, there is a decrease in the mean VA of approximately 4.6 (95% CI, −8.0 to −1.3) letters (Table 3). With each 100-μm increase in disruption of ELM and EZ, the mean VA decreased by 0.4 (95% CI, −0.8 to −0.1 and −0.7 to −0.1, respectively) letters. For each 100-μm increase in RTF and MRT, mean VA decreased by 2.4 (95% CI, −4.0 to −0.7) and 0.04 (95% CI, −0.08 to −0.01) letters, respectively. After adjustment for RTF, all the variables remained significant. The MRT variable showed a strong association with VA, with each 100-μm increase in retinal thickness resulting in a decrease of 13.1 letters (95% CI, –19.6 to –6.6; P < .001). The Figure shows the presence of disrupted ELM and EZ.
Table 3. Correlations Between Visual Acuity and Optical Coherence Tomography–Derived Linear Variablesa.
| Unadjusted | Adjusted for RTF | |||
|---|---|---|---|---|
| Variable | B (95%CI) | P Value | B (95%CI) | P Value |
| RTF per 100 μm | −2.4 (−4.0 to −0.7) | .005b | NA | NA |
| MRT per 100 μm | −0.04 (−0.08 to −0.01) | .01b | −13.1 (−19.6 to −6.6) | <.001b |
| Average global DRIL per 100 μm | −4.6 (−8.0 to −1.3) | .009b | −4.7 (−7.9 to −1.4) | .006b |
| ELM disruption at fovea per 100 μm | −0.4 (−0.8 to −0.1) | .01b | −0.5 (−0.9 to −0.1) | .01b |
| EZ disruption at fovea per 100 μm | −0.4 (−0.7 to −0.1) | .003b | −0.4 (−0.7 to −0.1) | .008b |
Abbreviations: DRIL, disorganization of inner retinal layers; ELM, external limiting membrane; EZ, ellipsoid zone; MRT, maximum retinal thickness; NA, not applicable; RTF, retinal thickness at fovea.
Results are reported from general linear regression model.
Statistically significant (P < .05).
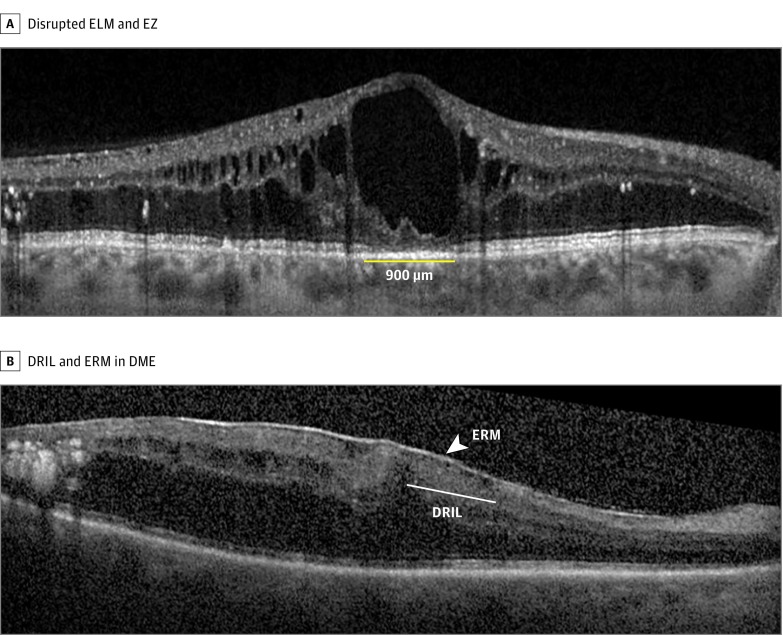
Figure. Spectral-Domain Optical Coherence Tomography Images of Disrupted Inner and Outer Retinal Layers.
A, Spectral-domain optical coherence tomography image showing disrupted external limiting membrane (ELM) and ellipsoid zone (EZ) in diabetic macular edema (DME). The yellow line denotes disruption of the ELM and EZ. B, Spectral-domain optical coherence tomography image showing the presence of disorganization of the retinal layers (DRIL) and epiretinal membrane (ERM) in DME. Arrow depicts the epiretinal membrane. The area of DRIL is denoted by a line where the retinal boundaries cannot be identified.
