Abstract
Background and Aims:
There are no systematic reviews comparing the use of endoscopic retrograde cholangiopancreatography (ERCP)-based brush cytology and forceps biopsy and endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) for the diagnosis of malignant biliary stricture; so in this revision, we will compare ERCP against EUS-FNA for tissue diagnosis of malignant biliary stricture.
Design:
A systematic review was conducted of comparative studies (prospective or retrospective) analyzing EUS and ERCP for tissue diagnosis of malignant biliary stricture.
Materials and Methods:
The databases Medline, EMBASE, Cochrane, LILACS, CINAHL, and Scopus were searched for studies dated previous to November 2014. We identified three prospective studies comparing EUS-FNA and ERCP for the diagnosis of malignant biliary stricture and five prospective studies comparing EUS-FNA with the same diagnosis of the other three studies. All patients were subjected to the same gold standard method. We calculated study variables (sensitivity, specificity, prevalence, positive and negative predictive values, and accuracy) and performed a meta-analysis using the Review Manager (RevMan) 5.3 software.
Results:
A total of 294 patients were included in the analysis. The pretest probability for malignant biliary stricture was 76.66%. The mean sensitivities of ERCP and EUS-FNA for tissue diagnosis of malignant biliary stricture were 49% and 75%, respectively; the specificities were 96.33% and 100%, respectively. The posttest probabilities positive predictive value (98.33% and 100%, respectively) and negative predictive value (34% and 47%, respectively) were determined. The accuracies were 60.66% and 79%, respectively.
Conclusion:
We found that EUS-FNA was superior to ERCP with brush cytology and forceps biopsy for diagnosing malignant biliary strictures. However, a negative EUS-FNA or ERCP test may not exclude malignant biliary stricture because both have low negative posttest probabilities.
Keywords: Bile duct neoplasm, cholangiocarcinoma, endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography (ERCP), Klatskin tumors
INTRODUCTION
Biliary strictures are always challenges for accurate diagnosis and management. At the onset of symptoms, the disease is typically already in an advanced stage.[1,2,3,4] Malignant strictures of the biliary tract are commonly caused by pancreatic cancer, periampullary cancer, or cholangiocarcinoma. Cholangiocarcinoma is the most common tumor of the biliary tract. The incidence varies based on geography, and the highest rates are seen in Southeast Asia.[5,6,7]
In the United States, approximately 5,000 cases are diagnosed annually.[8,9,10]
Cholangiocarcinomas can be classified using anatomical location as intrahepatic, perihilar (proximal), and extrahepatic (distal) cholangiocarcinomas.[11,12]
For unknown reasons, in recent years, the incidence and mortality of extrahepatic cholangiocarcinomas has decreased while that of intrahepatic cholangiocarcinomas has increased.[3,13,14]
Perihilar cholangiocarcinoma, also known as Klatskin tumor, involving the bifurcation of the hepatic duct is the most common, accounting for about 60%-80% of cholangiocarcinomas. Intrahepatic cholangiocarcinomas are the least common.[15,16,17]
Factors considered for therapeutic programming of biliary stricture include extension of the tumor, tumor anatomy, and results of histopathological study.
Noninvasive diagnosis of an indeterminate biliary stricture can be accomplished using serum tumor markers, radiological imaging such as ultrasound scan (USS), magnetic resonance (MR), positron emission tomography (PET), and computed tomography (CT) cholangiography, or endoscopic methods such as endoscopic retrograde cholangiopancreathography, endoscopic ultrasound (EUS), and cholangioscopy.[1,18,19,20,21]
The most common methods of obtaining tissue samples for diagnosis are endoscopic retrograde cholangiopancreatography (ERCP) with brush cytology and/or forceps biopsy and endoscopic ultrasound with fine-needle aspiration (FNA).
In the literature, results obtained using endoscopic brush cytology and biopsy are very heterogeneous. Brush cytology sensitivity and specificity varies from 26% to 72%, and for biopsy it ranges between 15% and 100%. Results of EUS-FNA are heterogeneous as well, varying in sensitivity from 27% to 83%.
Patients typically have biliary strictures of indeterminate etiology. If they are good candidates for surgery, the use of EUS could avoid ERCP. However, if they are not good candidates, ERCP could be employed to treat jaundice and to confirm diagnosis.
Because the diagnostic yield of EUS and ERCP is variable for indeterminate malignant biliary stricture and because there are uncertainties about the best method, we have decided to perform this review to determine which one is superior. To our knowledge, no formal quantitative review of the literature has been published comparing the diagnostic performances of ERCP and EUS for tissue diagnostics of suspected malignant biliary strictures. In this revision, we will compare ERCP against EUS-FNA for tissue diagnosis of malignant biliary stricture.
