Abstract
Acute generalised exanthematous pustulosis (AGEP) is a severe cutaneous adverse reaction and is attributed to drugs in more than 90% of cases. It is a rare disease, with an estimated incidence of 1–5 patients per million per year. The clinical manifestations characterised by the rapid development of sterile pustular lesions, fever and leucocytosis. Number of drugs has been reported to be associated with AGEP, most common being the antibiotics. Histopathologically there is intraepidermal pustules and papillary dermal oedema with neutrophilic and eosinophilic infiltrations. Systemic involvement can be present in more severe cases. Early diagnosis with withdrawal of the causative drug is the most important step in the management. Treatment includes supportive care, prevention of antibiotics and use of a potent topical steroid.
Keywords: Acute generalised exanthematous pustulosis, adverse drug reaction, pustular psoriasis, severe cutaneous adverse reaction
What was known?
Acute generalised exanthematous pustulosis (AGEP) is a severe cutaneous adverse reaction. With early diagnosis and withdrawal of the offending drug, this disease is often self limiting and has favourable prognosis.
Introduction
Acute generalised exanthematous pustulosis (AGEP) is a severe cutaneous adverse reaction, attributed to drugs in the majority (>90%) of the cases, but sometimes may be associated with acute viral infections and mercury. It is a severe cutaneous adverse reaction characterised by the rapid development of nonfollicular, sterile pustules on an erythematous base. The condition is characterised by an abrupt onset, generally occurring within 48 hour of ingesting the suspected medication, of fever and pustulosis with leukocytosis. Most cases have a spontaneous resolution and only a single episode. In severe cases, there can be mucous membrane and systemic organ involvement.[1] Typical histopathologic features include spongiform subcorneal and/or intraepidermal pustules, marked papillary oedema, and polymorphous perivascular infiltrate with neutrophils and exocytosis of some eosinophils.[2,3] Cases with similar clinical characteristics have been described using different names, such as generalised toxic pustuloderma, blistering drug eruptions, and generalised pustular dermatosis.[2]
History
AGEP was originally classified as a form of pustular psoriasis. Baker and Ryan detected, a subgroup of five patients, in a series of 104 cases of pustular psoriasis, who had no history of psoriasis and the episode of pustular eruption in those cases was acute, resolved spontaneously and never recurred. The authors called these patients exanthematic pustular psoriasis and suspected drugs or infections as trigger for these types of pustular eruption.[4]
In 1980, Beylot et al proposed the term AGEP for the first time in the medical literature as a separate entity.[5] However, the disease was mentioned in medical literature much earlier as many cases with similar clinical features were described under different denominations such as toxic pustuloderma and pustular drug rash or were interpreted as special variants of other pustular diseases.[6,7]
Epidemiology
AGEP is a very rare disease, with an estimated incidence of 1–5 patients per million patients per year.[8] The EuroSCAR study reported 97 validated cases of AGEP from different European countries; showed a mean age of 56 years and a female preponderance (male:female ratio of 0.8:3).[9] The female predominance was even higher in some other studies from Israel (76.9%) and Taiwan (68.7%). In these two small series from Israel and Taiwan, the mean age was found to be 40.8 and 40.9 years, respectively.[10,11] The female predominance in AGEP is consistent with the recognised female predominance in drug eruptions in general.[12] AGEP was also seen in children,[13] with a large paediatric series of 20 patients reported from China.[14] The period of onset after exposure is short for AGEP (usually 1–5 days) in the EuroSCAR study, and it may vary for different drugs. For antibiotics, including sulfonamides, the median latent period was 1 day, and for some other drugs, it was 11 days.[9] There may be some seasonal variation in the incidence of AGEP, as clustering of AGEP cases in the summer was reported in a series from Israel.[10]
Aetiology
Drug
AGEP is attributed to drugs in >90% of the cases.[1] EuroSCAR study revealed a range of causative agents for AGEP.[9] It is most commonly associated with the following drugs: pristinamycin (an antistaphylococcal used in Europe), ampicillin/amoxicillin, quinolones, hydroxychloroquine, sulfonamides, terbinafine, diltiazem, ketoconazole, and fluconazole.[9,15,16,17,18] Drugs with weaker associations include macrolides, oxicam nonsteroidal anti-inflammatory drugs, and antiepileptic drugs.[9] Some reports implicated corticosteroids as a possible causative agent in AGEP.[19] Some rarely incriminated drugs include terazosin hydrochloride, omeprazole, and sennoside [Table 1].[20,21,22]
Table 1.
Most frequently mentioned drugs causing acute generalised exanthematous pustulosis

Infection
Infectious agents such as parvovirus B19, Chlamydia pneumoniae, cytomegalovirus, and Coxsackie B4 are reported to have an aetiological association with AGEP.[18,23,24,25] Recurrent urinary tract infection with Escherichia coli was also reported as a cause of AGEP.[26] However, no significant risk for infection was found in the EuroSCAR study.[9] It may be difficult to predict association of AGEP with infection as it is always not possible to pinpoint infective agent and also may be the disease course is associated with antibiotics use.
