Abstract
Background
Salivary duct carcinoma, an aggressive subtype of salivary gland cancer, is mostly androgen receptor‐positive. Only limited data are available on androgen deprivation therapy (ADT).
Methods
Patients with advanced androgen receptor‐positive salivary duct carcinoma treated with first‐line ADT were retrospectively evaluated for clinical benefit (ie, partial response [PR] and stable disease, progression‐free survival [PFS] and overall survival [OS]). The OS was compared with patients with advanced salivary duct carcinoma who received best supportive care.
Results
Thirty‐four of 35 patients who were ADT‐treated were evaluable: 6 patients had a PR (18%) and 11 had stable disease (32%) leading to a clinical benefit ratio of 50%. The median PFS for the ADT‐treated patients was 4 months and the median duration of clinical benefit was 11 months. The median OS was 17 months versus 5 months in 43 patients receiving best supportive care (P = .02).
Conclusion
We recommend ADT in advanced androgen receptor‐positive salivary duct carcinoma given its response and clinical benefit. © 2017 Wiley Periodicals, Inc. Head Neck, 2017
Keywords: androgen deprivation therapy, androgen receptors, antineoplastic agents, hormonal, salivary duct carcinoma, salivary gland neoplasms
1. INTRODUCTION
Salivary gland cancers comprise a heterogeneous group of carcinomas. Salivary duct carcinoma is a very aggressive subtype of salivary gland cancer with a high risk of distant metastatic disease. The median overall survival (OS) is approximately 3 years after primary diagnosis.1, 2, 3
In case of distant metastatic disease, no standard treatment options are available and most of the treatments described in literature are based on small case series or case reports.
Most salivary duct carcinomas express the androgen receptor4; the percentage of androgen receptor positivity varies between 67% and 89%.5, 6, 7 Based on this androgen receptor expression, patients have been treated with androgen deprivation therapy (ADT), such as bicalutamide, an androgen receptor antagonist with a nonsteroidal structure, similar to the treatment with ADT for patients with prostate cancer. Due to the rarity of the disease, a clinical study has never been performed. In 2011, we reported on 10 patients with salivary duct carcinoma treated with ADT in a single center (Radboud University Medical Center), with a clinical benefit rate of approximately 50% and a median progression‐free survival (PFS) of 12 months in patients with clinical benefit.8 The purposes of this retrospective study are to determine the efficacy of ADT in a nationwide group of patients with incurable locally advanced or metastatic androgen receptor‐positive salivary duct carcinoma in terms of response, duration of response, and median OS. In addition, we aim to describe the outcome of a group of patients with incurable locally advanced or metastatic salivary duct carcinoma who received best supportive care during the same period.
2. PATIENTS AND METHODS
This study was performed in accordance with institutional ethical guidelines. In a retrospective search by the Nationwide Network and Registry of Histopathology and Cytopathology (PALGA) in The Netherlands, we collected all patients diagnosed with salivary duct carcinoma in the time period 1990‐2014.9 An experienced pathologist (U.F.) reviewed all pathology slides for central confirmation of the diagnosis. This enabled us to collect data of 177 patients with salivary duct carcinoma. In The Netherlands, treatment of patients with head and neck cancer is centralized in 8 head and neck cancer centers and 6 collaborating major hospitals. Clinical data of all these patients, including age, sex, primary tumor site, presenting symptoms, pathological report (all reviewed), pathological TNM classification, expression of androgen receptor, development of local or locoregional recurrence, or distant metastases, treatment, such as surgery, radiotherapy, or systemic treatment, response evaluation in case of systemic treatment, PFS, and OS, were obtained by reviewing medical records with permission of the treating physicians. By Dutch law, a review by a medical ethical committee was not needed due to the retrospective nature of these observations. Of all 177 patients, only the 86 patients with distant metastatic (n = 84) or incurable locally advanced salivary duct carcinoma (n = 2) were analyzed in further detail. Patients treated with ADT as first‐line palliative treatment were included for analysis about efficacy. Patients who received any other systemic treatment before ADT were excluded (chemotherapy, targeted therapy, or adjuvant systemic therapy before ADT). Ten patients of the current series were already described in an earlier report.8
2.1. Androgen receptor testing
For all 177 patients with salivary duct carcinoma, efforts were made to evaluate the androgen receptor. In 140 patients, the androgen receptor was determined using the androgen receptor polyclonal antibody of Santa Cruz, dilution 1:200, pretreatment with citrate (pH 6.0) for 10 minutes in a PT module. Immunostaining was carried out with the Powervision method by Immunologic. The androgen receptor was scored positive or negative based on diffuse nuclear staining, as described in the World Health Organization classification of salivary duct carcinoma.10 For the remaining patients, no tumor material was available for additional androgen receptor staining. Therefore, the results of androgen receptor determined during routine clinical care procedures were used.
