Abstract
Background and Objectives:
DNA molecular analysis has been suggested as a tool to evaluate pancreatic cysts. This study assesses whether the addition of DNA molecular analysis alters clinical management.
Methods:
This is a retrospective review of 46 consecutive patients who underwent EUS-FNA of pancreatic cysts with DNA molecular analysis at two major academic institutions. Cases were presented to two pancreaticobiliary surgeons first without and then with DNA molecular analysis data. The primary outcome was the frequency with which clinical management was altered with the addition of DNA molecular analysis.
Results:
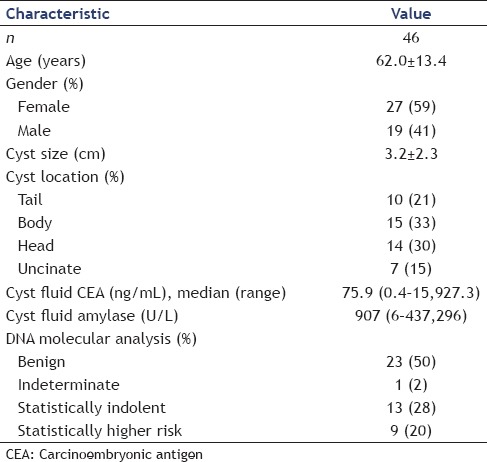
Forty-six patients with a mean age of 62.0 (±13.4) years and mean cyst size of 3.2 (±2.3) cm were included in the study. Cyst carcinoembryonic antigen (CEA) was available in 30 patients and ranged from 0.4 to 15,927 ng/mL. DNA molecular analysis was described as benign in 23 (50%), statistically indolent in 13 (28%), statistically higher risk in 9 (20%), and indeterminate in 1 (2%). Surgeon #1 changed the management in 13/46 cases (28%) and surgeon #2 changed the management in 12/46 cases (26%) with the addition of DNA molecular analysis. When organized by CEA concentration, those with an intermediate CEA (45–800 ng/mL) or without a CEA concentration had a management changed more frequently (40%) compared to all others (P < 0.05).
Conclusions:
The addition of DNA molecular analysis alters the clinical management of pancreatic cystic lesions most often when CEA levels are intermediate (45–800 ng/mL) or when no CEA concentration is available. Use of DNA molecular analysis can be considered in this cohort. Further study of molecular markers in pancreatic cystic lesions is recommended.
Keywords: CEA, DNA molecular analysis, pancreatic cyst
INTRODUCTION
Pancreatic cysts are increasingly encountered in clinical practice and have received significantly more attention in recent medical literature as a result. Studies of routine abdominal cross-sectional imaging have shown prevalence rates ranging from 1.2% to 2.4% in the general population.[1] Despite increased detection, the management of cystic lesions remains challenging given the difficulty in accurately identifying a preoperative diagnosis, the unknown natural history of many cystic lesions, and our inability to predict malignant potential. This study was designed to evaluate whether the clinical management is altered with the addition of DNA molecular analysis to our current diagnostic modalities.
There are a number of investigational tools to risk stratify pancreatic cysts; however, current guidelines provide only consensus statements on their use given a lack of evidence-based data.[2,3] Cross-sectional imaging alone affords a highly variable preoperative diagnosis with accuracy rates ranging from 20% to 82%.[4,5] Overall reported accuracy of EUS in determining mucinous cysts remains marginal at approximately 50%. The addition of FNA with cyst fluid analysis can be valuable. Carcinoembryonic antigen (CEA) values >192 ng/mL are predictive of a mucinous lesion with sensitivity of 75% and specificity of 84%;[6] however, CEA values have not been correlated with malignancy. Cytology remains of limited value with sensitivities ranging from 20% to 50%.[7] Needle-based confocal microscopy may offer improved specificity (100%) in detection of pancreatic cystic neoplasms but is limited by low sensitivity (59%).[8]
Limitations of diagnostic tests, particularly the inability to predict malignant potential, encouraged evaluation of molecular DNA analysis as an adjunctive tool. Khalid et al. determined that increased pancreatic cyst fluid DNA and loss of heterozygosity (LOH) of tumor suppressor genes and of k-ras mutations correlate with malignancy.[9] Furthermore, a recent study suggested that molecular analysis more accurately determined the malignant potential of pancreatic cysts when compared to the Sendai 2012 guidelines.[10] However, molecular DNA analysis remains expensive and the additional data provided by DNA molecular analysis may not alter pancreatic cyst management. Therefore, we sought to evaluate whether the addition of DNA molecular analysis alters the clinical management.
