Abstract
EUS-guided biliary drainage (EUS-BD) has emerged as a technique for gaining biliary access when ERCP fails. This article gives a comprehensive review on the role and technique of EUS-BD. Moreover, we propose an algorithm guiding the clinician when to consider EUS-BD after failed ERCP or in anticipated difficult cannulations.
Keywords: Biliary stricture, ERCP, EUS-guided, EUS-guided biliary drainage
INTRODUCTION
EUS-guided biliary drainage (EUS-BD) has emerged as a technique for gaining biliary access when ERCP fails. ERCP remains the first-line method for accessing the bile duct. ERCP fails in 5%–10% of cases due to inaccessible papilla or inability to cannulate the papilla.[1] Reasons for ERCP failure include altered anatomy, ampullary distortion, periampullary diverticulum, gastric outlet obstruction, or duodenal stents in situ. Conventionally, percutaneous transhepatic biliary drainage (PTBD) has been performed when ERCP fails. However, PTBD is associated with high adverse event rates that are seen in up to 33% and include bleeding, bile leak, dislocation of the external catheter, recurrent infection, and acute cholangitis. Catheter-related morbidity from the external drainage are well known and may also worsen the patient's quality of life.[2]
EUS-BD has emerged as a welcome alternative to PTBD or surgery when ERCP fails. EUS-BD was first described by Giovannini et al. in 2001.[3] Over the last decade, a wealth of data has surfaced demonstrating efficacy and safety of this technique. EUS-BD has several advantages. First, it is minimally invasive and can be performed directly after a failed ERCP in the same session by the same proceduralist. Second, drainage of both the intrahepatic and extrahepatic bile ducts may be achieved. Third, it is minimally invasive with minimal or no procedural pain. Fourth, as opposed to PTBD, there is no external drain that can dislocate or that limits patient's daily activities. In addition, a short hospital stay (similar to ERCP) is expected, and the reported adverse event rate is far lower than for PTBD.[4,5,6]
WHICH DRAINAGE ROUTE?
EUS-BD is a minimally invasive technique that can be performed in several ways.[7] Broadly, either an extra- or intra-hepatic approach can be used. The drainage can be achieved either with a transmural drainage route or transpapillary route. When using the extrahepatic approach, the bile duct can be accessed through the duodenal wall and either a choledochoduodenostomy (CDS) with a transluminal stent or a rendezvous or antegrade technique with transpapillary stent can be performed. Through the intrahepatic approach, the left lobe of the liver is accessed from the stomach and a transpapillary stent is placed through the antegrade or EUS-rendezvous technique (EUS-RV) or a transluminal stent is placed through a hepaticogastrostomy (HGS). There is no universal consensus on the optimal strategy to perform EUS-BD, and the decision is largely based on the patient's anatomy and level of obstruction.
Intrahepatic versus extrahepatic approach
Several studies have investigated the intrahepatic approach versus the extrahepatic approach showing different results. A large retrospective study, including 245 patients, revealed a similar success rate for the intrahepatic and extrahepatic approach.[8] Dhir et al. assessed the intrahepatic and extrahepatic approach for EUS-RV for distal common bile duct obstruction, and they confirmed that the success rates were equal.[9] However, the intrahepatic approach was associated with higher postprocedural pain, longer procedure time, and longer hospital admissions. The latter was confirmed by a retrospective analysis of 65 patients which showed that the intrahepatic approach was associated with more complications and three patients in whom the intrahepatic approach was used died after the procedure.[10] However, the success rate was the same for all techniques. In this same study, there was neither significant difference in complication rates among transluminal and transpapillary stent placements nor between direct and rendezvous stenting. This was confirmed in a prospective, international, multicenter study looking at the efficacy and safety of EUS-BD in which an extrahepatic approach was significantly associated with decreased procedure time, length of hospital stay, and risk of moderate adverse events.[11] In summary, although the published success rates are the same for the intrahepatic and extrahepatic approach, the latter seems to be safer.