2.2. Treatment protocol
Patients who received ADT were treated with 150 mg bicalutamide once daily (q.d.) or a combination of a luteinizing hormone‐releasing hormone (LHRH) analog (ie, goserelin 3.6 mg subcutaneously every 4 weeks, with 50 mg bicalutamide q.d.).
2.3. Response evaluation
Patients were evaluated approximately every 3 months. Tumor evaluation was measured using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.11 Clinical benefit was defined as: complete response (CR), partial response (PR), or stable disease. The PFS was defined as the time between the start of ADT until the documented date of progressive disease (PD) or death, whatever came first. The OS was counted from the date of confirmation of distant metastasis until the date of death of any cause. Patients alive at last follow‐up were included in the analysis as censored.
Clinical outcomes of ADT‐treated patients were compared with clinical outcomes for patients with distant metastasis or incurable locally advanced disease who received best supportive care.
2.4. Statistical analysis
The OS was estimated by the Kaplan‐Meier survival curves. The log‐rank test was used to compare OS between different groups of patients. A P value of < .05 was considered significant.
To study the association between treatment and survival, an association Cox regression model was fitted to the data. First, the regression model with only treatment as the independent variable was estimated. The hazard ratio in this model represents the association that is uncorrected for possible confounders (age, sex, androgen receptor status, primarily metastasized disease, and organ involvement). Next, the model was adjusted for possible confounders by a forward selection procedure. In every step, it was checked whether one of the possible confounders needed to be added to the model as an independent variable additional to the variables already included in the model. By including it into the model, the variable that causes the biggest change in the association between treatment and survival (estimate of the regression coefficient) but at least 10% and had a P value of, at the most, 10% (Wald‐test), was selected for inclusion to the model. This step was repeated until no variables could be included to the model. Analyses were performed using SPSS version 22.0.
3. RESULTS
3.1. Patient selection
A total of 177 patients with salivary duct carcinoma were retrieved from the nationwide search. Eighty‐six patients had incurable locally advanced (n = 2) or metastatic (n = 84) salivary duct carcinoma, of which 38 were treated with ADT. Of these 38 patients, 3 patients were excluded: 2 patients because they received chemotherapy as first‐line treatment before ADT, and another patient because of prior adjuvant ADT. Forty‐four patients received best supportive care, of which 1 patient was excluded because this patient received adjuvant ADT, which led to a total of 43 patients who received best supportive care only. The other 4 patients received chemotherapy and/or targeted therapy only and were not included in the analysis. Figure 1 shows the flow chart of these patients.
Figure 1.
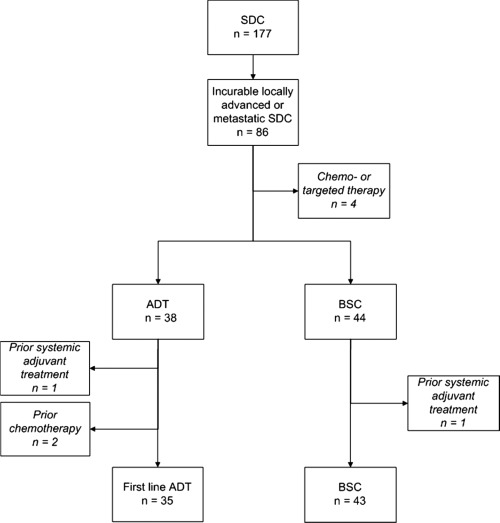
Flow chart of patients with salivary duct carcinoma (SDC; n = 177) and patients with incurable locally advanced or metastatic salivary duct carcinoma (n = 86). Patients treated with first‐line androgen deprivation therapy (ADT) and patients receiving best supportive care (BSC) are shown. Text boxes in cursive represent excluded patients
3.2. Patient characteristics and treatment
The baseline characteristics of the 35 patients receiving first‐line ADT and the 43 patients receiving best supportive care are displayed in Table 1. Patients were median 64 and 68 years of age, and 86% and 81% of the patients were men, respectively. The percentage of the sites of distant metastases and the number of sites of distant metastases per patient are shown in Table 1.