METHODS
We performed a retrospective review of all patients who had undergone EUS with FNA of pancreatic cysts and DNA molecular analysis at two major academic institutions from May 2010 to June 2011 and included follow-up of the lesions through 2015 when available. Exclusion criteria included age <18 or malignant cells visualized on cytology. IRB approval was obtained. All patients had undergone routine cross-sectional imaging with abdominal computed tomography (CT) or magnetic resonance imaging as part of their preprocedure workup. EUS procedures were performed with a linear echoendoscope by dedicated endoscopic ultrasonographers with advanced EUS training. FNA was performed with a 22- or 25-gauge needle. Cyst fluid was obtained and sent to the laboratory for CEA and amylase when sufficient fluid was available. DNA molecular analysis was performed on all cyst aspirates (RedPath Integrated Pathology, PathFinder TG™; Pittsburgh, PA, USA). Results included DNA quality, quantity, presence of K-ras point mutation, and allelic imbalance LOH. Reports included interpretation as benign, statistically indolent, aggressive, or indeterminate.
Each case was then reviewed by two experienced pancreatobiliary surgeons (RBA, TWB) with and without the DNA molecular analysis to evaluate the impact of molecular analysis on clinical decision-making. Both surgeons as standard of practice follow Sendai criteria for recommendation of management. Blinded to patient identifiers, both reviewers were independently given a brief clinical history, cross-sectional imaging, EUS reports, cyst CEA, cyst amylase, and cytology results. They were then asked to provide management recommendations in each case (surgery, observation, other). One month later, the cases were randomly represented to both surgeons with the addition of cyst aspirate DNA molecular analysis results and management recommendations recorded. We then compared the surgeons’ recommendations with and without DNA molecular analysis. Ten repeat cases were included with each group to assess for intraobserver variability, totaling 56 expertly reviewed cases (46 with DNA molecular analysis, 46 without DNA molecular analysis, and 10 repeat cases) per surgeon.
Multivariate analysis was performed on predictors of recommending surgery including age, gender, location of lesion (head/uncinate versus body/tail), CEA level >192 ng/mL, size ≥3.0 cm, and DNA molecular analysis of aggressive or indeterminate (i.e., not benign or statistically indolent). For multivariate analysis, recommendations for surgery by two surgeons were combined (n = 92).
Statistical analysis
All P values were two-sided, and a P = 0.05 or less was considered statistically significant. All statistical analysis was performed using SAS, version 9.4 (SAS Institute, Inc., North Carolina, USA).
RESULTS
Cases
A total of 46 patients (19 males and 27 females) with a mean age of 62.0 ± 13.4 years (range 27–79 years) were reviewed [Table 1]. The average cyst size was 3.2 cm (range 0.7–10.0 cm). Fourteen patients (30%) had cystic lesions in the head of the pancreas, 15 (33%) in the body, 10 (22%) in the tail, and 7 (15%) in the uncinate process. CEA concentrations were available in 30 cases, amylase values in 26, and cytology results in 45 of 46 cases. There was insufficient cyst fluid to perform CEA and amylase analyses in cases without these values. On follow-up in 2015, 24 (52%) did not have any further evaluation documented, 13 (28%) had surveillance imaging without significant changes, 5 (11%) underwent surgical therapy for the pancreas cyst, and 4 (9%) were deceased (3 from unrelated causes, 1 from pancreas cancer). The one person who developed pancreatic cancer had a cyst >3 cm in the pancreas head with “statistically higher risk” DNA molecular analysis.
Table 1.
Patient characteristics
Effect of molecular analysis on management recommendation
Excluding molecular analysis results, surgeon #1 chose surgical resection in 21 cases (45.6%), observation in 23 cases (50%), and other in 2 cases (4.4%) (CT-guided biopsy and cyst gastrostomy). With the addition of DNA molecular analysis, surgeon #1 changed his management in 28% of the cases (13/46), 10 of which were to recommend observation instead of surgery. Excluding molecular analysis results, surgeon #2 opted for surgical resection in 14/46 (30.4%) cases, observation in 29/46 (63%), and other in 3/46 (6.5%) including 2 recommendations to have informed discussion with patient and family regarding observation versus surgery and 1 request for EUS/FNA at region of duct caliber change. With the addition of DNA molecular analysis, surgeon #2 changed his management in 26% of cases (12/46), 8 of which were to recommend observation instead of surgery. Molecular analysis tended to change management decisions toward a more conservative approach (i.e., observation) as 18/25 (72%) of the changes in recommendations were to pursue observation rather than surgery [Table 2].
Table 2.