Contact sensitivity
Contact sensitivity to topical agents was suggested to have a role in the pathogenesis of AGEP. However, the evidences are limited. Hypersensitivity to mercury and bufexamac has been reported as a potential aetiological factor in rare instances of AGEP.[2,27] However, these were not identified as risk factors in the EuroSCAR study.[9]
Psoriasis and atopy
Generalised pustular psoriasis (GPP) may be difficult to distinguish from AGEP both clinically and histopathologically. Earlier times, there were some suggestions that AGEP can be a variant of pustular psoriasis that could be triggered by drugs or infections.[4] In some cases of AGEP, a personal or a family history of psoriasis could be elicited. In the EuroSCAR study, the percentage of individuals with a personal history of psoriasis (7%), family history of psoriasis (4%), or treatment for psoriasis (5%) was higher than but did not differ significantly from the control groups (3%, 4%, and 3%, respectively).[9]
There are some suggestions that bufexamac contact sensitivity induced AGEP may be precipitated by underlying atopy.
Additional causes of acute generalised exanthematous pustulosis
Some other causes have been suggested as an aetiologic agent of AGEP, which include spider bite, malignancy (chronic myeloid leukaemia), and pregnancy.[28,29,30]
Pathophysiology
Genetic background
There may be some genetic predisposition for the development of AGEP. Human leukocyte antigen B51, DR11, and DQ3 are found to be more common in AGEP patients. There seems to be a correlation between mutations in the interleukin-36RN (IL-36RN) gene, encoding the IL-36 receptor antagonist (IL-36Ra), and the development of generalised pustular eruptions after drug intake.[31] However, it is still unclear if mutations in IL-36RN lead to AGEP or a drug-induced GPP.[32] It had been postulated that a mutation in the IL-36RN gene leads to decreased or ineffective IL-36 receptor antagonist, resulting in an uncontrolled IL-36 pathway. Increased IL-36 signalling leads to increased production of IL-6, IL-8, IL-1a, and IL-1b and might predispose to pustular eruptions.[1]
Immune mechanisms
AGEP is now understood to be a T cell-mediated neutrophil-rich type IVd hypersensitivity reaction. The activation of drug-specific CD 4 and CD 8 cells play an important role, as indicated by the patch tests and in vitro tests.[33] Drug-specific cytotoxic T cells and cytotoxic proteins such as granzyme B and perforin induce the apoptosis of keratinocytes, leading to subcorneal vesicles.[34,35] Drug-specific T cells in AGEP patients produce significantly more chemokine (C-X-C motif) ligand 8/IL-8 which plays a central role in the formation of pustules by recruitment of neutrophils. High levels of IL-17, IL-22, and granulocyte–macrophage colony-stimulating factor (GM-CSF) in AGEP patients may also have some role in the strong neutrophilic activity.[36] Studies also indicated that innate cells might be involved in the pathogenesis of AGEP.
Pathogenesis
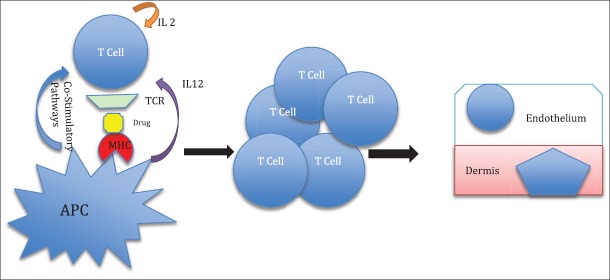
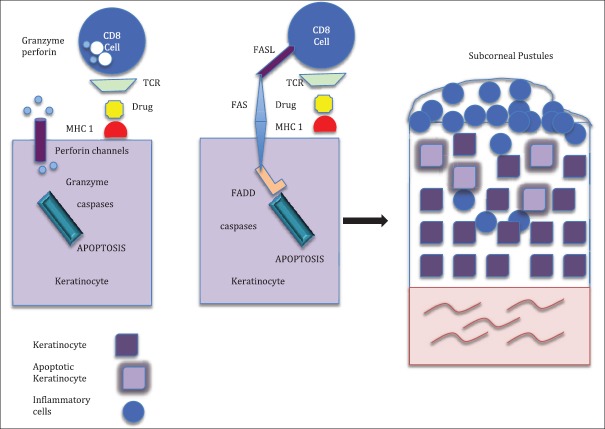
In the initial phase of the pathogenesis of AGEP, there is activation of drug-specific T-cells with subsequent migration of the CD4 and CD8 cells to the skin. The initial influx of CD8 cytotoxic T-cells results in apoptosis of keratinocytes and the formation of sub-corneal vesicles. The infiltrating CD4 cells and keratinocytes release CXCL-8, which results in the recruitment of neutrophils and GM-CSF, which prevents apoptosis of neutrophils. This results in the conversion of vesicles into pustules. CD4 cells also release Interferon (IFN) gamma, which stimulates keratinocytes to secrete CXCL-8. Resident Langerhans cells may present drug antigens to CD4 cells and keratinocytes may act as antigen presenting cells to CD8 cells, thereby augmenting the neutrophil-mediated inflammatory response[37] [Figures 1–3].