Hepaticogastrostomy versus choledochoduodenostomy
A retrospective study of 39 patients with obstructive jaundice caused by lower biliary obstruction and duodenal obstruction due to malignant tumors showed that EUS-HGS was associated with longer stent patency than EUS-CDS.[12] Moreover, CDS was the only risk factor associated with adverse events related to EUS-BD, in particular, reflux cholangitis (odds ratio 10.28; 95% confidence interval [CI] 1.686–62.733; P = 0.012). In a single-center prospective study, Artifon et al. randomized 49 patients with unresectable distal malignant biliary obstruction and failed ERCP to either HGS or CDS.[13] Both methods yielded similar technical success rates, safety, and procedure time (48 min). Moreover, a quality-of-life assessment revealed that no specific drainage route was superior. There was a minor trend in favor of HGS with regard to clinical success. However, this was not statistically significant. A recent systematic review showed no significant difference between transduodenal and transgastric approaches for EUS-BD with regard to efficacy and safety.[14] In summary, overall data show that EUS-HGS and EUS-CDS are equally effective and safe. However, there is limited data available in favor of EUS-HGS with regard to clinical success and safety.
Transpapillary versus direct transluminal approach
There is only limited data comparing transpapillary access versus transluminal access. In 2013, Khashab et al. compared the EUS-RV with the transluminal technique in 35 patients.[15] Technical and clinical success were comparable as well as the duration of hospitalization and decrease of bilirubin level. Moreover, the adverse events and long-term outcomes were similar between two groups, suggesting both techniques to be equally safe and effective. Dhir et al. also showed no difference in the success and complication rates between transpapillary and transluminal procedures.[10] A recent meta-regression analysis showed that transpapillary method of drainage was associated with a higher success rate and fewer complications.[16] There was no difference in technical success between either access route.
Individual-based approach
Taking into consideration all data presented above, there are no randomized controlled trials to support the best strategy for EUS-BD, and we believe the best strategy should be decided on a case-to-case basis according to the patient's anatomy and condition.
EUS-GUIDED BILIARY DRAINAGE VERSUS PERCUTANEOUS TRANSHEPATIC BILIARY DRAINAGE
As mentioned earlier, PTBD is associated with substantial morbidity.[2] There is only limited prospective, randomized data available evaluating the efficacy and safety of EUS-BD in comparison with PTBD. Artifon et al. were the first to compare the efficacy and safety of CDS versus PTBD in a small prospective randomized study including 25 patients.[17] They concluded both methods had equal technical success, clinical success, and adverse event profile. Giovannini et al. started another prospective multicenter study comparing EUS-BD with PTBD and randomized 41 patients.[5] They excluded patients with right-sided bile duct stenosis. Interim analysis showed a complication rate of 60% in the PTBD group versus 35% in the EUS-BD group and recruitment in the PTBD arm was consequently ceased thereafter. A retrospective study including 73 patients with failed ERCP showed that although technical success rate was higher in the PTBD group, clinical success was equivalent.[6] However, PTBD was associated with higher adverse event rate and higher costs. In a recent meta-analysis, there was no difference in technical success between EUS-BD and PTBD, but EUS-BD was associated with better clinical success and fewer postprocedural adverse events.[4] Importantly, EUS-BD was associated with lower reintervention rates and was more cost-effective.[4]
EUS-GUIDED BILIARY DRAINAGE VERSUS ERCP
At present, EUS-BD is mainly used when ERCP fails. No studies thus far have prospectively assessed the role of EUS-BD as a primary drainage technique in comparison to ERCP. However, a multicenter retrospective study comparing ERCP with EUS-BD suggested that both techniques were equally effective.[18] A small prospective clinical study including 18 patients showed that EUS-BD is safe and effective as a first-line BD therapy with success rates of 94% and a complication rate of 11%.[19] It is known that cannulation of difficult papillae is associated with increased adverse events. Difficult biliary access leads to increased manipulation, additional procedure time, and multiple attempts making the patients prone to adverse events, especially post-ERCP pancreatitis.[20,21] Pancreatitis rates are <3% for patients when cannulation is achieved within 5 min, but may exceed 10% when cannulation time exceeds 10 min or more than 10 attempts are made to achieve deep cannulation.[20,21] EUS-BD might therefore be a good primary alternative in patients with an expected difficult cannulation due to altered anatomy or malignant obstruction. Future randomized studies are needed to further explore this indication of EUS-BD as primary drainage. Moreover, the EUS-BD with antegrade stenting method has the advantage that the entire procedure can be carried out through an endoscopically created temporary fistula between the upper intestine and the intrahepatic bile ducts, without the need for the scope to reach the biliary orifice. A recently published pilot study showed that EUS-BD with antegrade stenting is also feasible and safe in patients with an altered anatomy.[22] In a small cohort of twenty patients with an altered anatomy, a Japanese group demonstrated a 95% technical and clinical success rate of EUS-guided antegrade stenting.