Table 1.
Baseline characteristics of patients with incurable locally advanced or metastatic salivary duct carcinomas
| ADT No. of patients = 35 | BSC No. of patients = 43 | |
|---|---|---|
| Median age, years, range | 64 (38‐83) | 68 (42‐84) |
| Sex, no. of patients (%) | ||
| Male | 30 (86) | 35 (81) |
| Female | 5 (14) | 8 (19) |
| Androgen receptor expression, no. of patients (%) | ||
| Positive | 35 (100) | 35 (81) |
| Negative | 0 | 2 (5) |
| Not performed | 0 | 6 (17) |
| Distant metastasis, no. of patients (%) | ||
| Presenting with distant metastases | 10 (29) | 1 (2) |
| Sequential presentation of distant metastases | 23 (66) | 42 (98) |
| Median time between diagnosis and metastases, months (range) | 15 (1‐69) | 14 (1‐46) |
| Sites of distant metastasis, no. of patients (%) | ||
| Lungs | 20 (57) | 19 (44) |
| Bones | 19 (54) | 16 (63) |
| Lymph nodes | 22 (63) | 8 (19) |
| Liver | 7 (20) | 12 (28) |
| Brain | 3 (9) | 8 (19) |
| Other | 5 (14) | 5 (12) |
| No. of involved organs per patient, no. of patients (%) | ||
| 0 involved organs | 2 (6) | 0 (0) |
| 1 involved organ | 12 (34) | 26 (61) |
| 2 involved organs | 6 (17) | 11 (26) |
| 3 involved organs | 10 (29) | 4 (9) |
| 4 involved organs | 3 (9) | 2 (5) |
| 5 involved organs | 2 (6) | 0 (0) |
Abbreviation: ADT, androgen deprivation therapy; BSC, best supportive care
Patients receiving ADT and best supportive care (BSC) are mentioned separately.
Androgen receptor status was determined using the polyclonal androgen receptor antibody from Santa Cruz in 17 of 35 ADT‐treated patients and 33 of 43 best supportive care‐treated patients. For the remaining patients, no tumor material was available for additional androgen receptor staining. Therefore, the results of androgen receptor as determined during routine clinical care were used. In 6 best supportive care‐treated patients, the androgen receptor expression was not determined and, therefore, unknown.
Of the ADT‐treated patients, 28 patients (80%) received single‐agent bicalutamide, whereas 7 patients (20%) received an LHRH analog in combination with low‐dose bicalutamide.
Although ADT was usually well tolerated, 1 patient switched to another form of ADT and 2 male patients (6%) stopped because of presumed toxicity (ie, fatigue and loss of appetite).
3.3. Response to androgen deprivation therapy and survival
Of the 35 ADT‐treated patients, 1 patient requested to discontinue treatment before the first evaluation and was left out of the response evaluations. Six of 34 evaluable patients (18%) had a PR, 11 (32%) had stable disease, and 17 (50%) had PD (Table 2). All patients with a PR were treated with bicalutamide monotherapy. Figure 2 shows the CT imaging at baseline and after 15 months of ADT in a patient with PR. In total, 50% had clinical benefit and stayed on treatment for a median duration of 11 months (95% confidence interval [CI] 6‐15 months). Median PFS for all ADT‐treated patients was 4 months (95% CI 3‐5 months; Table 2). At the time of analysis, 6 patients were still on first‐line ADT.
Table 2.