Relationship Between DRIL and Other OCT Features of DME and DR
Binary logistic regression showed that the odds of having DRIL was greater in eyes with disrupted ELM (odds ratio [OR], 4.4; 95% CI, 1.6-12.0; P = .003) and disrupted EZ (OR, 2.7; 95% CI, 1.0-7.2; P = .03), presence of ERM (OR, 2.8; 95% CI, 1.0-7.4; P = .03), and increase in RTF (OR, 1.6; 95% CI, 1.1-2.2; P < .001) (Table 4). Eyes with PDR were more likely to show evidence of DRIL (OR, 7.3; 95% CI, 1.7-31.4; P = .007) (Table 4). The Figure shows an example of DRIL in an eye with ERM.
Table 4. Association Between DRIL and Other Retinal Morphological Features of DME and DRa.
| Variable | DRIL, No. | OR (95% CI) | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Type of DME | ||||
| IRF only | 17 | 55 | 1 [Reference] | .75 |
| IRF and SRF | 6 | 23 | 0.8 (0.2-2.4) | |
| Type of IRF | ||||
| Diffuse | 4 | 17 | 1 [Reference] | NA |
| Cystoid | 5 | 21 | 1.0 (0.2-4.3) | .98 |
| Mixed | 14 | 40 | 1.0 (0.2-4.3) | .53 |
| Cystoid abnormalities in inner retina | ||||
| Yes | 20 | 61 | 1.8 (0.4-7.0) | .36 |
| No | 3 | 17 | 1 [Reference] | |
| Septae in the cystoid abnormalities | ||||
| Yes | 13 | 46 | 0.6 (0.2-1.7) | .36 |
| No | 7 | 15 | 1 [Reference] | |
| IRF symmetry at fovea | ||||
| Symmetrical | 17 | 52 | 0.7 (0.2-2.0) | .51 |
| Asymmetrical | 6 | 26 | 1 [Reference] | |
| DME shape | ||||
| Dome | 14 | 37 | 0.5 (0.2-1.4) | .26 |
| Fusiform | 9 | 41 | 1 [Reference] | |
| ERM | ||||
| Yes | 11 | 19 | 2.8 (1.0-7.4) | .03b |
| No | 12 | 59 | 1 [Reference] | |
| ELM disrupted | ||||
| Yes | 15 | 23 | 4.4 (1.6-12.0) | .003b |
| No | 8 | 55 | 1 [Reference] | |
| EZ disrupted | ||||
| Yes | 14 | 28 | 2.7 (1.0-7.2) | .03b |
| No | 9 | 50 | 1 [Reference] | |
| RTF, linear variable | 1.6 (1.1-2.2) | <.001b | ||
| DR severity scale | ||||
| Mild NPDR | 9 | 44 | 1 [Reference] | |
| Moderate NPDR | 8 | 29 | 1.3 (0.4-3.8) | .50 |
| PDR | 6 | 4 | 7.3 (1.7-31.4) | .007b |
Abbreviations: DME, diabetic macular edema; DR, diabetic retinopathy; DRIL, disorganization of the inner retinal layers; ELM, external limiting membrane; ERM, epiretinal membrane; EZ, ellipsoid zone; IRF, intraretinal fluid; NA, not applicable; NPDR, nonproliferative diabetic retinopathy; OR, odds ratio; PDR, proliferative diabetic retinopathy; RTF, retinal thickness at fovea; SRF, subretinal fluid.
Results are reported form binary logistic regression with DRIL presence as the dependent variable.
Statistically significant (P < .05).
Discussion
We systematically graded the tomographic images of the retina and sought associations between the presence and severity of a number of morphological features of DME, including DRIL, a novel and recently described biomarker, with severity of DR and visual function. Results from this study suggest that centrally located DRIL is correlated with VA in eyes with center-involved DME and was strongly associated with the disruption of ELM and EZ layers and presence of ERM. Worsening of VA was seen with increasing DR severity and may be related to the presence of DRIL. Centrally located DRIL and the extent of disruption were both associated with worse VA. These findings are similar to those of previous studies that found DRIL to be a predictive marker for VA outcomes in eyes with DME that resolved after treatment. An important finding from our analysis has been the elucidation of the association between the horizontal extent of DRIL and VA. We have shown that for each 100-μm increase in DRIL there is a negative impact of approximately 6 letters, which is more than 1 line on the Early Treatment Diabetic Retinopathy Study chart. It has been hypothesized that disorganization of the inner retina occurs when bipolar axons snap when their elasticity limit has been exceeded because of edema. It has also been suggested that DRIL represents loss of bipolar, amacrine, or horizontal cells within the inner retinal layers. Key findings from the present analysis also include the highly significant associations between presence of DRIL and outer retinal changes. Disorganization of the retinal layers was strongly associated with the disruption of ELM and EZ layers. In our study, RTF was significantly increased in the presence of DRIL. Taken together, these findings suggest that the mechanisms that create the conditions for inner retinal disorganization may also be responsible for concurrent disruption of the outer retinal architecture.
