Abstract
Background:
Tinea versicolor (TV) is characterised by the appearance of maculosquamous lesions sometimes associated with mild erythema and pruritus in characteristic areas of the body. Eberconazole and terbinafine though drugs of different classes provide both mycological and clinical cure.
Aim:
This study aims to compare the efficacy and safety of eberconazole versus terbinafine in patients of TV.
Materials and Methods:
An open-label, randomised, comparative clinical trial was conducted on 60 patients. The patients were randomly divided into two study groups. Group A: Eberconazole 1% cream once daily and Group B: Terbinafine 1% cream once daily for 2 weeks. Efficacy assessment was done by observing signs and symptoms, i.e., Physician assessment 4-point scale, microscopic KOH examination, Wood's lamp examination, global clinical response assessment, and patient's assessment on visual analog scale at the end of 2 weeks and subsequently patients were reassessed at the end of 4 and 8 weeks to check any relapse. Safety assessment was also done.
Results:
There was a significant improvement in all the parameters in both groups over a period of 2 weeks. Both the treatment groups, i.e., eberconazole and terbinafine were found to be safe and efficacious at the end of 2 weeks, and no statistically significant difference was observed between the two groups regarding complete cure, i.e., mycological and clinical cure (80% vs. 63.33%), respectively. However, early response (at the end of week 1) was observed with eberconazole. No relapse was seen with eberconazole, but one patient had relapse at 8 weeks with terbinafine. Both drugs had similar safety profile.
Conclusion:
Although both the drugs cured the disease, eberconazole showed better response as clinical cure and mycological cure were observed earlier and no patient relapsed in the follow-up.
Keywords: Eberconazole, terbinafine, tinea versicolor
What was known?
Topical terbinafine is indicated for use in tinea versicolor and yields cure rate of more than 80%. Azoles for e.g clotrimazole, ketoconazole are quite efficacious in treatment of tinea versicolor. However, only fewer studies are available depicting the efficacy of a newer azole; eberconazole (topical) in tinea versicolor which is found to be effective in other fungal infections (dermatophytoses and candidiasis).
Introduction
Tinea versicolor (TV) or pityriasis versicolor, also known as Peter Elam's disease, is one of the most common infectious skin diseases that is seen in abundance during summer. It is a chronically recurring fungal infection of the stratum corneum characterised by scaly, hypo- or hyper-pigmented, irregular macules usually located on the trunk and proximal extremities.[1] It is caused by a fungus, Malassezia furfur which is an opportunistic organism, which changes from the saprophytic phase to the pathogenic mycelial phase under certain conditions, such as increased temperature, greasy skin, sweating, and immunosuppression.[2]
Treatment with topical antifungal therapy has several advantages over systemic management, including fewer side effects, fewer drug interactions, localisation of treatment and lower cost. The azole antifungal compounds which have been used for the treatment of tinea versicolor are clotrimazole, miconazole, econazole, ketoconazole, sulconazole, sertaconazole, etc.
Terbinafine is an allylamine antifungal which inhibits the enzyme squalene epoxidase in the fungal cell membrane, thereby blocking the biosynthesis of ergosterol and leads to accumulation of squalene which accounts for its fungicidal activity while deficiency of ergosterol is associated with its fungistatic activity. Topical terbinafine is indicated for use in dermatophytoses and TV. Once daily application of topical terbinafine 1% solution, cream or gel, has been effective in TV.[3] It efficiently penetrates the stratum corneum and may persist for up to 7 days after application.[4]
Eberconazole, an imidazole derivative is a newer antimycotic agent. It is a broad spectrum antifungal agent used as a topical preparation in the management of cutaneous mycoses. It prevents fungal growth by inhibiting lanosterol 14α-demethylase enzyme that is responsible for the formation of 14α-methylsterols (precursor of ergosterols).[5] Eberconazole exerts fungicidal or fungistatic activity depending upon concentration, being fungicidal at higher and fungistatic at lower concentrations.[5] Topical eberconazole has good local and general tolerability without detectable systemic drug levels.[6]
A similar study comparing the efficacy and safety of eberconazole versus terbinafine had already been done on patients with tinea corporis and tinea cruris.[7] The present study was undertaken to compare the efficacy and safety of eberconazole versus terbinafine in TV.