Response evaluation, progression‐free survival, and overall survival of androgen deprivation therapy‐treated patients
| ADT No. of patients = 35a | BSC No. of patients = 43 | |
|---|---|---|
| Response evaluation, no. of patients (%) | ||
| PR | 6 (18) | |
| Stable disease | 11 (32) | |
| PD | 17 (50) | |
| Not evaluable | 1 | |
| Median PFS in months [95% CI] | 4 [3‐5] | |
| Median PFS in patients with PR or stable disease in months [95% CI] | 11 [6‐15] | |
| Median OS in months [95% CI] | 17 [10‐24] | 5 [1‐9] |
Abbreviations: ADT, androgen deprivation therapy; BSC, best supportive care;CI, confidence interval; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response.
The OS for best supportive care patients is also shown.
There were 34 evaluable patients.
Figure 2.
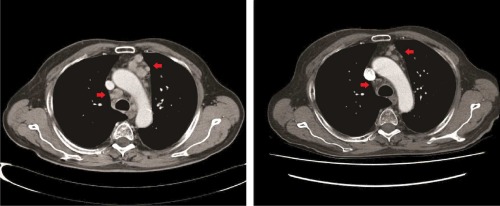
One of the 6 patients with a partial response on androgen deprivation therapy (ADT). Baseline CT imaging of mediastinal lymph nodes (left) and after 15 months of ADT (right). The patient was treated with bicalutamide alone [Color figure can be viewed at wileyonlinelibrary.com]
The median follow‐up time was 10 months (range 1‐64 months). The median OS for ADT‐treated patients was 17 months (95% CI 10‐24 months). The median OS for the ADT‐treated patients with clinical benefit was 29 months (95% CI 8‐51 months) and for patients with PD this was 8 months (95% CI 5‐10 months; Table 2). Two of 5 female patients had a PR on ADT. Possibly because the numbers are small, we did not observe significant differences in OS or PFS between male and female patients treated with ADT, nor did we observe a significant difference between patients treated with single‐agent bicalutamide or LHRH analogs with bicalutamide.
The median OS for patients treated with best supportive care was 5 months (95% CI 1‐9 months; Table 2). The association between treatment and survival was investigated by means of estimating a Cox regression model. No confounders were found. The estimated survival curve for ADT‐treated patients in a Cox regression model is shown in Figure 3. The ADT‐treated patients had a significantly better OS than best supportive care patients in this Cox regression model (P = .024; hazard ratio 0.53; 95% CI 0.30‐0.92).
Figure 3.
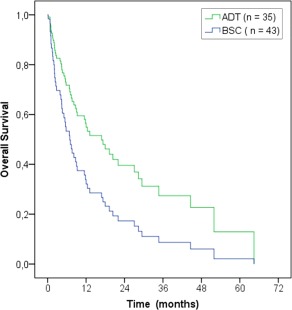
Cox regression survival curves for all androgen deprivation therapy (ADT)‐treated versus best supportive care (BSC) patients. The ADT‐treated patients had significantly better overall survival compared with patients receiving best supportive care (P = .024; hazard ratio 0.53; 95% confidence interval 0.30‐0.92) [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Consecutive systemic treatments
After PD on ADT as first‐line treatment, 11 patients received second‐line ADT. Depending on the agents the patients received as first line, patients were treated with an LHRH analog, either as monotherapy, or combined with bicalutamide and/or a 5‐alfa‐reductase‐inhibitor. Of the 10 patients treated with first‐line bicalutamide monotherapy, 2 patients were consecutively treated with an LHRH analog, 7 patients with an LHRH analog in combination with low‐dose bicalutamide (50 mg), and 1 patient with a combination of an LHRH analog, bicalutamide, and 5‐alfa‐reductase‐inhibitor. One patient treated with an LHRH analog in combination with low‐dose bicalutamide in first‐line, was treated in second‐line with an LHRH analog.
Six patients had stable disease for a median of 9 months (95% CI 6‐12 months), 4 patients had PD, and 1 patient did not have the first evaluation yet. The 6 patients with stable disease on second‐line ADT previously had stable disease (4 patients) or PD (2 patients) on first‐line ADT. One patient received third‐line ADT and had stable disease for another 7 months, after also having stable disease, on first‐line and second‐line ADT.
Ten patients received chemotherapy as second‐line or third‐line treatment after first‐line ADT; 2 of those patients received anti‐human epidermal growth factor receptor 2 (HER2) therapy in combination with docetaxel; 1 patient was treated only with trastuzumab, and the other patient was treated with a combination of trastuzumab and pertuzumab. Five patients had a PR, 3 patients had stable disease and 2 patients had PD.