OBJECTIVES
The aim of this study was to perform a structured meta-analysis of all eligible studies to compare the tissue diagnostic abilities of ERCP and EUS in cases of suspected malignant biliary stricture. To address the diagnoses of indeterminate malignant biliary strictures, clinical trials and observational studies were searched.
MATERIALS AND METHODS
Protocol and registration
This systematic review of the literature was conducted in accordance with preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations.[22] The review was registered in the PROSPERO international database under number CRD42014015411.[23]
Eligibility criteria
Types of studies — Clinical trials and observational studies were searched and targeted for a posterior selection process.
Types of participants — We chose studies with patients who had indeterminate malignant biliary strictures and with similar population characteristics (age, sex, abnormal liver function tests, and evidence of biliary obstruction).
Types of intervention — We chose trials that used either ERCP or EUS-FNA in diagnostics. There were no restrictions regarding the modality of diagnosis in each.
Types of outcome measures — The main outcomes were accuracy, sensitivity, specificity, positive predictive value, and negative predictive value.
Information sources
Studies were identified by searching electronic databases and scanning reference lists of articles. No limits were applied as far as language was concerned. This search was applied to Medline.[24] In EMBASE, a resumed strategy was needed.[25] The Cochrane, LILACS (via BVS), Scopus, and CINAHL (via EBSCO), databases were also reviewed.[26,27] The last search was performed on November 10, 2014.
Search
The following search strategy was used in the Medline database.
Cholangiocarcinoma or cholangiocarcinomas or cholangiocellular carcinoma or bile duct neoplasm or neoplasm, bile duct or neoplasms, bile duct or bile duct cancer or bile duct cancers or cancer, Bile duct or cancers, bile duct or cancer of the bile duct or cancer of bile duct or Klatskin or Klatskin tumor or Klatskin tumors and endosonography or endosonographies or endoscopy, echo or echo endoscopies or endoscopies, echo or ultrasonic endoscopy or echo-endoscopy or echo endoscopy or echo-endoscopies or endoscopy, ultrasonic or endoscopies, ultrasonic or ultrasonic endoscopies or ultrasonography, endoscopic or endoscopic ultrasonography or endoscopic ultrasonographies or ultrasonographies, endoscopic.
In the EMBASE, Cochrane, LILACS, Scopus, and CINAHL databases, the search was “bile duct neoplasm and ERCP and endoscopic ultrasound.”
Study selection
Eligibility assessment and selection of screened records were performed independently in an unblinded standardized manner by two reviewers. Disagreements between the reviewers were resolved by a consensus.
To summarize the study selection processes, an adapted PRISMA flow diagram was used.[22]
Data collection process
The method of data extraction from each included study consisted of collecting data on information sheets after reading the paper. A QUADAS-based checklist was used and the data were analyzed using Openepi and Catmaker tables.[28,29,30] One author extracted data from included studies and another checked the extracted data. Disagreements were resolved by discussion between the authors.
Data items
Population characteristics (patients included in the analysis with suspected malignant biliary strictures and clinical indications for the test), study design, test methods, gold standard used, EUS-FNA versus gold standard, and ERCP versus gold standard were obtained from the published trials. The study populations were first classified according to the suspect lesion in the biliary tract, and then patients diagnosed with malignant lesions were considered to be true positives, whereas patients with benign lesions were considered to be negative.
Risk of bias in individual studies
To evaluate the risk of bias and applicability of primary diagnostic accuracy, we used the QUADAS-2 tool.[28] It consists of four key domains: Patient selection, index test, reference standard, and flow and timing, each assessed in terms of risk of bias. The first three domains are also assessed in terms of applicability. Signaling questions are included to assist in judgments about risk of bias [Table 2]. The principal four questions of QUADAS-2 are:
Table 2.
