Abstract
We examine trends in incidence, mortality and survival of penile squamous cell carcinoma (SCC) in Norway over 60 years. Data on all cases of penile cancer diagnosed in Norway during 1956–2015 were obtained from the Cancer Registry of Norway. Trends in age‐standardized rates of penile SCC incidence, mortality and 5‐year relative survival were assessed by the annual percentage change statistic and joinpoint regression. A total of 1,596 penile cancer cases were diagnosed during 1956–2015, among which 1,474 (92.4%) were SCC. During 2011–2015, the age‐standardized incidence and mortality of penile SCC were 0.91 (95% confidence interval (CI): 0.78; 1.05) and 0.50 (0.42; 0.60) per 100,000, respectively, and the 5‐year relative survival was 61.6% (41.9; 76.4). The incidence of SCC increased during 1956–2015, with an average annual percentage change (AAPC) of 0.80% (0.46; 1.15). The increase was strongest among men diagnosed at a relatively early age (age<=64 years; AAPC: 1.47% (0.90; 2.05)). Mortality also increased over the study period (AAPC: 0.47% (0.10; 0.85)), whereas 5‐year relative survival did not change (AAPC: 0.08% (−0.19; 0.36)). We conclude that the incidence of penile SCC has increased at a moderate and constant rate during 1956–2015, and that the most consistent increase occurred among younger men. Mortality also increased during the study period. However, survival did not change, thus changes in diagnostics and treatment had little impact on survival from penile SCC. Since a substantial proportion of penile SCC is caused by human papillomavirus (HPV), the incidence increase may in part be attributed to increased exposure to HPV in the population.
Keywords: epidemiology, relative survival, incidence, mortality, penile cancer
Short abstract
What's new?
Current trends in penile cancer incidence are unclear. Some studies suggest that the disease is on the rise, while others indicate the opposite. Our study examined changes in penile squamous cell carcinoma (SCC) incidence in Norway from 1956 to 2015. The results show that penile SCC incidence climbed steadily over the 60‐year period, especially among younger men. Mortality associated with penile PCC rose somewhat, while survival did not change significantly, indicating that changes in treatment have only marginally benefited survival. The observed increase in incidence may be associated with increased human papillomavirus (HPV) transmission, which is preventable through HPV vaccination.
Introduction
Penile cancer is a rare disease, reported to occur at rates around 1 per 100,000 men in several Western countries,1, 2, 3 while somewhat higher rates have been reported from countries in Africa, Asia and South‐America. The total number of cases worldwide has been estimated to 26,300 per year.4 Squamous cell carcinomas (SCC) account for the vast majority of penile cancer cases.5 Penile cancer mostly affects older men although it occasionally also may present in younger men.6, 7 Prognostic factors for penile cancer include advanced age, tumor stage, histologic grade and subtype, presence of perineural and lymphatic infiltration, depth of infiltration and lymph node involvement at diagnosis.5, 8, 9 The treatment of penile cancer is often mutilating,10 and may negatively impact on the quality of life and sexual functioning of patients.11, 12 The treatment cost per case of penile cancer is substantial, and comparable to that of other urological cancers.13
Strong risk factors for penile cancer include phimosis and chronic inflammatory conditions.14 The increased risk of penile cancer among men with phimosis is associated with lichen sclerosis or inadequate penile hygiene, smegma retention and thus infection. A recent meta‐analysis showed that childhood circumcision may have a protective effect against penile cancer,15 possibly due to a reduction in the susceptibility to infection by hyperkeratosis on the glans penis and/or facilitated penile hygiene. The condition most frequently associated with penile cancer is sexually transmitted human papillomavirus (HPV) infection. Systematic reviews of studies on HPV prevalence in penile cancer show that 47–48% of tumors tested positive for HPV. Moreover, HPV 16, 18 and 6/11 are the most frequently detected virus types in penile SCC, and HPV is most strongly associated with basaloid and warty subtypes of SCC.16, 17 A higher number of sexual partners and a history of genital warts also increase penile cancer risk,18 most likely through association with exposure to oncogenic HPV. Smoking and penile trauma are other risk factors for penile cancer,18, 19 which may be associated with increased susceptibility to infection.20
An increase in the incidence of some HPV‐related carcinomas has been reported, most consistently for carcinomas of the anus, oropharynx and cervical adenocarcinoma.4 Trend analyses for penile cancer seem less clear‐cut, some studies reporting a decrease,6 some no change21 and some an increase.3 Few studies have examined trends in penile cancer mortality and/or survival, but the existing evidence suggests that any changes for these outcomes are small and may also differ between geographical regions.2, 22
The main objective of our study is to examine trends in incidence, mortality and survival of penile SCC in Norway during the period 1956–2015. We focus on SCC because it is the predominant type of penile cancer, and because it is associated with HPV and thus may be prevented by HPV vaccination.23 We also describe characteristics of all primary cases of penile tumors diagnosed in Norway during this 60‐year period.