4. DISCUSSION
Salivary duct carcinoma is a rare and aggressive subtype of salivary gland cancer. Results on ADT in androgen receptor‐positive incurable locally advanced or metastatic salivary duct carcinomas are scarce. We aimed to collect data of patients with salivary duct carcinoma over a period of 25 years in The Netherlands. In this way, we were able to describe the largest retrospective series of patients with salivary duct carcinoma treated with ADT so far and describe a separate group of patients who received best supportive care during the same time period. In 35 patients treated with first‐line ADT for salivary duct carcinoma, 18% had PR and 32% had stable disease, resulting in a clinical benefit rate of 50% with a median treatment duration of 11 months for patients with clinical benefit. The median PFS and OS of the ADT‐treated patients were 4 months and 17 months, respectively. The OS of ADT‐treated patients was significantly better than of best supportive care patients. However, as not all patients responded to ADT, future research should focus on biomarkers that predict the response of patients to ADT and mechanisms of intrinsic and acquired resistance to ADT.12 One may assume that female patients could have less benefit from ADT due to the lower physiological presence of androgen, and, thus, less stimulation of androgen receptor‐positive tumor cells. However, 2 of 5 female patients had sustained PR and no differences in OS and PFS for male and female patients were found.
The incidence of adverse events of ADT‐treated patients seemed to be low, although this may be due to underreporting in this retrospective case series. On the other hand, ADT is a commonly used therapy for patients with prostate cancer, with a well‐known low toxicity profile.13
Obviously, this study has some limitations. The first one is its retrospective design, which is largely due to the rarity of the disease. Performing studies in such rare cancer types is notoriously difficult and, with a central pathological review, we have done the best attempt as possible to collect data of a homogeneous group of patients with salivary duct carcinoma. Second, we compared OS in ADT‐treated patients with best supportive care patients, which could suggest that bias played a role. Unfortunately, we could not retrieve the World Health Organization performance score of patients included in this analysis to investigate this issue. However, we did perform a Cox regression analysis on OS to adjust for possible confounders, measured at diagnosis of incurable locally advanced or metastatic disease. Age, sex, androgen receptor status, metastasized disease at diagnosis, and organ involvement were evaluated as possible confounders, but no confounders were found. Additionally, the majority of patients who received ADT were treated in 1 hospital (74%), because part of the hospitals may not have been familiar with ADT in patients with androgen receptor‐positive salivary duct carcinoma, which pleads against selection bias.
Our objective response rate and clinical benefit rate on ADT in patients with androgen receptor‐positive locally advanced or metastatic salivary duct carcinoma might be slightly lower than case series described in literature (Table 3).8, 14, 15 Locati et al14 reported on 17 patients with androgen receptor‐positive salivary gland cancer (ie, salivary duct carcinoma and also other adenocarcinomas) treated with ADT and found an overall response rate of 65%. Of the 8 patients with salivary duct carcinoma, 2 patients had CR, 2 patients had PR, and 3 patients had stable disease. Furthermore, Yajima et al15 reported on 8 patients with salivary duct carcinoma, with 2 patients with PR and 3 patients with stable disease.
Table 3.
Overview of studies on treatment with androgen deprivation therapy for patients with androgen receptor‐positive salivary duct carcinoma
| Author | No. of patients | ADT | Results | ||||
|---|---|---|---|---|---|---|---|
| CR (%) | PR (%) | Stable disease (%) | PD (%) | Clinical benefit, % | |||
| Current papera | 35b | Bicalutamide + /‐ LHRH analogue | 0 | 6 (18) | 11 (32) | 17 (50) | 50 |
| Jaspers et al8 | 10 | Bicalutamide + /‐ LHRH analogue | 0 | 2 (20) | 3 (30) | 5 (50) | 50 |
| Locati et al14 | 8 | Bicalutamide or cyproterone acetate (1 patient) + LHRH analogue | 2 (25) | 2 (25) | 3 (38) | 1 (13) | 87 |
| Yajima et al15 | 8 | LHRH analogue | 0 | 2 (25) | 3 (38) | 3 (38) | 63 |
Abbreviations: ADT, androgen deprivation therapy; CR, complete response; LHRH, luteinizing hormone‐releasing hormone; PD, progressive disease; PR, partial response.