In addition, EUS-BD can be used as an alternative to precut sphincterotomy. A recent retrospective study showed that the ERCP failure rate decreases when EUS-BD is available.[23] The success for EUS-BD (95.1%, 95% CI, 89.7–100) was significantly higher than for precut (75.3%, 95% CI, 68.2–82.4), P < 0.001 which supports the role for EUS-BD as an alternative to precut after failed cannulation.
EUS-GUIDED BILIARY DRAINAGE IN BENIGN BILIARY STRICTURES
Data on EUS-BD in benign strictures are scarce as most studies only included malignant strictures or did not evaluate the difference between malignant and benign strictures. In a recently published meta-analysis, only four out of a total of 483 patients had a benign indication for EUS-BD.[4] In a multicenter, nonrandomized study of 240 patients, including 194 patients with malignant disease and 44 with benign disease, a higher success rate was noted in malignant diseases compared with benign diseases (90.2% vs. 77.3%, P = 0.02), although the adverse event rates were the same.[8] A possible explanation for this difference is that in malignant disease, the bile duct was more dilated and the bile duct might have been fixated to the duodenum or stomach by the tumor. Another explanation might be the type of stent that has been used. The choices of metallic versus plastic stents are generally made by the etiology and stage of the disease; for benign indications plastic stents are often used. A recent systematic review and meta-analysis showed that the clinical success rate of malignant strictures was higher than for benign strictures, whereas the technical success rate was the same between both groups.[14] It is worth noting that many studies have focused on malignant diseases and the numbers of patients with benign diseases are small. Further prospective studies are needed to establish the role of EUS-BD in benign strictures.
ADVERSE EVENTS
The adverse events for EUS-BD that have been reported range from 3.4% to 38.6% with an average reported adverse event rate of 17% to 18.9%.[16,24] However, it is worth noting that recently published studies show lower complication rates.[18,25,26] Reported complications include bile leak (3%), bleeding (2.7%), cholangitis (0.3%), sepsis and peritonitis (3.5).[4] Serious complications such as stent migration in the peritoneal cavity and fatal perforations are rare but have been reported.[4,27,28] In the published literature, there is no consistency in the definition of clinically significant adverse events. For example, some studies include pneumoperitoneum as an adverse event. However, pneumoperitoneum is a known sequelae of the procedure in patients undergoing transluminal puncture, and perhaps, this should not be considered an adverse event.
The lower complication rates in recent studies may be partially explained by the increasing use of lumen apposing metal stents that are especially designed for this indication and may decrease the incidence of bile leakage. In the past, plastic stents were used for EUS-BD, which are associated with higher rates of bile leakage. Khashab et al. performed an international multicenter study on HGS compared with CDS and demonstrated that adverse events were significantly more common in patients who underwent plastic stenting than metallic stenting (43% vs. 13%).[29] Gupta et al. showed that the use of plastic stents was associated with higher rates of cholangitis.[8] A recent meta-analysis confirmed that the adverse events were lower in metal stents compared with plastic stents.[14] Currently, metal stents are recommended for EUS-BD, which may have had an impact on the adverse event rates as well as technical success rates and procedure time. Moreover, use of noncoaxial electrocautery was independently associated with more adverse events.