QUADAS-2 questions and answers for the included studies
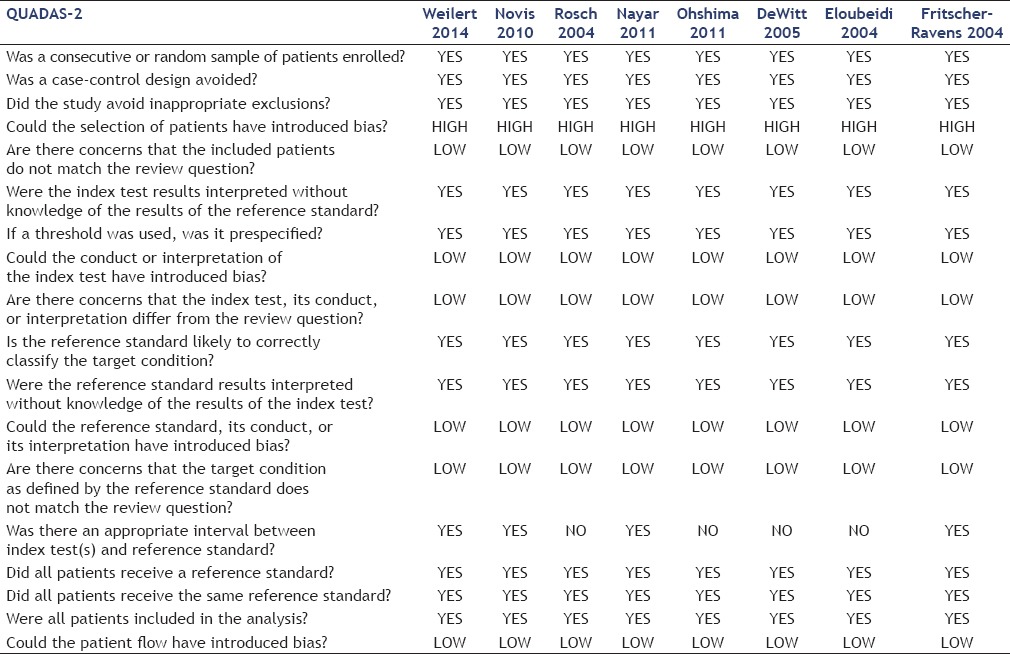
Did the study avoid inappropriate exclusion?
Could the conduct or interpretation of the index test have introduced bias?
Could the reference standard, its conduct, or its interpretation have introduced bias?
Could the patient flow have introduced bias? These questions were answered to evaluate the risk of bias in the studies.
Summary measures
The analyses of sensitivity, specificity, pretest probability, positive and negative predictive values, and accuracy of EUS-FNA and ERCP for detection of a malignant lesion were the primary outcome measures. These values were calculated from the data provided in the original papers. Also, averages and standard deviations (SDs) of the main outcomes were analyzed using Review Manager (RevMan) 5.3, obtained from the website of Cochrane Informatics and Knowledge Management Department. Averages and SDs were obtained using Microsoft Excel Software for Windows version 2013.[31,32]
Synthesis of results
Data entered (true positives, false positives, true negatives, and false negatives) were converted to percentage values and graphs by the RevMan software package.
Risk of bias across studies
Publication bias is the influence on what is likely to be published, among what is available to be published. A problematic and much discussed bias is the tendency of researchers and editors to focus on positive results of trials, and actions such as deleting inconclusive results leads to a bias that increases the number of positive results.
Attention to selection bias, performance, detection, and confusion, for example, triggers a targeted and focused analysis.
Additional analyses
A common receiver operating characteristic (ROC) curve was used to facilitate interpretation of the results.
RESULTS
Study selection
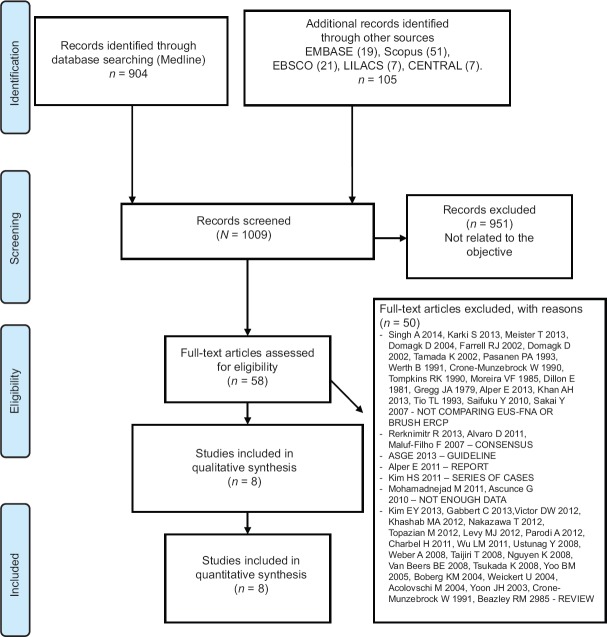
One thousand and nine (1,009) studies were screened and assessed for eligibility after the titles and abstracts were read. Of these, 951 were excluded because they were not related to our objective. Of the remaining 58, 50 were excluded. A total of eight studies were included for qualitative and quantitative analyses. This process is summarized in Figure 1.
Figure 1.