Materials and Methods
This was an open-label, randomised, comparative clinical trial conducted on sixty patients. An informed consent was obtained from all patients enrolled for the study. The study was approved by Institutional Review Board.
An adequate number of patients were screened and selected as per the inclusion and exclusion criteria for the study. The eligible patients were randomly divided into two study groups, i.e. Group A (eberconazole 1% cream once daily for 2 weeks) and Group B (terbinafine 1% cream once daily for 2 weeks) with the help of computer-generated random numbers. Each study group had thirty patients who had completed the study as per protocol.
The inclusion criteria were: Patients of either gender over 18 year of age, patients having lesions on the face, neck and thorax, patients with a clinical diagnosis of TV, confirmed by microscopic KOH test and patients who were ready to give written informed consent. The exclusion criteria were: Pregnant and lactating females, all other types of tinea infections, patients with a history of intolerance or hypersensitivity to the study drugs, patients who had received systemic or topical antifungal treatment within a month, patients who had serious concomitant illness which could prevent the completion of study, patients who had extensive involvement with TV and patients with contact dermatitis, atopic dermatitis, psoriasis, or any other skin disease.
Efficacy assessment was done by observing signs and symptoms, i.e. pruritus, scaling and erythema on a scale of 0–3 (3-severe, 2-moderate, 1-mild, and 0-absent), i.e., physician assessment 4-point scale, microscopic potassium hydroxide (KOH) examination, Wood's lamp examination, global clinical response assessment and patient's assessment on visual analog scale at the end of 1 and 2 weeks and subsequently patients were reassessed at the end of 4 and 8 weeks to check for any relapse. Safety assessment was also done.
Patients were considered as having clinical cure who were not having any of the three symptoms, i.e., erythema, scaling, and pruritus. Mycological cure was considered for negative KOH. Complete cure was considered in patients with clinical as well as mycological cure.
A provision was made for escape treatment to those patients who were not mycologically cured with the study drugs at the end of 2 weeks. Those patients were decided to be treated with 1% clotrimazole as per the standard treatment guidelines.
Data were expressed as mean ± standard error of the mean unless specified otherwise. Intragroup analysis for repeated measures was done using ANOVA for parametric data. Intergroup analysis was done using unpaired t-test for numerical data. Categorical data were analyzed using Chi-square test. P < 0.05 was considered as statistically significant.
Results
Enrolment of patients for the study is shown in Figure 1. The baseline characteristics in terms of age, gender, and marital status in both groups were comparable.
Figure 1.
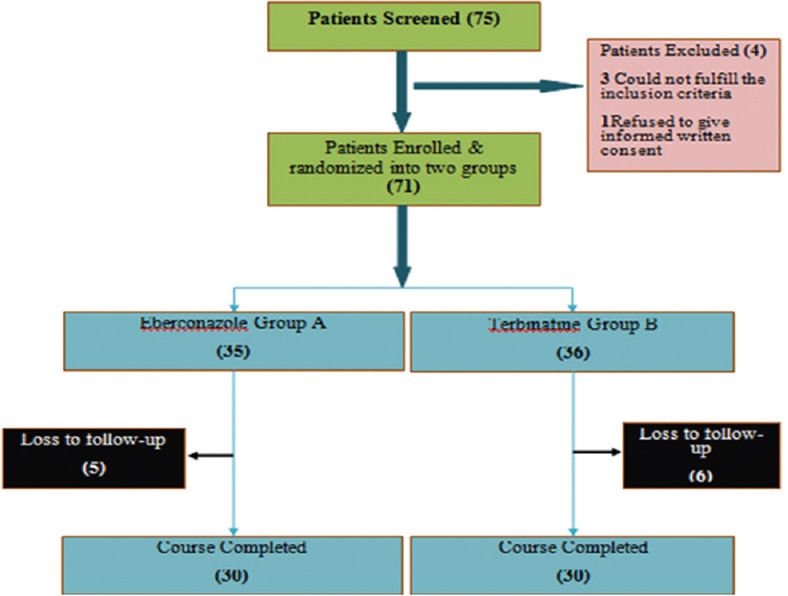
Enrolment of study population
No statistically significant difference in improvement regarding physician assessment 4-point scale was seen on comparing both groups at week 2. However, statistically significant (P < 0.05) improvement was seen with eberconazole as compared to terbinafine in pruritus score at the end of week 1.