Figure 1.
The first phase of the pathogenesis of AGEP; activation and expansion of drug-specific T-cells with subsequent migration to the skin. APC: antigen presenting cells. IL: interleukin; MHC: major histocompatibility complex
Figure 3.
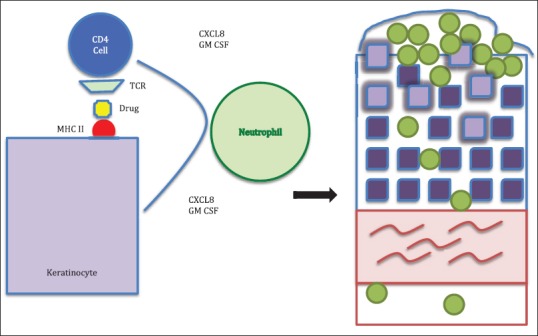
Presentation of the drug bound to major histocompatibility complex Class II by keratinocytes to CD4 cells resulting in (a) the release of CXC chemokine ligand 8 and granulocyte–macrophage colony-stimulating factor by both CD4 cells and keratinocytes which lead to (b) the migration of neutrophils into the epidermis and the conversion of the subcorneal blister into a sterile pustule. TCR: T-cell receptor.
Figure 2.
Influx of drug-specific cytotoxic T-cells and presentation of the drug bound to major histocompatibility complex Class I by keratinocytes result in apoptosis of the keratinocyte from perforin and granzyme release and the Fas-Fas ligand. These result in the formation of acantholysis and subcorneal blister. TCR: T-cell receptor. FADD: Fas associated death domain
Histopathology
A histopathological examination should be performed to distinguish AGEP from other pustular eruptions. Histopathological hallmark of AGEP is the presence of the intracorneal, subcorneal, or intraepidermal pustules. There are also oedema at the papillary dermis and perivascular infiltrates containing neutrophils and eosinophils. Spongiform changes are found in both the intracorneal and subcorneal pustules. Epidermis also has spongiosis along with exocytosis of neutrophils and presence of necrotic keratinocytes.[38] In some cases, necrotic keratinocytes and leukocytoclastic vasculitis may also be found. Unlike plaque psoriasis, papillary dermal dilated blood vessels are infrequent in AGEP. In a study by EuroSCAR and RegiSCAR study group, there was no statistically significant difference in histopathology between a subgroup of AGEP with a personal history of psoriasis as compared with AGEP with no history of psoriasis.[39] The presence of eosinophils, necrotic keratinocytes, a mixed interstitial and mid-dermal perivascular infiltrate and the absence of tortuous or dilated blood vessels favour AGEP, while the presence of psoriasiform acanthosis is characteristic of generalised pustular psoriasis.
Clinical Features
Onset
AGEP is a severe cutaneous adverse reaction with rapid onset. Characteristically, patients of AGEP develop an acute rash with pinhead sized pustules on erythematous base starting in the flexural area and spreading quickly within a few hours to involve trunk and limbs. The time period, from the drug intake to the disease onset, is usually 24–48 h.[9]
Morphology
The mucocutaneous features of AGEP include few to hundreds of small, sterile, nonfollicular pustules on an erythematous base with absent or minimal mucous membrane involvement. The usual morphology is an acute oedematous erythema, followed by dozens of small nonfollicular sterile pustules with a predilection for the larger flexural areas. Mild, nonerosive mucous membrane involvement may occur in about 20% of the cases, usually confined to a single site, most often the lips or buccal mucosa [Figure 4]. Uncommonly and atypically, other features may occur, including oedema of the face, purpura, blisters, or target-like lesions.[2]
Figure 4.