PRISMA flow diagram
Study characteristics
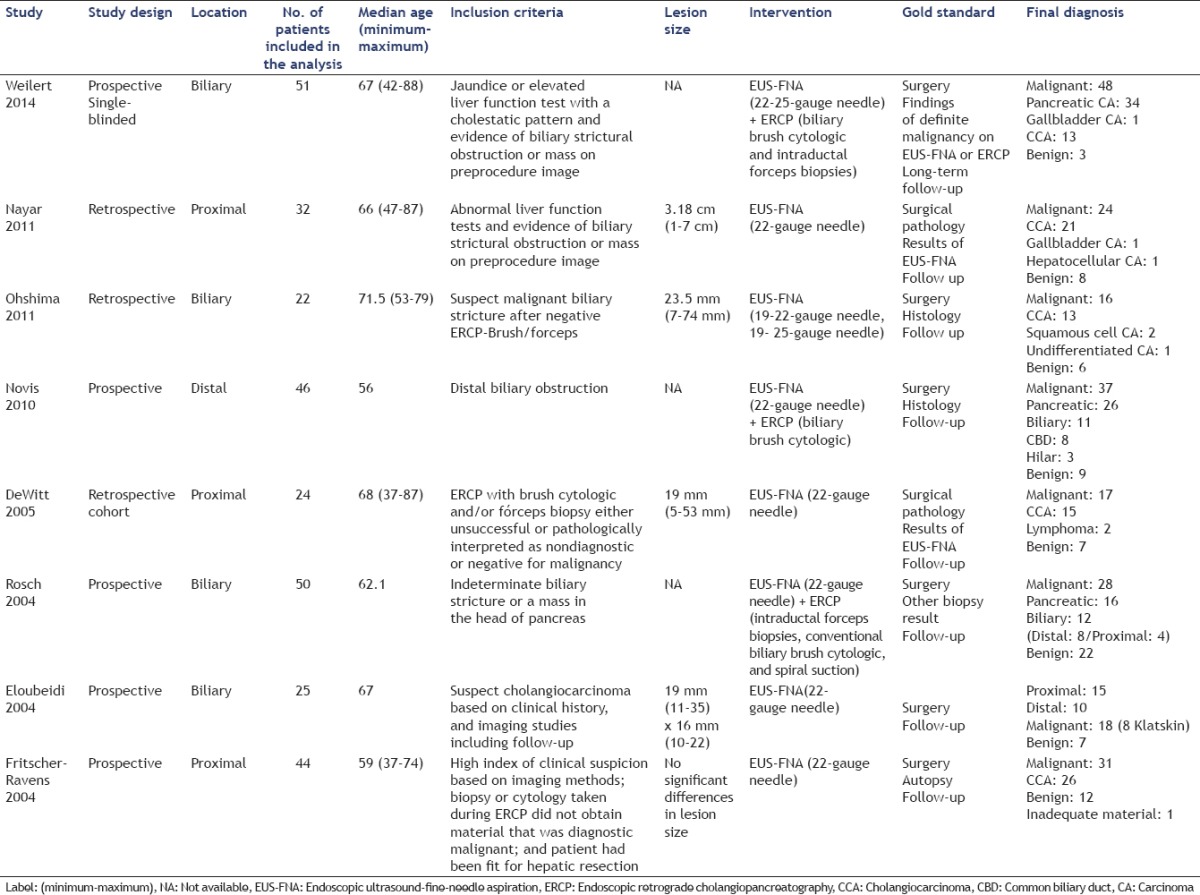
The important characteristics of the selected studies are summarized in Table 1. These values were extracted through a careful reading of included papers. The design, conduct, and gold standard analysis of these studies were similar. The main objective of these studies was evaluating the performance of EUS-FNA and ERCP for the detection of malignant biliary stricture.
Table 1.
Characteristics of the studies
Risk of bias within studies
Using QUADAS-2, we found that most studies did not impose bias [Table 2]. We noted that all the studies followed the same pattern of exclusion, with similar results, except for the one by Ohshima et al., which was the only study with 100% specificity and sensitivity.
The gold standard does not introduce bias in any study, and all trials were considered to have the same gold standard, histopathology (surgery or the index test), and follow-up.
There was no introduction of bias in terms of the selection of patients but there were differences in the size of the suspect lesions, which may have facilitated the diagnosis. For example, Ohshima et al., presented the best results, but also had the highest average lesion size.
The greatest bias in this review is in the location of the lesion, as only Weilert et al., Ohshima et al., Rosch et al., and Eloubedi et al. did not refer lesions location. Nayar et al., DeWitt et al., and Fritscher-Ravens et al. selected proximal lesions while Novis et al. selected distal lesions.