Material and Methods
Data
Penile cancer data was extracted from the Cancer Registry of Norway (CRN). Since 1953, the CRN has registered virtually all new cancer cases in the entire Norwegian population. Notification of cancer diagnoses is compulsory by law, and the CRN receives data from clinicians, pathology laboratories and the Cause of Death Registry, ensuring high data completeness and quality.24 Subjects are identifiable in the registries by a unique personal identification number given to each Norwegian citizen at birth or immigration. For all incident cases of penile cancer registered in the CRN during the period 1956–2015, we extracted the patient's date of birth and vital status (alive per 31.12.2015, emigration date or death date), and the tumor localization, morphology, stage and date of diagnosis. Patients were followed up until death, migration or end of study (31.12.2015), whichever occurred first. Total population data was obtained from the National Registry.
Penile cancer cases were identified by topography codes C60.0 (preputium), C60.1 (glans penis), C60.2 (penile corpus), C60.8 (overlapping sites of penis) and C60.9 (penis NOS), and morphology codes from ICD‐O‐3. Basal cell carcinomas and pre‐malignant lesions were not included. Cancer stage at diagnosis was categorized as localized (without metastases), regional spread (any infiltration into surrounding areas or regional metastases), distant metastases or unknown. Tumor morphology was categorized as SCC or other (Table 1).
Table 1.
Characteristics of all cases of invasive penile cancer diagnosed in Norway 1956–2015
| <=44 | 45–54 | 55–64 | 65–74 | >=75 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | |
| Diagnose year | |||||||||||
| 1956–1965 | 10 | (5.3) | 16 | (8.5) | 42 | (22.2) | 52 | (27.5) | 69 | (36.5) | 189 |
| 1966–1975 | 9 | (4.5) | 14 | (7.0) | 38 | (19.0) | 82 | (41.0) | 57 | (28.5) | 200 |
| 1976–1985 | 16 | (6.7) | 25 | (10.4) | 54 | (22.5) | 68 | (28.3) | 77 | (32.1) | 240 |
| 1986–1995 | 21 | (9.0) | 20 | (8.6) | 43 | (18.5) | 61 | (26.2) | 88 | (37.8) | 233 |
| 1996–2005 | 20 | (6.3) | 30 | (9.5) | 55 | (17.5) | 101 | (32.1) | 109 | (34.6) | 315 |
| 2006–2015 | 21 | (5.0) | 60 | (14.3) | 93 | (22.2) | 101 | (24.1) | 144 | (34.4) | 419 |
| Anatomical site | |||||||||||
| C60.0 preputium | 27 | (7.7) | 40 | (11.5) | 92 | (26.4) | 99 | (28.4) | 91 | (26.1) | 349 |
| C60.1 glans penis | 52 | (5.6) | 100 | (10.7) | 171 | (18.3) | 280 | (30.0) | 329 | (35.3) | 932 |
| C60.2 penile corpus | 3 | (7.3) | 3 | (7.3) | 8 | (19.5) | 14 | (34.1) | 13 | (31.7) | 41 |
| C60.8 overlapping penile sites | 7 | (7.0) | 11 | (11) | 21 | (21.0) | 28 | (28.0) | 33 | (33.0) | 100 |
| C60.9 penis, NOS | 8 | (4.6) | 11 | (6.3) | 33 | (19.0) | 44 | (25.3) | 78 | (44.8) | 174 |
| Cancer type | |||||||||||
| Squamous cell carcinoma (SCC)a | 91 | (6.2) | 162 | (11) | 301 | (20.4) | 428 | (29.0) | 492 | (33.4) | 1,474 |
| Other typesb | 6 | (4.9) | 3 | (2.5) | 24 | (19.7) | 37 | (30.0) | 52 | (42.6) | 122 |
| Disease stage | |||||||||||
| 1. Localized tumors | 59 | (5.5) | 111 | (10.3) | 224 | (20.8) | 301 | (28.0) | 381 | (35.4) | 1,076 |
| 2. Regional spread | 26 | (7.4) | 40 | (11.4) | 69 | (19.7) | 119 | (34.0) | 96 | (27.4) | 350 |
| 3. Distant metastases | 7 | (9.6) | 8 | (10.9) | 19 | (26.0) | 11 | (15.1) | 28 | (38.4) | 73 |
| 4. Unknown stage | 5 | (5.1) | 6 | (6.2) | 13 | (13.4) | 34 | (35.1) | 39 | (40.2) | 97 |