Clinical cure at the end of week 1 and 2 was better with eberconazole than terbinafine, but it was not statistically significant (P > 0.05).
Mycological cure at the end of week 2 was also better with eberconazole than terbinafine, but the difference was not statistically significant (P = 0.313). However, the statistically significant response was observed with eberconazole than terbinafine at the end of week 1 (P = 0.009), indicating an early response with eberconazole.
Wood's lamp examination was negative in 100% of the patients at the end of 2 weeks in both groups showing an equivocal response.
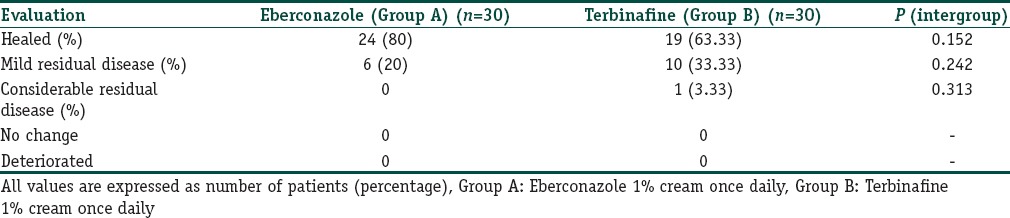
On global clinical assessment at the end of 2 weeks as shown in Table 1, better response was seen with eberconazole group as 80% were completely cured (clinically and mycologically clear) whereas in terbinafine group 63.33% were completely cured. All the patients in eberconazole group, i.e., 100% were responders (healed and mild residual disease) while in terbinafine group 96.66% were responders.
Table 1.
Comparison of global clinical assessment in both groups
Although there was statistically significant reduction in VAS score in both groups at the end of 2 weeks, but there was no statistically significant difference between them (57.67% vs. 45.72%; P = 0.533).
None of the patients in eberconazole group was given escape treatment, but one patient in terbinafine group was given escape treatment at the end of week 2 as he was found to be positive on microscopic examination.
Relapse was seen in only one patient in terbinafine group at the end of 8 weeks while none of the patients relapsed at the end of 4 weeks in both groups.
Safety assessment was carried out in the two study groups at the end of 1st and 2nd week and further re-assessed at the end of 4th and 8th week for follow-up. No patient in either of the two groups complained of any side effects at any time during the study. Laboratory investigations were done at baseline and at the end of 2 weeks and subsequently repeated at the end of 8 weeks. The assessment was done by assessing parameters such as haematological investigations (complete haemogram), biochemical investigations including liver function tests. The laboratory parameters for all the patients were within normal limits in both groups before and after the treatment.
Discussion
In the present study, clinical cure was present in 80% in eberconazole group versus 63.33% in terbinafine group. The mycological cure was seen in 100% patients in eberconazole group as compared to terbinafine (96.66%). In our study, on global clinical assessment, all the patients in eberconazole group, i.e., 100% were responders (healed and mild residual disease) while in terbinafine group 96.66% were responders. In eberconazole group, 80% were completely cured (clinically and mycologically clear) whereas in terbinafine group 63.33% were completely cured.
In a study done by Chopra and Jain,[8] twenty-five patients were distributed to each group randomly and treated either with 2% ketoconazole cream (Group A) or 1% terbinafine cream (Group B) once daily for 2 weeks. In this study, the clinical cure was seen in 100% of the patients in both groups while the mycological cure was seen in 96% of the patients in terbinafine group as compared to 88% in ketoconazole group. On global assessment, 80% in Group A and 96% in Group B were considered healed, leaving 20% in Group A and 4% in Group B with mild residual disease.
Repiso Montero et al[9] compared the efficacy of eberconazole 1% cream with miconazole 2% cream applied twice daily for 4 weeks in the treatment of dermatophytosis, it was observed that clinical efficacy with eberconazole was 76.1% versus 75% in miconazole group.
In a study done by del Palacio et al[10] eberconazole was more effective than clotrimazole against dermatophytosis with overall efficacy of 72% for eberconazole and 61% for clotrimazole. There was no significant difference in relapse in both groups.
In another study done by Choudhary et al,[7] patients with tinea corporis and tinea cruris were treated with topical 1% terbinafine hydrochloride and 1% eberconazole nitrate cream respectively, twice daily for 3 weeks. There was 100% cure rate in both groups at the end of 3 weeks. It was concluded that eberconazole nitrate 1% cream was as effective as terbinafine hydrochloride 1% cream in tinea corporis and cruris.