Results of individual studies
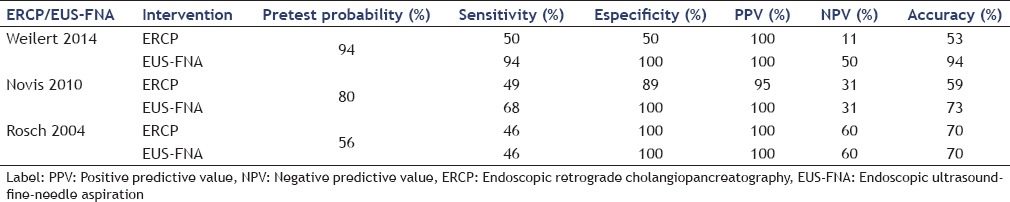
We assessed pretest probability, sensitivity, specificity, positive predictive value, negative predictive values, and accuracy. During our evaluation, we found that the specificity and positive predictive values of both tests were excellent.
We noted that EUS-FNA appeared to be more sensitive than ERCP except in the study of Rosch et al. where it was the same. Both Rosch et al. and Nayar et al. showed no significant sensitivity for the diagnosis of indeterminate malignant biliary stricture using EUS-FNA.
Unfortunately, most of the studies showed low negative predictive values; the notable exceptions were Ohshima et al. and Fritscher-Ravens et al. who reported negative predictive values of 100% and 90%, respectively. All reports had pretest probability superiors higher than up to 50%.
Synthesis of results
Analyzing the results of Table 3, we found that sensitivity had a value of around 50% for ERCP, showing that EUS-FNA was more sensitive. In addition, EUS-FNA was more accurate, specific, and had higher positive predictive values but ERCP had low negative predictive values.
Table 3.
Performance of ERCP compared to EUS-FNA for indeterminate biliary stricture
The accuracies, sensitivities, specificities, prevalences, positive predictive values, and negative predictive values in EUS-FNA are reported in Table 4, showing that it had specificities of 100% as well as positive predictive values of 100% in all the relevant studies. In addition, the average sensitivity was 75% and the average accuracy was greater than 75%. As with ERCP, EUS-FNA had low negative predictive values, with the exception of Ohshima et al. and Ravens Fritscher et al.
Table 4.
Performance of EUS-FNA for indeterminate malignant biliary stricture
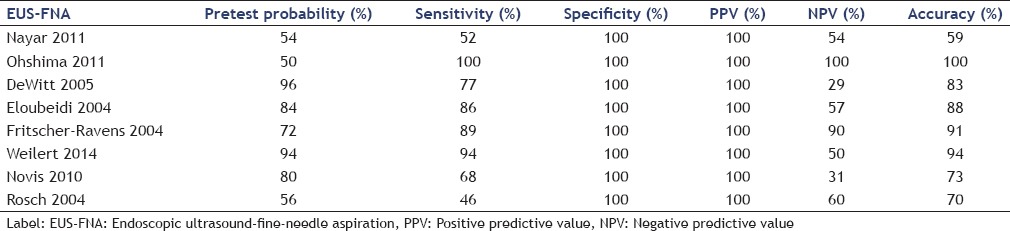
Tables 5 and 6 show the sensitivities and specificities of EUS-FNA and ERCP.
Table 5.
Diagnosis of suspected malignant biliary stricture in EUS-FNA.
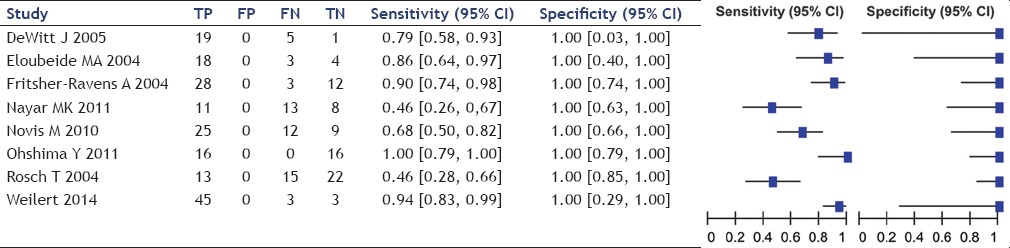
Table 6.
Diagnosis of suspected malignant biliary stricture in ERCP
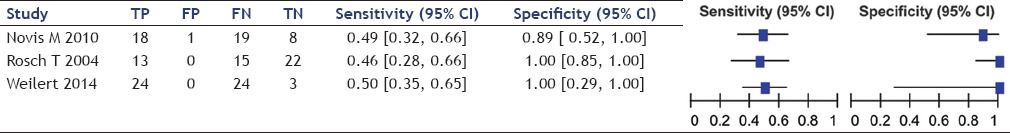
The sensitivity of EUS-FNA ranged between 46% and 100%, averaging 75% while that of ERCP ranged between 46% and 50%, averaging 49%. Specificity of EUS-FNA was 100% in all studies while that of ERCP ranged from 89% to 100%, averaging 96%.