| Total | 97 | (6.1) | 165 | (10.3) | 325 | (20.4) | 465 | (29.1) | 544 | (34.1) | 1,596 |
ICD‐O‐3 morphology: 80513,80702,80703,80713,80723,80733,80743,80753,80763,80812,80833,81233.
ICD‐O‐3 morphology: 69999,80003,80103,80203,80502,80503,80702,80812,81203,81403,82313,83903,85423,87203,87213,87433,88003,88103,88323,88503, 88903,91403.
Statistics
We calculated incidence and mortality rates per 100,000 person‐years with corresponding 95% confidence intervals (CI), using the World Standard Population25 for age‐standardization. Age‐standardization allows comparison of summary rates over time despite potential differences in the age structure between populations. Five‐year relative survival was calculated as the ratio of the proportion of observed survivors among the penile cancer patients to the proportion of survivors in the comparable total male population. Observed survival was calculated using the actuarial method and expected survival was calculated using Ederer II estimators,26 applying national population life‐tables for males by age‐group and diagnosis period. Overall survival estimates were age‐standardized using the International Cancer Survival Standard weights.27 When the standard weighting could not be used due to sparse data, age‐standardized estimates were obtained by the alternative weighting method proposed by Brenner.28 The cohort‐approach was used for patients who had completed 5‐year follow‐up. The period‐approach was used to estimate 5‐year relative survival for the most recent cohorts (2011–2015).29 Only patients with localized tumors and regional spread were included in the 5‐year relative survival analysis by stage. Patients with tumors of unknown stage (n = 97) or with distant metastases (n = 73) were excluded from these analyses because they were too few for separate analyses.
Penile SCC trends for incidence, mortality and survival were assessed by yearly rates using joinpoint regression. The analysis identifies periods with distinct linear slopes that can be separated by joinpoints, and determines how many (if any) joinpoints should be used to best describe trends in the data.30 The annual percentage change (APC) with 95% CI was estimated for each trend by fitting a regression to the logarithm of the rates by calendar year. The minimum and maximum number of joinpoints allowed in the time series were zero and four, respectively, and there had to be at least four data points between any joinpoints, and at least three data points from a joinpoint to either end of the data series. We also estimated the average annual percentage change (AAPC) over the whole 60‐year period 1956–2015. For analyses with identified joinpoints during the period, the AAPC is the average of the individual APCs weighted by the length of each segment.31 For overall analyses with no identified joinpoints, the AAPC and the APC are the same. For the survival trend analyses stratified by cancer stage, we used 5‐yearly rates due to a low number of stage‐specific cases in some calendar years. For the incidence trend analyses stratified by age at diagnoses, we used the age strata <=64, 65–74 and >=75, which each contained approximately a third of the penile SCC cases. Significance tests of joinpoint regressions were performed by Monte Carlo permutation with 4,499 replicates. Two‐sided p‐values were considered significant when they were <0.05.