Ten patients described in the current article were published before.8
There were 34 evaluable patients.
Clinical benefit is CR + PR + stable disease.
In addition to ADT, other therapies may be considered. In a retrospective series of 18 patients with salivary duct carcinoma treated with carboplatin and paclitaxel, they had a median PFS of 6.5 months and median OS of 34.7 months.16 In case of HER2‐positive salivary duct carcinoma, which is observed in 21%‐44% of patients with salivary duct carcinoma, trastuzumab may be added to chemotherapy.17, 18 In fact, trastuzumab‐based treatment regimens have shown some promising responses. In 2 separate reports, a total of 8 patients were treated with paclitaxel, carboplatin, and trastuzumab. Two patients had CR (duration 36 and 52 months) and 2 patients had PR (duration 20 and 36 months).19, 20
Basket studies may offer a promising way to investigate new treatment options for patients with salivary duct carcinoma with targetable mutations. Two case reports mentioned response on temsirolimus and bevacizumab in patients with a somatic phosphotidylinositol‐3‐kinase mutation, and on vemurafenib in a patient with a somatic B‐type Raf mutation.21, 22 Whole genome sequencing may reveal other targetable mutations.
Currently, no treatment algorithms exist for patients with metastatic salivary duct carcinoma. Based on our results and literature, we suggest as first‐line treatment ADT in androgen receptor‐positive salivary duct carcinoma due to efficacy and tolerability. The choice of bicalutamide monotherapy or LHRH analog with low‐dose bicalutamide depends on patient characteristics, such as age and sex. Multiple lines of ADT may be given before considering chemotherapy. In patients with a more aggressive course of the disease, chemotherapy may be considered. In HER2‐positive salivary duct carcinoma, trastuzumab and pertuzumab may be added to chemotherapy. One of the patients treated with docetaxel, pertuzumab, and trastuzumab after ADT was discussed in a separate paper.23 Finally, patients can be analyzed for targetable mutations and treated accordingly.
For future research, combining chemotherapy with ADT in androgen receptor‐positive salivary duct carcinoma may be interesting as an analog to recent insights in prostate cancer (CHAARTED trial).24 In this randomized trial, first‐line ADT was compared to docetaxel plus ADT and showed a significant OS benefit for the latter.
A randomized phase II European Organisation for Research and Treatment of Cancer trial (clinical trial number NCT01969578) is currently including patients with androgen receptor‐positive recurrent or metastatic salivary gland carcinomas. Patients are randomized between chemotherapy (either cisplatin with doxorubicin or carboplatin with paclitaxel) and ADT (triptorelin and bicalutamide). The results of this trial will probably give more insight in treatment for androgen receptor‐positive salivary gland cancer.
One may wonder, with the aggressive behavior of salivary duct carcinoma, and the shown efficacy of ADT in the metastatic setting, if adjuvant treatment with ADT is warranted. The benefit of adjuvant ADT will, however, be difficult to demonstrate.
In conclusion, treatment with ADT in androgen receptor‐positive salivary duct carcinoma can be recommended given its response, clinical benefit, and median OS benefit as compared with patients treated with best supportive care. Further research to select patients with androgen receptor‐positive salivary duct carcinoma who are most likely to benefit from ADT is warranted.
ACKNOWLEDGMENTS
We thank M.H. Liedenbaum for providing the radiology images in this article. We also thank all the pathologists and clinicians for their contribution to patient selection and inclusion. Pathologist, Affiliation: J. Meijer, Rijnstate, Arnhem, The Netherlands; J.E. van der Wal: Martini, Groningen, The Netherlands; L. Arensman, Meander MC, Amersfoort, The Netherlands; S.M. Willems, University Medical Center Utrecht, Utrecht, The Netherlands; E. Bloemena, VU University Medical Center, Amsterdam, The Netherlands; L.A. Smit, Netherlands Cancer Institute, Amsterdam, The Netherlands; the Tissue Bank, University Medical Center Groningen, and the Stichting Laboratorium Pathologie Oost‐Nederland, The Netherlands.