In our study also, both the treatment groups, i.e., eberconazole and terbinafine were found to be efficacious and safe at the end of 2 weeks, and no statistically significant difference was observed between the two groups regarding complete cure, i.e., mycological and clinical cure (80% vs. 63.33%), respectively. However, early response (at the end of week 1) was observed with eberconazole.
Limitations of the study were; the sample size was small and the duration of the study was short. Further research with larger groups and longer study periods is required to support these findings.
Conclusion
Both the treatment groups, i.e., eberconazole and terbinafine were found to be safe and efficacious in patients suffering from TV (led to improvement in clinical cure and mycological cure). On comparing both treatment groups, better response was observed with eberconazole as patients had earlier clinical and mycological cure and no patient relapsed in the follow-up.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Eberconazole, a newer azole yielded earlier response regarding the clinical as well as mycological cure as compared to terbinafine in patients of tinea versicolor. Hence, it can be a new addition in the armamentarium for the treatment of tinea versicolor.
References
- 1.Goslen JB, Kobayashi GS. Mycologic infections. In: Fitzpatrick TB, Eisen AZ, Wolf K, Freedberg IM, Austen KF, editors. Dermatology in General Medicine. 3rd ed. New York: McGraw Hill Book Company; 1987. pp. 2197–200. [Google Scholar]
- 2.Roberts SO. Pityriasis versicolor: A clinical and mycological investigation. Br J Dermatol. 1969;81:315–26. doi: 10.1111/j.1365-2133.1969.tb13990.x. [DOI] [PubMed] [Google Scholar]
- 3.Faergemann J, Hersle K, Nordin P. Pityriasis versicolor: Clinical experience with Lamisil cream and Lamisil DermGel. Dermatology. 1997;194(Suppl 1):19–21. doi: 10.1159/000246178. [DOI] [PubMed] [Google Scholar]
- 4.High W, Fitzpatrick J. Topical antifungal agents. In: Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Leffel D, editors. Fitzpatrick's Dermatology in General Medicin. 7th ed. New York: McGraw Hill; 2007. pp. 2116–21. [Google Scholar]
- 5.Moodahadu-Bangera LS, Martis J, Mittal R, Krishnankutty B, Kumar N, Bellary S, et al. Eberconazole – Pharmacological and clinical review. Indian J Dermatol Venereol Leprol. 2012;78:217–22. doi: 10.4103/0378-6323.93651. [DOI] [PubMed] [Google Scholar]
- 6.Barbanoj MJ, Antonijoan R, García-Gea C, Puntes M, Gich I, Jané F, et al. Eberconazole cream: Topical and general tolerability, sensitisation potential, and systemic availability. Methods Find Exp Clin Pharmacol. 2005;27:227–34. doi: 10.1358/mf.2005.27.4.893581. [DOI] [PubMed] [Google Scholar]
- 7.Choudhary SV, Aghi T, Bisati S. Efficacy and safety of terbinafine hydrochloride 1% cream vs. eberconazole nitrate 1% cream in localised tinea corporis and tinea cruris. Indian Dermatol Online J. 2014;5:128–31. doi: 10.4103/2229-5178.131079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chopra V, Jain VK. Comparative study of topical terbinafine and topical ketoconazole in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2000;66:299–300. [PubMed] [Google Scholar]
- 9.Repiso Montero T, López S, Rodríguez C, del Rio R, Badell A, Gratacós MR, et al. Eberconazole 1% cream is an effective and safe alternative for dermatophytosis treatment: Multicenter, randomized, double-blind, comparative trial with miconazole 2% cream. Int J Dermatol. 2006;45:600–4. doi: 10.1111/j.1365-4632.2006.02841.x. [DOI] [PubMed] [Google Scholar]
- 10.del Palacio A, Ortiz FJ, Pérez A, Pazos C, Garau M, Font E, et al. A double-blind randomized comparative trial: Eberconazole 1% cream versus clotrimazole 1% cream twice daily in Candida and dermatophyte skin infections. Mycoses. 2001;44:173–80. doi: 10.1046/j.1439-0507.2001.00632.x. [DOI] [PubMed] [Google Scholar]