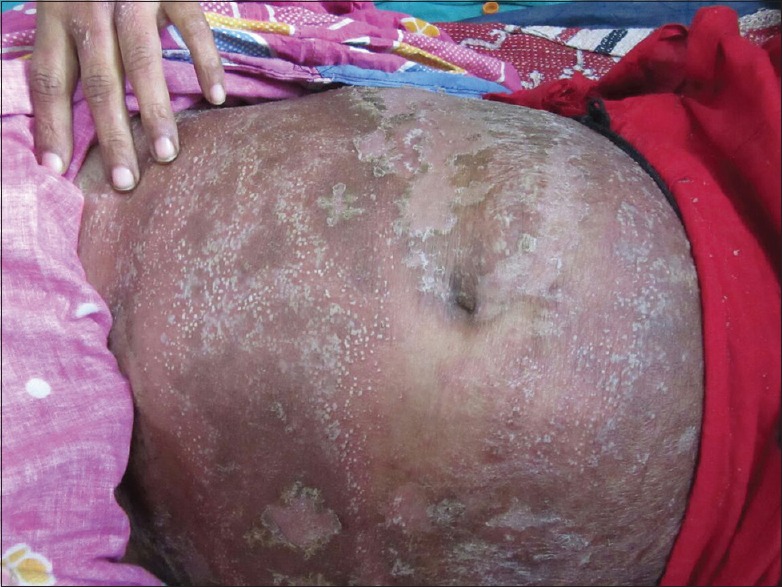
Acute generalised exanthematous pustulosis caused by broad spectrum antibiotic
Symptoms
AGEP is typically pruritic. The skin symptoms of AGEP are almost always accompanied by fever (above 38°C).
Systemic involvement
Systemic involvements are uncommon in AGEP. In a study of 58 patients, 17% of cases had internal organ involvement. Leukocytosis with an elevated neutrophil count (>7500/microliter) and fever (38°C) are constant features of AGEP. Elevated absolute neutrophil count and C-reactive protein levels were associated with systemic organ involvement.[40]
If systemic involvement occurs; liver, kidney, and lung are the most commonly affected organs in the patients of AGEP. Elevated liver enzymes can either be a hepatocellular pattern with high aspartate aminotransferase and alanine aminotransferase or a cholestatic pattern with elevated alkaline phosphatase and g-glutamyltransferase. Abdominal ultrasound of individuals with hepatic involvement may reveal steatosis or hepatomegaly.[1,41]
Lung involvement in AGEP includes bilateral pleural effusion resulting in hypoxaemia, requiring supplemental oxygen. In the case of multiple organ dysfunction a patient of AGEP should be treated under intensive care.[1,41]
Atypical systemic presentations of acute generalised exanthematous pustulosis
Toxic epidermal necrolysis (TEN)-like AGEP: there are several reports of AGEP mimicking TEN and also several overlap cases have been described. The so-called “TEN-AGEP overlap” cases, described in the literature, where patients had diffuse exfoliation and bullae formation along with mucosal involvement akin to TEN and demonstrate AGEP on histopathology. However, its worth remembering that in up to 20% of cases of AGEP, the mucosa may be involved; cases of AGEP presenting with atypical targets or bullae have also been reported. Medications implicated in “TEN-AGEP overlap” cases include hydroxychloroquine, valdecoxib, paracetamol, infliximab and clindamycin, beta lactam antibiotics, famotidine, carbamazepine, lamotrigine, and estazolam.[42]
AGEP-drug-induced hypersensitivity syndrome (AGEP-DIHS) overlap: Cases of AGEP overlapping with DIHS have also been described. AGEP-DIHS overlap occurs most commonly in association with anti-epileptics such as carbamazepine and phenytoin and has also been described with allopurinol. DIHS with features of AGEP have also been found in association with ibuprofen and mexiletine.[42]
Atypical localised presentations of acute generalised exanthematous pustulosis
Localised AGEP has been reported over a mid-sternal SCAR and on the face, cheeks, and lips.[42,43] Diltiazem has been reported to induce AGEP limited to the distal limbs with sparing of the trunk and flexural folds.[44] AGEP-like allergic contact dermatitis to hydrocortisone butyrate propionate has also been described.[45] AGEP-like contact dermatitis to methylchloroisothiazolinone has been described, in the third-trimester pregnant female, which was consistent with AGEP on histopathology.[46] AGEP was also described in a photosensitive distribution.[42]
Course and Prognosis
The clinical course of AGEP is characterised by spontaneous resolution on drug withdrawal. Resolution is marked by a characteristic desquamation. On discontinuation of the causative agent, resolution of the cutaneous features generally occurs within 15 days. AGEP has a favourable prognosis, although organ involvement, mucosal involvement, secondary infection, delay in diagnosis, and medical comorbidities may result in poor outcome. The reported mortality is 5%. When death does occur, it is generally a result of multiple organ dysfunction and disseminated intravascular coagulation. AGEP can recur after the reintroduction of the causative drug.[1,40]
Diagnosis
Diagnosis of AGEP depends on clinical and histological criteria. The EuroSCAR study group developed a standardised AGEP validation score. The AGEP validation score includes the morphology of skin lesions, the presence of fever, the clinical course, and the laboratory and histopathological findings. It is a standardised scheme that classifies patients with suspected AGEP as having definite, probable, possible, or no AGEP [Table 2]. To identify the responsible drug in case of polymedication, a patch test can be performed after complete skin resolution. The sensibility of the patch test in AGEP is higher than in other drug reactions such as Stevens-Johnson syndrome (SJS) or TEN (58% positive in AGEP vs. 24% positive in SJS/TEN). A positive result often shows small pustules at the location of testing.