Boon E, van Boxtel W, Buter J, et al. Androgen deprivation therapy for androgen receptor‐positive advanced salivary duct carcinoma: A nationwide case series of 35 patients in The Netherlands. Head & Neck. 2018;40:605–613. https://doi.org/10.1002/hed.25035
This article was presented at the annual meeting of the American Society of Clinical Oncology (ASCO) Chicago, Illinois, June 3‐7, 2016.
REFERENCES
- 1. Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Löning T. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103(12):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 2. Guzzo M, Di Palma S, Grandi C, Molinari R. Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck. 1997;19(2):126‐133. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert MR, Sharma A, Schmitt NC, et al. A 20‐year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142(5):489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Locati LD, Perrone F, Losa M, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs). Oral Oncol. 2009;45(11):986‐990. [DOI] [PubMed] [Google Scholar]
- 5. Di Palma S, Simpson RH, Marchiò C, et al. Salivary duct carcinomas can be classified into luminal androgen receptor‐positive, HER2 and basal‐like phenotypes. Histopathology. 2012;61(4):629‐643. [DOI] [PubMed] [Google Scholar]
- 6. Nardi V, Sadow PM, Juric D, et al. Detection of novel actionable genetic changes in salivary duct carcinoma helps direct patient treatment. Clin Cancer Res. 2013;19(2):480‐490. [DOI] [PubMed] [Google Scholar]
- 7. Williams MD, Roberts D, Blumenschein GR Jr, et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol. 2007;31(11):1645‐1652. [DOI] [PubMed] [Google Scholar]
- 8. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor‐positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473‐e476. [DOI] [PubMed] [Google Scholar]
- 9. Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El‐Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. (eds). WHO Classification of Head and Neck Tumours 4th edition Lyon, France, IARC, 2017. [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 12. Schalken J, Fitzpatrick JM. Enzalutamide: targeting the androgen signalling pathway in metastatic castration‐resistant prostate cancer. BJU Int. 2016;117(2):215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825‐836. [DOI] [PubMed] [Google Scholar]
- 14. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor‐positive salivary gland cancers. Head Neck. 2016;38(5):724‐731. [DOI] [PubMed] [Google Scholar]
- 15. Yajima Y, Kobayashi T, Ishiki H, Hayashi R, Tahara M. Antiandrogen therapy for the patients with recurrent and/or metastatic salivary duct carcinoma expressing androgen receptors: a retrospective study. Ann Oncol. 2012;23(Suppl 9):abstract 1706P. [Google Scholar]
- 16. Nakano K, Sato Y, Sasaki T, et al. Combination chemotherapy of carboplatin and paclitaxel for advanced/metastatic salivary gland carcinoma patients: differences in responses by different pathological diagnoses. Acta Otolaryngol. 2016;136(9):948‐951. [DOI] [PubMed] [Google Scholar]
- 17. Clauditz TS, Reiff M, Gravert L, et al. Human epidermal growth factor receptor 2 (HER2) in salivary gland carcinomas. Pathology. 2011;43(5):459‐464. [DOI] [PubMed] [Google Scholar]
- 18. Masubuchi T, Tada Y, Maruya S, et al. Clinicopathological significance of androgen receptor, HER2, Ki‐67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol. 2015;20(1):35‐44. [DOI] [PubMed] [Google Scholar]
- 19. Limaye SA, Posner MR, Krane JF, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. 2013;18(3):294‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29(10):907‐912. [DOI] [PubMed] [Google Scholar]
- 21. Piha‐Paul SA, Cohen PR, Kurzrock R. Salivary duct carcinoma: targeting the phosphatidylinositol 3‐kinase pathway by blocking mammalian target of rapamycin with temsirolimus. J Clin Oncol. 2011;29(26):e727‐e730. [DOI] [PubMed] [Google Scholar]
- 22. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Boxtel W, Boon E, Weijs WLJ, van den Hoogen FJA, Flucke UE, van Herpen CML. Combination of docetaxel, trastuzumab and pertuzumab or treatment with trastuzumab‐emtansine for metastatic salivary duct carcinoma. Oral Oncol. 2017;72:198‐200. [DOI] [PubMed] [Google Scholar]
- 24. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med. 2015;373(8):737‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]