Table 7 shows a comparison between the average and variance of diagnostic variables.
Table 7.
Average and variance of diagnostic variables
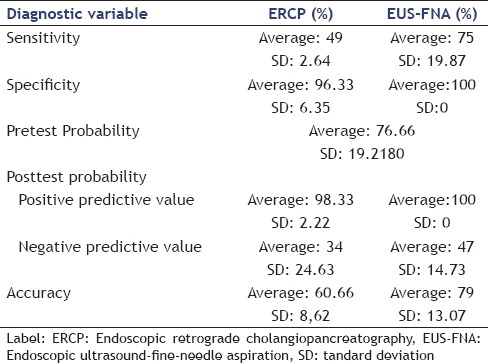
In diagnosing suspected malignant biliary stricture, the sensitivity of EUS-FNA was superior to ERCP, averaging 75% (SD, 19.87) versus 49% (SD, 2.64). Its specificity was slightly superior, averaging 100% (SD, 0) versus 96.33% (SD, 6.35). Its mean positive predictive value was also superior to that of ERCP, averaging 100% (SD, 0) versus 98.33% (SD 2.22). Likewise, its mean negative predictive value was higher, averaging 47% (SD, 14.73) versus 34% (SD, 24.63). Finally, the aggregated accuracy of EUS-FNA was higher, averaging 79% (SD, 13.07) versus 60.66% (SD, 8.62).
Risk of bias across studies
The risks of bias were minimal because the articles followed the same patterns. The greatest bias was related to the lesion size and secondarily to the lesion location. The size of the trials varied, facilitating the chance of suitable material for pathological studies, which could introduce bias.
Additional analyses
The ROC curve comparing EUS-FNA and ERCP (brush cytology and forceps biopsy) are shown in Figure 2.
Figure 2.
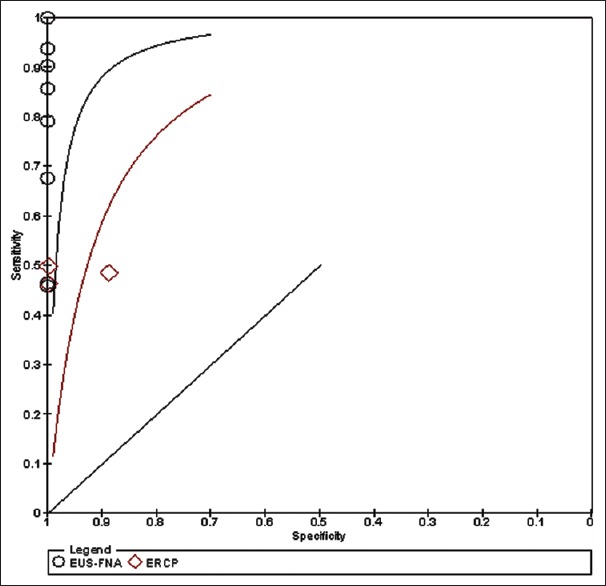
ROC curve
Figure 2 shows the ROC curve in which the best sensitivity and specificity are shown for both diagnostic arms. The EUS-FNA sensitivity and specificity were 87% and 90%, respectively, whereas for ERCP they were 75% and 81%, respectively.
Using Medcalc statistical software, we calculated the positive posttest probability and the accuracy of both methods, which for EUS-FNA were 96.5% and 87.7%, respectively. For ERCP, the values were 92.5% and 74.4%, respectively.[33]
A larger area below the line is seen for EUS-FNA; therefore, we conclude that it is a better method than ERCP for diagnosing suspected malignant biliary stricture.
DISCUSSION
Summary of evidence
EUS has an important role in the evaluation of patients with indeterminate biliary strictures and has advantages over ERCP because it has fewer complications, can identify adjacent structures, and can identify secondary causes of stenosis involving lymph nodes or neoplasms.