Table 2.
Diagnostic score for acute generalised exanthematous pustulosis from EuroSCAR study
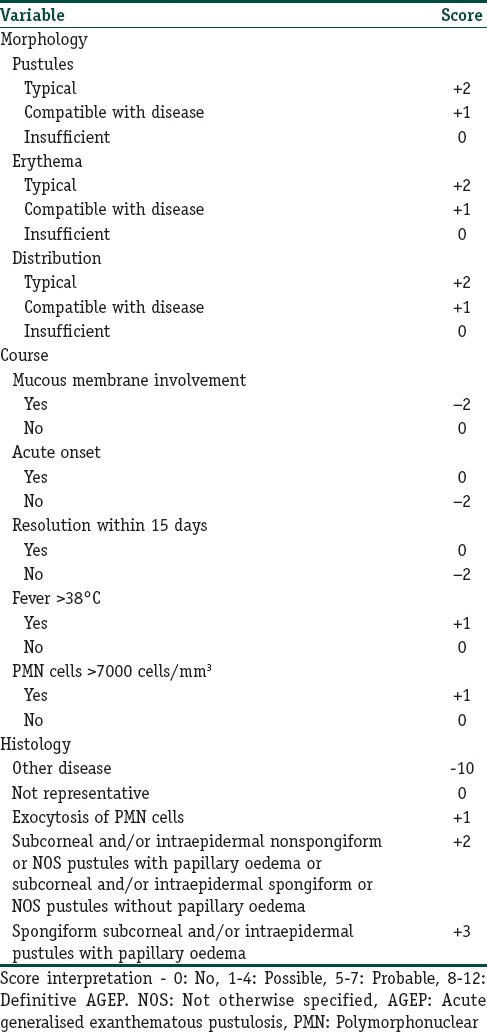
Diagnostic tests
In vivo (patch tests) and in vitro tests can help in the identification of culprit drug in AGEP.
Patch tests: there have been reports of a high rate of strongly positive patch test reactions with the offending drugs (diltiazem, bufexamac, etoricoxib, fluindione, prednisolone, and tetrazepam) in AGEP patients. In a study, patch tests were found positive in seven of 14 AGEP cases. AGEP induced by corticosteroids, the patch tests have showed pustular reactions.[1]
In vitro tests: the in vitro lymphocyte proliferation responses and the in vivo patch test reactions in patients with AGEP are consistent in some cases. The in vitro drug-induced release of the Th1-type cytokine IFN gamma may have some diagnostic role in AGEP. However, poor availability data regarding the diagnostic role of in vitro tests in AGEP, limits its use in real life scenario.[1]
Differential Diagnosis
A wide spectrum of cutaneous diseases or reactions is associated with pustular eruptions. In view of the self-limiting character of AGEP, it is essential to differentiate it from the wide spectrum of cutaneous diseases or reactions, which are associated with pustular eruptions [Table 3]. The principal differential diagnoses of AGEP consist of pustular psoriasis, Sweet's syndrome, pustular erythema multiforme, TEN, drug rash with eosinophilia and systemic symptoms (DRESS), subcorneal pustulosis (Sneddon-Wilkinson syndrome), pustular vasculitis, and bullous impetigo.[47]
Table 3.