Change in pancreatic cyst management with the addition of molecular analysis stratified by carcinoembryonic antigen level
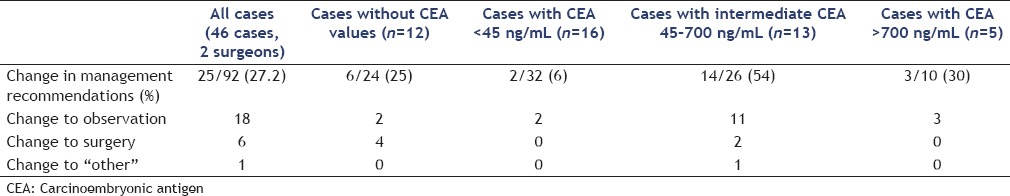
When grouped by CEA concentration and combining surgeons #1 and #2 management recommendations, cases with low CEA (0–45 ng/mL) or high CEA (>800 ng/mL) had recommendation changes in 5/42 (11.9%) after the addition of DNA molecular analysis. However, those with intermediate CEA levels (45–800 ng/mL) or no CEA available had management recommendations change in 20/50 (40%) cases after the addition of DNA molecular analysis (P < 0.05). DNA molecular analysis had the highest rate of recommendation change in those with intermediate CEA levels (54%) which was statistically higher (P < 0.05) than all other groups [Table 2].
Predictors of recommendation for surgery
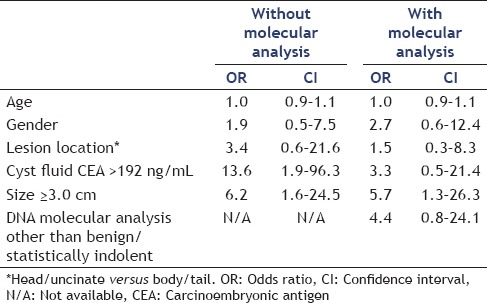
Location of a cystic lesion did not seem to play a significant role in either initial clinical management or management with the addition of DNA analysis. Surgery was recommended in 42% of lesions involving the body, 32% involving the head, 14% involving the uncinated process, and 38% involving the tail. Management changes were made in 17% of lesions involving the body, 36% involving the head, 14% involving the uncinate process, and 35% involving the tail (P = NS). On multivariate analysis, cyst fluid CEA >192 and cyst ≥3.0 cm were predictive of recommendation for surgical management [Table 3]. Age, gender, and cyst location did not predict recommendation of surgery. With the addition of molecular analysis, cyst size >3 cm remained statistically significant in predicting recommendation for surgery. A molecular analysis that was not benign or statistically indolent trended toward recommendation of surgery although was not statistically significant [Table 3].
Table 3.
Multivariate analysis of recommendation for surgery
Interobserver and intraobserver agreement
Interobserver agreement for the recommendation of surgery versus observation/other was moderate (kappa = 0.60) without molecular analysis and moderate (kappa = 0.43) with molecular analysis. Out of the 10 repeat cases presented to the surgeons to assess for intraobserver variation, only one case differed in management resulting in a 95% intraobserver agreement.
DISCUSSION
DNA molecular analysis has been used as a tool to help differentiate premalignant and malignant lesions from those with low or no oncogenic potential; however, it remains unclear if the addition of this information changes management.[11,12,13] There are currently no consensus guidelines on the use of DNA molecular analysis in the evaluation of pancreatic cysts and its use is often based on personal or institutional preferences. One recent study suggests that use of molecular analysis is a reliable predictor of benign and potentially malignant lesions. When compared to the use of Sendai 2012 guidelines, molecular analysis was more accurately in determining the malignant potential of pancreatic cysts. However, a selection bias likely exists in this comparison as all patients in this recent study had EUS with FNA and molecular analysis.[10]
Our study was designed to determine the likelihood of molecular analysis altering immediate clinical management of cystic pancreatic lesions when cytology did not reveal malignancy. Overall surgical recommendations were changed in 25/96 (26%) individual cases of pancreatic cystic lesions with the addition of molecular analysis. However, a majority of these changes occurred in the setting of intermediate CEA levels.
In the landmark publication, Brugge et al.[6] reported a mean CEA level of 8400 ng/mL for mucinous pancreatic cysts with malignancy, 683.9 ng/mL for benign mucinous cysts, 36.8 ng/mL for inflammatory cysts, and 2.7 ng/mL for serous cysts. Although the study reported a sensitivity and specificity of 75% and 84% for the diagnosis of mucinous cysts when CEA >192 ng/mL, it also reported wide ranges of CEA concentrations and considerable overlap among each cyst group. Other studies have shown optimal CEA levels >300 ng/mL, >400 ng/mL, and >800 ng/mL in distinguishing mucinous from nonmucinous lesions.[14,15,16] Therefore, we defined intermediate CEA values as 45 ng/mL to 800 ng/mL. Our study included 13 cases with CEA values within this range. Of these, DNA molecular analysis changed the management with greatest frequency (54%).