This review shows that EUS-FNA is a better method than ERCP for diagnosing suspected malignant biliary strictures. However, this study did not consider the site of lesion; it included studies of both distal and proximal lesions. Some authors have shown better results using EUS-FNA in distal lesions and ERCP in proximal lesions, primarily taking into account the type of the lesion.[34,35,36,37] Small lesions (<10 mm) are more difficult to sample using EUS-FNA and when the lesions present with wall thickening, this method has very low sensitivity; so the use of ERCP and cholangioscopy are preferable. In larger lesions, where masses and/or nodules appear, EUS-FNA is easier to perform and has better results.[9,38,39,40]
In cases of pancreatic tumors, EUS-FNA has high sensitivity and high specificity while ERCP has low sensitivity, ranging from 32% to 50%. In cases of papillary tumors, biopsy can be performed during duodenoscopy with high sensitivity and specificity.[15,41,42]
Our review shows that both methods have high specificities and high positive predictive values in diagnosing suspected biliary strictures, assuming that a positive test is trusted. Both have low negative predictive values; when any of the tests have negative values, the disease is not excluded. Therefore, if the first test is negative, combination with another method is the best way to diagnose a suspected malignant biliary stricture.
LIMITATIONS
The main limitation of this review is that none of the studies were randomized trials. Another limitation is that the negative predictive value is very high, which in the case of a negative test, means that one cannot exclude the disease.
CONCLUSIONS
This study demonstrates that EUS-FNA is better than ERCP for the detection of suspected malignant biliary stricture as it has superior sensitivity, specificity, positive posttest probability, and accuracy. A negative test using EUS-FNA or ERCP does not exclude a malignant biliary stricture because both have low negative posttest probabilities.
Financial support and sponsorship
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Anderson MA, Appalaneni V, Ben-Menachem T, et al. American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77:167–74. doi: 10.1016/j.gie.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Boberg KM, Schrumpf E. Diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2004;6:52–9. doi: 10.1007/s11894-004-0026-1. [DOI] [PubMed] [Google Scholar]
- 3.Nayar MK, Manas DM, Wadehra V, et al. Role of EUS/EUS-guided FNA in the management of proximal biliary strictures. Hepatogastroenterology. 2011;58:1862–5. doi: 10.5754/hge10531. [DOI] [PubMed] [Google Scholar]
- 4.Rösch T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390–6. doi: 10.1016/s0016-5107(04)01732-8. [DOI] [PubMed] [Google Scholar]
- 5.Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific Working Group on Hepatobiliary Cancers. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593–607. doi: 10.1111/jgh.12128. [DOI] [PubMed] [Google Scholar]
- 6.Novis M, Ardengh JC, Libera ED, et al. Prospective comparative study of ERCP brush cytology and EUS-FNA for the differential diagnosis of biliary strictures. Rev Col Bras Cir. 2010;37:190–8. doi: 10.1590/s0100-69912010000300006. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg MJ. Cholangiocarcinoma. Dis Mon. 2004;50:540–4. doi: 10.1016/j.disamonth.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Garrow D, Miller S, Sinha D, et al. Endoscopic ultrasound: A meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616–23. doi: 10.1016/j.cgh.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Victor DW, Sherman S, Karakan T, et al. Current endoscopic approach to indeterminate biliary strictures. World J Gastroenterol. 2012;18:6197–205. doi: 10.3748/wjg.v18.i43.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang KJ. State of the art lecture: Endoscopic ultrasound (EUS) and FNA in pancreatico-biliary tumors. Endoscopy. 2006;38(Suppl 1):S56–60. doi: 10.1055/s-2006-946654. [DOI] [PubMed] [Google Scholar]
- 11.Fritscher-Ravens A, Broering DC, Knoefel WT, et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45–51. doi: 10.1046/j.1572-0241.2003.04006.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohshima Y, Yasuda I, Kawakami H, et al. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011;46:921–8. doi: 10.1007/s00535-011-0404-z. [DOI] [PubMed] [Google Scholar]
- 13.Mohamadnejad M, DeWitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest Endosc. 2011;73:71–8. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Yoo BM. Endoscopic staging of hilar cholangiocarcinoma. Korean J Gastroenterol. 2005;46:16–9. [PubMed] [Google Scholar]