We noted that these outer retinal changes were also associated with poor VA, and our findings are consistent with those from previous studies. Breakdown of the blood-retinal barrier in DME could create the conditions for damage to the ELM and EZ. Unlike DRIL, for which mechanical forces have been considered in its pathogenesis, the disrupted ELM has been postulated to be a consequence of impaired retinal function. It is presently unknown whether the EZ line seen on OCT images truly corresponds to the histologic junction of the inner and outer segments. An important consideration with the growing literature regarding the EZ is how to objectively and consistently evaluate disruption. Maheshwary et al concluded that both the status of inner segment and outer segment disruption and the percentage disruption are important predictors of VA in a patient. Spaide and Curcio speculated that this highly reflective band was located at the ellipsoid in the inner segments, considering the correlation between the microstructure on the SD-OCT images and the histologic findings. Our present work not only reinforces the clinical relevance of an intact EZ but also suggests that the same pathogenic pathways that create conditions for DRIL may also disrupt the outer retinal architecture.
Cystic changes that appear within the macula represent focal areas of coalesced extracellular fluid. These foci of fluid likely result from disturbed cellular function of the Müller cells, which are thought to act as metabolic pumps to keep the macula dehydrated. Based on the univariate analysis, we noted that VA was better in eyes with septae within the cystoid abnormalities compared with those without. Our findings support those of previous investigators.
Many studies have shown that ERM is common in eyes with DME, and we observed a high frequency of ERM in the present series. We systematically graded for ERMs that are recognized on OCT as thin, hyperreflective bands anterior to the retina or bright red bands in the pseudocolor representation on OCT. Gandorfer et al showed that in eyes with DME, multilayered membranes are situated on a layer of native vitreous collagen, predominantly coupled with fibroblasts and fibrous astrocytes. Removal of the ERM by vitrectomy and membrane peel can result in resolution of the DME and improvements in VA. To our knowledge, no study has examined the associations between ERM, DRIL, and VA, and our observations of a strong association between these features suggest a common pathological process. Histological studies are required to understand the pathogenetic mechanisms underpinning these inner retinal morphological manifestations.
A prospective study investigated the correlation between the features of OCT and the severity of retinopathy and found that the prevalence of DME with serous retinal detachment and with vitreomacular traction was higher in eyes with severe nonproliferative diabetic retinopathy or proliferative diabetic retinopathy than in eyes with mild to moderate nonproliferative diabetic retinopathy. It is also known that increasing DR severity is associated with macular thickening. While the present study also shows similar findings, it is the first we know of to report an association between DRIL and increasing severity of DR. Our finding of worsening of VA with increasing DR severity may be related to the presence of DRIL.
A biomarker of interest is the visibility of the cone outer receptor segments (COSTs). It has been shown that there is reduced visibility of COSTs in patients with DME. During grading we observed that COST lines could not be delineated consistently; therefore, we did not grade for this biomarker. Our experience is similar to that of Rii et al, who found that these outer retinal layers could not be segmented even in normal scans of healthy eyes.
The strengths of this study are the systematic grading of color and OCT images and the homogeneous sample of patients with DME naive to anti–vascular endothelial growth factor treatments.
Limitations
An important limitation of the study is its cross-sectional design. We are unable to predict the impact on function over time of the various OCT markers that we identified. Also, our study is retrospective and the sample size is modest. Furthermore, the data were acquired as part of routine clinical care and thus many patients had prior exposure to laser photocoagulation, which can alter the appearance of the retinal layers. However, we believe our findings do not arise as a consequence of laser treatment because the foveal retina is avoided while undertaking macular laser treatment.
In summary, we have systematically studied both inner and outer retinal morphology in DME and associations with both VA and increasing severity of DR.