Principal differential diagnoses of acute generalised exanthematous pustulosis
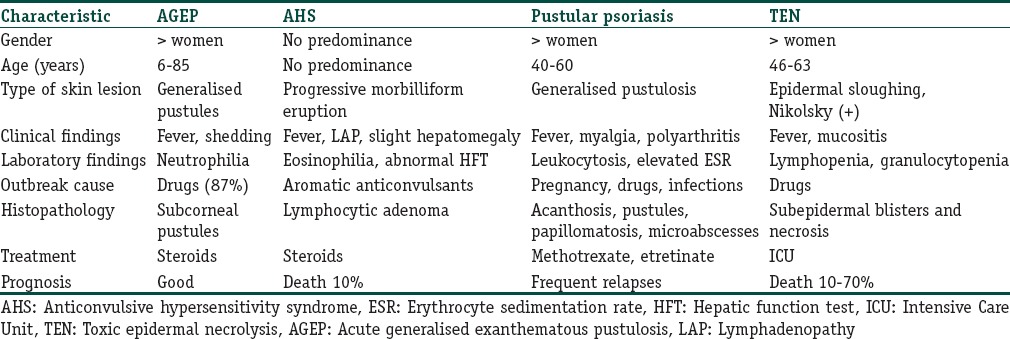
Nonfollicular pustules characterise AGEP, which distinguishes it from follicular pustular diseases such as bacterial folliculitis. Generalised pustular psoriasis, which is the most important differential of AGEP, can be more difficult to differentiate from AGEP. However certain features like slow onset, association with pregnancy, drugs, and infection, and a personal or family history of psoriasis; favour a diagnosis of pustular psoriasis. Salient points in favour of pustular psoriasis histopathologically, include parakeratosis, increased mitotic figures, Munro's microabscess, and tortuous, dilated dermal blood vessels. DRESS typically has an erythematous morbilliform rash but occasionally can develop pustules. The onset of DRESS is insidious (2–6 weeks), compared with 1–2 days for AGEP. Internal organ involvement is nearly a rule in DRESS. Epidermal sloughing, a positive Nikolsky sign, and mucous membrane involvement characterise SJS and TEN.[1,41]
Treatment
AGEP is a self-limiting disease with a favourable prognosis in most cases. In the majority of cases withdrawal of the offending drug and supportive therapy result in recovery. Moist dressings and antiseptic solutions are appropriate during the pustular phase to help prevent infection. Antibiotics should be avoided unless there is a sign of superinfection. Topical corticosteroids reduce pruritus and inflammation. Treatment with potent topical corticosteroids may help in decreasing the duration of hospitalisation.[1] In a study from Israel, of nine AGEP cases, the majority (7 cases) received only supportive care, and the rest 2 cases received corticosteroids.[10] However, in a series of 16 AGEP cases from Taiwan, the treatment in most patients began with intravenous hydrocortisone, with others receiving oral or topical corticosteroids. There was no significant difference between treatment regimens in terms of the course or the duration of the disease.[11] It should be stated that the evidence for systemic corticosteroids to reduce disease duration is extremely thin. Infliximab therapy was reported in three cases of overlap AGEP and TEN. In all three cases, the treatment caused rapid and complete resolution.[48]
Conclusion
AGEP is a T cell-mediated reaction characterised by acute onset of the sterile pustular lesions with a predilection to large folds. Numerous drugs have been implicated as causative agent. The disease needs to be differentiated from other pustular lesions, especially GPP. Once the correct diagnosis is made and the offending drug is withdrawn the disease usually has a favourable prognosis with majority going to spontaneous recovery in 2 weeks time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Many drugs have been described as the causative agent with antibiotics being the most common. In rare cases it can be fatal, especially when there is severe form of the disease with multi-organ involvement.
References
- 1.Szatkowski J, Schwartz RA. Acute generalised exanthematous pustulosis (AGEP): A review and update. J Am Acad Dermatol. 2015;73:843–8. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalised exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333–8. [PubMed] [Google Scholar]
- 3.Burrows NP, Russell Jones RR. Pustular drug eruptions: A histopathological spectrum. Histopathology. 1993;22:569–73. doi: 10.1111/j.1365-2559.1993.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 4.Baker H, Ryan TJ. Generalised pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771–93. doi: 10.1111/j.1365-2133.1968.tb11947.x. [DOI] [PubMed] [Google Scholar]
- 5.Beylot C, Bioulac P, Doutre MS. Pustuloses exanthematiques aigues generalisees. A propos de 4 cas. Ann Dermatol Venereol. 1980;107:37–48. [PubMed] [Google Scholar]
- 6.Staughton RC, Payne CM, Harper JI, McMichen H. Toxic pustuloderma – A new entity? J R Soc Med. 1984;77(Suppl 4):6–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Macmillan AL. Generalised pustular drug rash. Dermatologica. 1973;146:285–91. doi: 10.1159/000251978. [DOI] [PubMed] [Google Scholar]
- 8.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalised exanthematous pustulosis (AGEP) – A clinical reaction pattern. J Cutan Pathol. 2001;28:113–9. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 9.Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalised exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157:989–96. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 10.Davidovici B, Dodiuk-Gad R, Rozenman D, Halevy S, Israeli RegiSCAR Network Profile of acute generalised exanthematous pustulosis in Israel during 2002-2005: Results of the RegiSCAR study. Isr Med Assoc J. 2008;10:410–2. [PubMed] [Google Scholar]