- 15.Tsukada K, Takada T, Miyazaki M, et al. Japanese Association of Biliary Surgery; Japanese Society of Hepato-Biliary-Pancreatic Surgery; Japan Society of Clinical Oncology. Diagnosis of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:31–40. doi: 10.1007/s00534-007-1278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karki S, Joshi KS, Regmi S, et al. Role of ultrasound as compared with ERCP in patient with obstructive jaundice. Kathmandu Univ Med J (KUMJ) 2013;11:237–40. doi: 10.3126/kumj.v11i3.12512. [DOI] [PubMed] [Google Scholar]
- 17.Van Beers BE. Diagnosis of cholangiocarcinoma. HPB (Oxford) 2008;10:87–93. doi: 10.1080/13651820801992716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charbel H, Al-Kawas FH. Cholangiocarcinoma: Epidemiology, risk factors, pathogenesis, and diagnosis. Curr Gastroenterol Rep. 2011;13:182–7. doi: 10.1007/s11894-011-0178-8. [DOI] [PubMed] [Google Scholar]
- 19.Alper E, Arabul M, Buyrac Z, et al. The use of radial endosonography findings in the prediction of cholangiocarcinoma in cases with distal bile duct obstructions. Hepatogastroenterology. 2013;60:678–83. [PubMed] [Google Scholar]
- 20.Wu LM, Jiang XX, Gu HY, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy in the evaluation of bile duct strictures and gallbladder masses: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:113–20. doi: 10.1097/MEG.0b013e3283426313. [DOI] [PubMed] [Google Scholar]
- 21.Khan AH, Austin GL, Fukami N, et al. Cholangiopancreatoscopy and endoscopic ultrasound for indeterminate pancreaticobiliary pathology. Dig Dis Sci. 2013;58:1110–5. doi: 10.1007/s10620-012-2471-2. [DOI] [PubMed] [Google Scholar]
- 22. [Last accessed on 2014 Nov 14]. Available from: http://www.prisma-statement.org/
- 23. [Last accessed on 2014 Nov 14]. Available from: http://www.crd.york.ac.uk/prospero/
- 24. [Last accessed on 2014 Nov 14]. Available from: http://www.ncbi.nlm.nih.gov/pubmed .
- 25. [Last accessed on 2014 Nov 14]. Available from: http://www.embase.com/info/helpfiles/
- 26. [Last accessed on 2014 Nov 14]. Available from: http://www.bireme.br/php/index.php .
- 27. [Last accessed on 2014 Nov 14]. Available from: https://www.ebsco.com/
- 28.Whiting J, Rutjes AW, Dinnes J, et al. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Tech Assess. 2004;8(iii):1–234. doi: 10.3310/hta8250. [DOI] [PubMed] [Google Scholar]
- 29. [Last accessed on 2014 Nov 14]. Available from: http://www.openepi.com/Menu/OE_Menu.htm .
- 30. [Last accessed on 2014 Nov 14]. Available from: http://www.cebm.net/catmaker-ebm-calculators/
- 31. [Last accessed on 2014 Nov 14]. Available from: http://www.cochrane.org/news/tags/authors/revman-53-now-available-training-webinars-3-july .
- 32. [Last accessed on 2014 Nov 14]. Available from: https://products.office.com/pt-br/excel?legRedir=trueandCorrelationId=735faf7b-ed40-4ca1-93e6-65be0dfa2a94 .
- 33.Medcalc statistical software. [Last accessed on 2014 Nov 14]. Avaiable from: http://www.medcalc.org .
- 34.Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: Results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97–104. doi: 10.1016/j.gie.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Topazian M. Endoscopic ultrasonography in the evaluation of indeterminate biliary strictures. Clin Endosc. 2012;45:328–30. doi: 10.5946/ce.2012.45.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tio TL, Reeders JW, Sie LH, et al. Endosonography in the clinical staging of Klatskin tumor. Endoscopy. 1993;25:81–5. doi: 10.1055/s-2007-1009129. [DOI] [PubMed] [Google Scholar]
- 37.Tompkins RK, Saunders K, Roslyn JJ, et al. Changing patterns in diagnosis and management of bile duct cancer. Ann Surg. 1990;211:614–21. [PMC free article] [PubMed] [Google Scholar]
- 38.Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–13. doi: 10.1016/s1542-3565(04)00005-9. [DOI] [PubMed] [Google Scholar]
- 39.DeWitt J, Misra VL, Leblanc JK, et al. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325–33. doi: 10.1016/j.gie.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 40.Khashab MA, Fockens P, Al-Haddad MA. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepatic cholangiocarcinoma (with videos) Gastrointest Endosc. 2012;76:1024–33. doi: 10.1016/j.gie.2012.04.451. [DOI] [PubMed] [Google Scholar]
- 41.Crone-Münzebrock W, Rowedder A, Meyer-Pannwitt U, et al. Comparative efficacy of sonography, computed tomography, ERCP and angiography in the diagnosis of primary papillary carcinomas. Rontgenblatter. 1990;43:266–9. [PubMed] [Google Scholar]
- 42.Moreira VF, Meroño E, del Olmo L, et al. Endoscopic retrograde cholangiopancreatography in the diagnosis of carcinomas of Vater's ampulla (ampulloma) Rev Esp Enferm Apar Dig. 1985;67:524–9. [PubMed] [Google Scholar]