Special considerations
It is important to note that all published studies originate from tertiary care centers. A cohort study from a tertiary care center showed that EUS-BD was required in only 0.6% of patients with a native papilla undergoing therapeutic biliary ERCP.[30] The overall exposure to EUS-BD is therefore limited. In addition, all procedures in published studies were performed by experts with significant EUS and ERCP skills. A French experience showed that there is a significant learning curve that is directly related to the number of adverse events.[31] Lower technical success rates and higher complication rates have been reported during the first 20 EUS-BD procedures.[32] Oh et al. investigated the learning curve for EUS-HGS in a prospective study involving 129 patients.[33] They demonstrated that procedure time and adverse events were shorter after 24 cases, and stabilized at 33 cases of EUS-HGS, respectively. This learning curve might also explain the lower complication rates in more recent studies. Therefore, prospective studies are needed to determine true adverse event rates. Finally, if the right and left intrahepatic ducts are not connected, EUS-BD cannot access the dilated right system and PTBD is indicated.[5]
CONCLUSION
EUS-BD is an emerging technique with demonstrated safety and efficacy. It is increasingly used as a better alternative for PTBD after failed ERCP. There are no randomized control trials to support the best strategy for EUS-BD, and we believe the best approach should be decided on a case-to-case basis according to the patient's anatomy and condition. Considering the data presented above, we propose an algorithm guiding the clinician when to consider EUS-BD [Figure 1], after failed ERCP or in anticipated difficult cannulations. Moreover, in the near future, there might be an important role for EUS-BD as a primary biliary draining technique.
Figure 1.
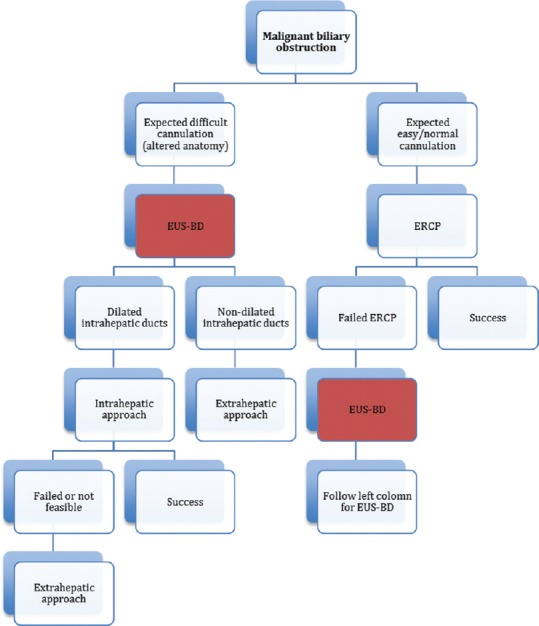
Individual-based approach to EUS-guided biliary drainage
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Enochsson L, Swahn F, Arnelo U, et al. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest Endosc. 2010;72:1175–84. doi: 10.1016/j.gie.2010.07.047. 1184.e1-3. [DOI] [PubMed] [Google Scholar]
- 2.Nennstiel S, Weber A, Frick G, et al. Drainage-related complications in percutaneous transhepatic biliary drainage: An analysis over 10 years. J Clin Gastroenterol. 2015;49:764–70. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 3.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 4.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M. Multicenter randomized phase II study: Percutaneous biliary drainage vs. EUS guided biliary drainage: Results of interim analysis. Gastrointest Endosc. 2015;81:AB171. [Google Scholar]
- 6.Khashab MA, Valeshabad AK, Afghani E, et al. Acomparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–65. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 7.Artifon EL, Ferreira FC, Otoch JP, et al. EUS-guided biliary drainage: A review article. JOP. 2012;13:7–17. [PubMed] [Google Scholar]
- 8.Gupta K, Perez-Miranda M, Kahaleh M, et al. Endoscopic ultrasound-assisted bile duct access and drainage: Multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80–7. doi: 10.1097/MCG.0b013e31828c6822. [DOI] [PubMed] [Google Scholar]
- 9.Dhir V, Bhandari S, Bapat M, et al. Comparison of transhepatic and extrahepatic routes for EUS-guided rendezvous procedure for distal CBD obstruction. United European Gastroenterol J. 2013;1:103–8. doi: 10.1177/2050640613480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhir V, Artifon EL, Gupta K, et al. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: Choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430–5. doi: 10.1111/den.12153. [DOI] [PubMed] [Google Scholar]