- 11.Chang SL, Huang YH, Yang CH, Hu S, Hong HS. Clinical manifestations and characteristics of patients with acute generalised exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363–5. doi: 10.2340/00015555-0438. [DOI] [PubMed] [Google Scholar]
- 12.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 13.Ersoy S, Paller AS, Mancini AJ. Acute generalised exanthematous pustulosis in children. Arch Dermatol. 2004;140:1172–3. doi: 10.1001/archderm.140.9.1172. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JL, Chen X, Li J, Xie HF. Clinical analysis of childhood acute generalised exanthematous pustulosis. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10:497–9. [PubMed] [Google Scholar]
- 15.Miteva L, Kadurina M, Schwartz RA. Childhood acute generalised exanthematous pustulosis induced by oral ketoconazole. Acta Dermatovenereol Croat. 2010;18:267–70. [PubMed] [Google Scholar]
- 16.Vassallo C, Derlino F, Brazzelli V, D’Ospina RD, Borroni G. Acute generalised exanthematous pustulosis: Report of five cases and systematic review of clinical and histopathological findings. G Ital Dermatol Venereol. 2014;149:281–90. [PubMed] [Google Scholar]
- 17.Di Lernia V, Ricci C. Fluconazole-induced acute generalised exanthematous pustulosis. Indian J Dermatol. 2015;60:212. doi: 10.4103/0019-5154.152572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calistru AM, Lisboa C, Cunha AP, Bettencourt H, Azevedo F. Acute generalised exanthematous pustulosis to amoxicillin associated with parvovirus B19 reactivation. Cutan Ocul Toxicol. 2012;31:258–61. doi: 10.3109/15569527.2011.645978. [DOI] [PubMed] [Google Scholar]
- 19.Buettiker U, Keller M, Pichler WJ, Braathen LR, Yawalkar N. Oral prednisolone induced acute generalised exanthematous pustulosis due to corticosteroids of group A confirmed by epicutaneous testing and lymphocyte transformation tests. Dermatology. 2006;213:40–3. doi: 10.1159/000092837. [DOI] [PubMed] [Google Scholar]
- 20.Speck LM, Wilkerson MG, Perri AJ, Kelly BC. Acute generalised exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395–7. [PubMed] [Google Scholar]
- 21.Nantes Castillejo O, Zozaya Urmeneta JM, Valcayo Peñalba A, Martínez-Peñuela Virseda JM. Acute generalised exanthematous pustulosis induced by omeprazole. Gastroenterol Hepatol. 2008;31:295–8. doi: 10.1157/13119883. [DOI] [PubMed] [Google Scholar]
- 22.Sugita K, Nishio D, Kabashima K, Tokura Y. Acute generalised exanthematous pustulosis caused by sennoside in a patient with multiple myeloma. J Eur Acad Dermatol Venereol. 2008;22:517–9. doi: 10.1111/j.1468-3083.2007.02378.x. [DOI] [PubMed] [Google Scholar]
- 23.Manzano S, Guggisberg D, Hammann C, Laubscher B. Acute generalised exanthematous pustulosis: First case associated with a chlamydia pneumoniae infection. Arch Pediatr. 2006;13:1230–2. doi: 10.1016/j.arcped.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Haro-Gabaldón V, Sánchez-Sánchez-Vizcaino J, Ruiz-Avila P, Gutiérrez-Fernández J, Linares J, Naranjo-Sintes R, et al. Acute generalised exanthematous pustulosis with cytomegalovirus infection. Int J Dermatol. 1996;35:735–7. doi: 10.1111/j.1365-4362.1996.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 25.Feio AB, Apetato M, Costa MM, Sá J, Alcantâra J. Acute generalised exanthematous pustulosis due to coxsackie B4 virus. Acta Med Port. 1997;10:487–91. [PubMed] [Google Scholar]
- 26.Klein N, Hartmann M, Helmbold P, Enk A. Acute generalised exanthematous pustulosis associated with recurrent urinary tract infections. Hautarzt. 2009;60:226–8. doi: 10.1007/s00105-008-1604-1. [DOI] [PubMed] [Google Scholar]
- 27.Belhadjali H, Mandhouj S, Moussa A, Njim L, Amri M, Zakhama A, et al. Mercury-induced acute generalised exanthematous pustulosis misdiagnosed as a drug-related case. Contact Dermatitis. 2008;59:52–4. doi: 10.1111/j.1600-0536.2007.01306.x. [DOI] [PubMed] [Google Scholar]
- 28.Davidovici BB, Pavel D, Cagnano E, Rozenman D, Halevy S; EuroSCAR, et al. Acute generalised exanthematous pustulosis following a spider bite: Report of 3 cases. J Am Acad Dermatol. 2006;55:525–9. doi: 10.1016/j.jaad.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz M, Kreuzer KA, Baskaynak G, Dörken B, le Coutre P. Imatinib-induced acute generalised exanthematous pustulosis (AGEP) in two patients with chronic myeloid leukemia. Eur J Haematol. 2002;69:254–6. doi: 10.1034/j.1600-0609.2002.02830.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto Y, Okubo Y, Yamamoto T, Ito T, Tsuboi R. Case of acute generalised exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362–4. doi: 10.1111/j.1346-8138.2008.00484.x. [DOI] [PubMed] [Google Scholar]