In clinical practice, obtaining CEA and amylase values requires >1 mL of fluid which may not always be possible either because of cyst size or viscosity; whereas DNA molecular analysis only requires 0.2 mL of cyst fluid to perform. In 12/46 of our cases (26.1%), no CEA concentration was available. This seems to be consistent with one of the larger trials evaluating DNA molecular analysis of cyst fluid in which CEA data were not available in 33% of patients enrolled.[9] Among those 12 cases without CEA values, management changed in 6/24 cases (25%) compared with 5/42 (12%) cases with either low (<45 ng/mL) or high (>800 ng/mL) CEA concentration. These results suggest the use of DNA molecular analysis in patients with intermediate CEA values and those without fluid CEA concentrations may be useful.
Interobserver concordance with management recommendations was only moderate in this study. This is not surprising given the frequent inability to make a definitive preoperative diagnosis which often translates into ambiguity in treatment recommendations. It is also worthy to note that location of cystic lesions did not seem to affect surgical decision-making. Distal pancreatectomy is technically less challenging and may influence surgeons to be more aggressive in their management of distal lesions. In this study, surgical removal of 38% of tail lesions was recommended compared to 37% of lesions involving the head/body of pancreas. The predictors of a recommendation for surgery included cyst size >3 cm and CEA >192 ng/mL without molecular analysis.
There are several limitations to this study. First, the design was to evaluate the change in management afforded by the addition of DNA molecular analysis; however, management recommendations are often complex and include factors such as patient age, medical comorbidities, and malignant potential. All of the patients included were consecutively seen and evaluated at a time when each institution was performing DNA analysis on all cyst fluid. In this way, we attempted to create a patient population similar to those encountered in clinical practice. The conclusions of this study are limited by its size (46 cases) and number of reviewers for surgical management two although both are experienced pancreatobiliary surgeons with over 10 years of clinical experience. Furthermore, there is a possibility of memory bias on review with DNA molecular analysis 1 month after initial exposure. We attempted to account for this by randomly changing the order that the cases were presented.
CONCLUSIONS
Our current ability to preoperatively predict the malignant potential of pancreatic cystic lesions is limited. To our knowledge, this is the first study to demonstrate a change in clinical management with the addition of DNA molecular analysis. Those patients with intermediate CEA or no CEA concentrations had management changed significantly more often. DNA molecular analysis should be considered in patients when cyst aspirate has an intermediate CEA level and when insufficient fluid is aspirated for CEA and amylase investigation. Further study as to the utility of molecular markers in pancreatic cyst fluid is recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Kehagias D, Smyrniotis V, Kalovidouris A, et al. Cystic tumors of the pancreas: Preoperative imaging, diagnosis, and treatment. Int Surg. 2002;87:171–4. [PubMed] [Google Scholar]
- 5.Procacci C, Biasiutti C, Carbognin G, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906–12. doi: 10.1097/00004728-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Al-Haddad M, DeWitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79–87. doi: 10.1016/j.gie.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–13. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 9.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Al-Haddad MA, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy. 2015;47:136–42. doi: 10.1055/s-0034-1390742. [DOI] [PubMed] [Google Scholar]
- 11.Al-Haddad M, El Hajj II, Eloubeidi MA. Endoscopic ultrasound for the evaluation of cystic lesions of the pancreas. JOP. 2010;11:299–309. [PubMed] [Google Scholar]
- 12.Toll AD, Kowalski T, Loren D, et al. The added value of molecular testing in small pancreatic cysts. JOP. 2010;11:582–6. [PubMed] [Google Scholar]
- 13.Shen J, Brugge WR, Dimaio CJ, et al. Molecular analysis of pancreatic cyst fluid: A comparative analysis with current practice of diagnosis. Cancer. 2009;117:217–27. doi: 10.1002/cncy.20027. [DOI] [PubMed] [Google Scholar]
- 14.Shami VM, Sundaram V, Stelow EB, et al. The level of carcinoembryonic antigen and the presence of mucin as predictors of cystic pancreatic mucinous neoplasia. Pancreas. 2007;34:466–9. doi: 10.1097/mpa.0b013e318033fa12. [DOI] [PubMed] [Google Scholar]
- 15.Hammel P, Voitot H, Vilgrain V, et al. Diagnostic value of CA 72-4 and carcinoembryonic antigen determination in the fluid of pancreatic cystic lesions. Eur J Gastroenterol Hepatol. 1998;10:345–8. doi: 10.1097/00042737-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 16.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc. 2005;62:383–9. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]