- 11.Khashab MA, Van der Merwe S, Kunda R, et al. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc Int Open. 2016;4:E487–96. doi: 10.1055/s-0042-102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura T, Chiba Y, Masuda D, et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy. 2016;48:156–63. doi: 10.1055/s-0034-1392859. [DOI] [PubMed] [Google Scholar]
- 13.Artifon EL, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest Endosc. 2015;81:950–9. doi: 10.1016/j.gie.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Khashab MA, Valeshabad AK, Modayil R, et al. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: Rendezvous versus direct transluminal techniques (with videos) Gastrointest Endosc. 2013;78:734–41. doi: 10.1016/j.gie.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: A Systematic review and meta-analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 17.Artifon EL, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: Endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–74. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 18.Dhir V, Itoi T, Khashab MA, et al. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913–23. doi: 10.1016/j.gie.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Hara K, Yamao K, Hijioka S, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392–6. doi: 10.1055/s-0032-1326076. [DOI] [PubMed] [Google Scholar]
- 20.Bailey AA, Bourke MJ, Williams SJ, et al. A prospective randomized trial of cannulation technique in ERCP: Effects on technical success and post-ERCP pancreatitis. Endoscopy. 2008;40:296–301. doi: 10.1055/s-2007-995566. [DOI] [PubMed] [Google Scholar]
- 21.Halttunen J, Meisner S, Aabakken L, et al. Difficult cannulation as defined by a prospective study of the Scandinavian Association for Digestive Endoscopy (SADE) in 907 ERCPs. Scand J Gastroenterol. 2014;49:752–8. doi: 10.3109/00365521.2014.894120. [DOI] [PubMed] [Google Scholar]
- 22.Iwashita T, Yasuda I, Mukai T, et al. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: Single-center prospective pilot study. Dig Endosc. 2017;29:362–8. doi: 10.1111/den.12800. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, Aditi A, Bhat YM, et al. Endoscopic ultrasound-guided biliary access versus precut papillotomy in patients with failed biliary cannulation: A retrospective study. Endoscopy. 2017;49:146–53. doi: 10.1055/s-0042-120995. [DOI] [PubMed] [Google Scholar]
- 24.Dhir V, Isayama H, Itoi T, et al. Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig Endosc. 2017;29:472–85. doi: 10.1111/den.12818. [DOI] [PubMed] [Google Scholar]
- 25.Tyberg A, Desai AP, Kumta NA, et al. EUS-guided biliary drainage after failed ERCP: A novel algorithm individualized based on patient anatomy. Gastrointest Endosc. 2016;84:941–6. doi: 10.1016/j.gie.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Kunda R, Pérez-Miranda M, Will U, et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc. 2016;30:5002–8. doi: 10.1007/s00464-016-4845-6. [DOI] [PubMed] [Google Scholar]
- 27.Minaga K, Kitano M, Yamashita Y, et al. Stent migration into the abdominal cavity after EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2017;85:263–4. doi: 10.1016/j.gie.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: Fatal complication. Endoscopy. 2010;42(Suppl 2):E126–7. doi: 10.1055/s-0029-1243911. [DOI] [PubMed] [Google Scholar]
- 29.Khashab MA, Messallam AA, Penas I, et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. Choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175–81. doi: 10.1055/s-0041-109083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt BA, Hawes R, Hasan M, et al. Biliary drainage: Role of EUS guidance. Gastrointest Endosc. 2016;83:160–5. doi: 10.1016/j.gie.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 32.Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc. 2012;76:1133–41. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]