- 31.Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N, Barker JN, Capon F, Creamer D, et al. Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalised exanthematous pustulosis. J Invest Dermatol. 2013;133:1904–7. doi: 10.1038/jid.2013.44. [DOI] [PubMed] [Google Scholar]
- 32.Gabay C, Towne JE. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J Leukoc Biol. 2015;97:645–52. doi: 10.1189/jlb.3RI1014-495R. [DOI] [PubMed] [Google Scholar]
- 33.Britschgi M, Pichler WJ. Acute generalised exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol. 2002;2:325–31. doi: 10.1097/00130832-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Schmid S, Kuechler PC, Britschgi M, Steiner UC, Yawalkar N, Limat A, et al. Acute generalised exanthematous pustulosis: Role of cytotoxic T cells in pustule formation. Am J Pathol. 2002;161:2079–86. doi: 10.1016/S0002-9440(10)64486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlapbach C, Zawodniak A, Irla N, Adam J, Hunger RE, Yerly D, et al. NKp46+cells express granulysin in multiple cutaneous adverse drug reactions. Allergy. 2011;66:1469–76. doi: 10.1111/j.1398-9995.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 36.Kabashima R, Sugita K, Sawada Y, Hino R, Nakamura M, Tokura Y, et al. Increased circulating th17 frequencies and serum IL-22 levels in patients with acute generalised exanthematous pustulosis. J Eur Acad Dermatol Venereol. 2011;25:485–8. doi: 10.1111/j.1468-3083.2010.03771.x. [DOI] [PubMed] [Google Scholar]
- 37.Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87–92. doi: 10.1111/j.1440-0960.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 38.Kardaun SH, Kuiper H, Fidler V, Jonkman MF. The histopathological spectrum of acute generalised exanthematous pustulosis (AGEP) and its differentiation from generalised pustular psoriasis. J Cutan Pathol. 2010;37:1220–9. doi: 10.1111/j.1600-0560.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 39.Halevy S, Kardaun SH, Davidovici B, Wechsler J, EuroSCAR and RegiSCAR Study Group The spectrum of histopathological features in acute generalised exanthematous pustulosis: A study of 102 cases. Br J Dermatol. 2010;163:1245–52. doi: 10.1111/j.1365-2133.2010.09967.x. [DOI] [PubMed] [Google Scholar]
- 40.Hotz C, Valeyrie-Allanore L, Haddad C, Bouvresse S, Ortonne N, Duong TA, et al. Systemic involvement of acute generalised exanthematous pustulosis: A retrospective study on 58 patients. Br J Dermatol. 2013;169:1223–32. doi: 10.1111/bjd.12502. [DOI] [PubMed] [Google Scholar]
- 41.Halevy S. Acute generalised exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322–8. doi: 10.1097/ACI.0b013e32832cf64e. [DOI] [PubMed] [Google Scholar]
- 42.Kostopoulos TC, Krishna SM, Brinster NK, Ortega-Loayza AG. Acute generalised exanthematous pustulosis: Atypical presentations and outcomes. J Eur Acad Dermatol Venereol. 2015;29:209–14. doi: 10.1111/jdv.12721. [DOI] [PubMed] [Google Scholar]
- 43.Sim HS, Seol JE, Chun JS, Seo JK, Lee D, Sung HS, et al. Acute localized exanthematous pustulosis on the face. Ann Dermatol. 2011;23:S368–70. doi: 10.5021/ad.2011.23.S3.S368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gesierich A, Rose C, Brocker EB, Trautmann A, Leverkus M. Acute generalised exanthematous pustulosis with subepidermal blisters of the distal extremities induced by diltiazem. Dermatology. 2006;213:48–9. doi: 10.1159/000092840. [DOI] [PubMed] [Google Scholar]
- 45.Tohgi N, Eto H, Maejima H, Saito N, Nakamura M, Katsuoka K, et al. Allergic contact dermatitis induced by topical hydrocortisone butyrate propionate mimicking acute generalised exanthematous pustulosis. Eur J Dermatol. 2009;19:518–9. doi: 10.1684/ejd.2009.0744. [DOI] [PubMed] [Google Scholar]
- 46.Córdoba S, García-Donoso C, Villanueva CA, Borbujo J, Moreno A. Allergic contact dermatitis from methylchloroisothiazolinone, with acute exanthematous pustulosis-like histopathologic changes. Dermatitis. 2011;22:60–1. [PubMed] [Google Scholar]
- 47.Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, Tlacuilo-Parra A. Acute generalised exanthematous pustulosis: Report of 12 cases and literature review. Int J Dermatol. 2009;48:253–8. doi: 10.1111/j.1365-4632.2009.03908.x. [DOI] [PubMed] [Google Scholar]
- 48.Meiss F, Helmbold P, Meykadeh N, Gaber G, Marsch WCh, Fischer M, et al. Overlap of acute generalised exanthematous pustulosis and toxic epidermal necrolysis: Response to antitumour necrosis factor-alpha antibody infliximab: Report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717–9. doi: 10.1111/j.1468-3083.2006.02026.x. [DOI] [PubMed] [Google Scholar]