Conclusions
Disorganization of the inner retinal layers correlated with the integrity of outer retinal architecture and the presence of ERM. The odds of having DRIL increased with DR severity. Disorganization of the inner retinal layers requires further investigation in large clinical trials across multiple sites with prospective data collection with adjustment for multiple potential confounders, including VA and other morphological features on OCT, to be considered as a noninvasive marker.
eTable 1. Grading of SD- OCT images in DME-definitions
eTable 2. Relationship of visual acuity with age and systemic factors
eTable 3. Associations between VA and retinal morphological features of DME and DR
eTable 4. Correlations between VA and OCT linear variables
eFigure 1. SD-OCT image showing both cystoid and diffuse DME
eFigure 2. DME with sub retinal fluid
eFigure 3. Asymmetrical shape of DME
References
- 1.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105(6):998-1003. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, XV: the long-term incidence of macular edema. Ophthalmology. 1995;102(1):7-16. [DOI] [PubMed] [Google Scholar]
- 3.Browning DJ, Glassman AR, Aiello LP, et al. ; Diabetic Retinopathy Clinical Research Network . Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C; Diabetic Retinopathy Research Group Vienna . Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116(5):914-920. [DOI] [PubMed] [Google Scholar]
- 5.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150(1):63-67, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otani T, Yamaguchi Y, Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina. 2010;30(5):774-780. [DOI] [PubMed] [Google Scholar]
- 7.Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):61-70. [DOI] [PubMed] [Google Scholar]
- 8.Sun JK, Lin MM, Lammer J, et al. . Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309-1316. [DOI] [PubMed] [Google Scholar]
- 9.Sun JK, Radwan SH, Soliman AZ, et al. . Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015;64(7):2560-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 2015;133(7):820-825. [DOI] [PubMed] [Google Scholar]
- 11.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786-806. [PubMed] [Google Scholar]
- 12.Pelosini L, Hull CC, Boyce JF, McHugh D, Stanford MR, Marshall J. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011;52(5):2741-2748. [DOI] [PubMed] [Google Scholar]
- 13.Forooghian F, Stetson PF, Meyer SA, et al. . Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina. 2010;30(1):63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol. 2011;151(2):310-317. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31(8):1609-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol. 2008;2(4):919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deák GG, Bolz M, Ritter M, Prager S, Benesch T, Schmidt-Erfurth U; Diabetic Retinopathy Research Group Vienna . A systematic correlation between morphology and functional alterations in diabetic macular edema. Invest Ophthalmol Vis Sci. 2010;51(12):6710-6714. [DOI] [PubMed] [Google Scholar]
- 18.Panozzo G, Parolini B, Gusson E, et al. . Diabetic macular edema: an OCT-based classification. Semin Ophthalmol. 2004;19(1-2):13-20. [DOI] [PubMed] [Google Scholar]
- 19.Gandorfer A, Rohleder M, Grosselfinger S, Haritoglou C, Ulbig M, Kampik A. Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol. 2005;139(4):638-652. [DOI] [PubMed] [Google Scholar]
- 20.Sakimoto S, Saito Y, Nakata K, Sakamoto Y, Tatebayashi M. Surgical outcomes of epiretinal membrane removal after successful pars plana vitrectomy for retinal diseases. Jpn J Ophthalmol. 2008;52(3):227-230. [DOI] [PubMed] [Google Scholar]
- 21.Alkuraya H, Kangave D, Abu El-Asrar AM. The correlation between optical coherence tomographic features and severity of retinopathy, macular thickness and visual acuity in diabetic macular edema. Int Ophthalmol. 2005;26(3):93-99. [DOI] [PubMed] [Google Scholar]
- 22.Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2008;115(3):533-539, e2. [DOI] [PubMed] [Google Scholar]
- 23.Rii T, Itoh Y, Inoue M, Hirakata A. Foveal cone outer segment tips line and disruption artifacts in spectral-domain optical coherence tomographic images of normal eyes. Am J Ophthalmol. 2012;153(3):524-529, e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Grading of SD- OCT images in DME-definitions
eTable 2. Relationship of visual acuity with age and systemic factors
eTable 3. Associations between VA and retinal morphological features of DME and DR
eTable 4. Correlations between VA and OCT linear variables
eFigure 1. SD-OCT image showing both cystoid and diffuse DME
eFigure 2. DME with sub retinal fluid
eFigure 3. Asymmetrical shape of DME