Results
All penile cancer
In total, 1,596 cases of penile cancer were diagnosed in Norway during the period 1956–2015. Penile cancer diagnosed among men below age 55 was relatively rare, and accounted for 16.4% of all cases, while 34.1% of all penile cancers occurred among men age 75 or older (Table 1). The number of cases more than doubled from the first to the last decade of the study period, from a total of 189 cases in 1956–1965 to 419 cases in 2006–2015 (Table 1). The majority of tumors originated from the glans penis (58.4%) or the preputium (21.9%), while only 2.6% originated from the penile corpus (Table 1). The tumor origin for the remaining cases was overlapping between penile subsites (6.3%), or unknown (10.9%). SCC was by far the most common histological type and accounted for 92.4% of the total penile cancer cases (Table 1). The remaining types included malignant melanoma (1.8%), Paget's disease (0.7%), adenocarcinoma (0.6%), sarcoma (0.3%) and a diverse group of other histological types (4.2%) (Table 1). At diagnosis, 67.4% of tumors were localized, 21.9% had spread regionally and 4.6% had distant metastases. The remaining 6.1% had an unknown tumor stage at diagnosis (Table 1).
Penile SCC
The median age at diagnosis of penile SCC was 69 years (1st, 3rd quartile: 59, 78 years). The number of penile SCC cases diagnosed peaked around age 70, after which it declined (Fig. 1), while the age‐specific incidence rate increased with age and was highest among men age 85 or older, at 10.5 per 100,000. Moreover, for most age‐groups, the number of penile SCC cases was higher during 1996–2015 than during the previous decades (Fig. 1).
Figure 1.
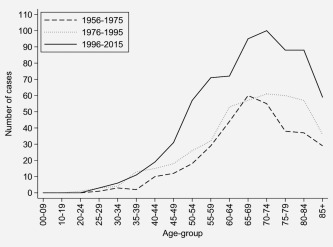
Age‐specific number of cases of penile SCC in Norway by calendar period.
The age‐standardized incidence rate of penile SCC for the most recent 5‐year period (2011–2015) was 0.91 per 100,000 person‐years (95% CI: 0.78, 1.05). The number of cases and the age‐standardized incidence rates fluctuated during 1956–2000, while the three most recent 5‐year periods (2001–2015) had somewhat elevated incidence rates compared to the previous periods (Table 2). However, the best model of the yearly incidence trend in penile SCC did not include any joinpoints, and hence describes a constantly increasing age‐standardized incidence during the period 1956–2015 (Fig. 2 a). The increasing incidence trend was statistically significant, with an AAPC of 0.80% (95% CI: 0.46; 1.15) (Table 3). Age‐stratified analyses showed that the average increase over the whole 60‐year period was significant only for men diagnosed at age 64 years or younger (AAPC: 1.47% (95% CI: 0.90; 2.05)). The increase was less pronounced and non‐significant for men diagnosed at age 65–74 years (AAPC: 0.83% (95% CI: −2.60; 4.37)) and age 75 years or older (AAPC: 0.26% (95% CI: −0.27; 0.80)). The incidence trend for men diagnosed at age 65–74 years included joinpoints in 1971, 1991 and 1996 (Table 3).
Table 2.
Age‐standardized incidence, mortality and 5‐year relative survival for penile SCC in Norway 1956–2015
| Period | Cases (n) | Incidence/100,000 (95% CI) | Cases (n) | Mortality/100,000 (95% CI) | 5‐year RSP (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| 1956–1960 | 75 | 0.63 | (0.49; 0.79) | 29 | 0.22 | (0.15; 0.33) | 66.1 | (48.6; 78.9) |
| 1961–1965 | 95 | 0.74 | (0.59; 0.91) | 53 | 0.39 | (0.29; 0.52) | 67.2 | (52.4; 78.3) |
| 1966–1970 | 84 | 0.63 | (0.50; 0.78) | 52 | 0.36 | (0.26; 0.48) | 61.4 | (45.5; 74.0) |
| 1971–1975 | 84 | 0.56 | (0.45; 0.71) | 64 | 0.41 | (0.31; 0.52) | 73.0 | (55.5; 84.5) |
| 1976–1980 | 109 | 0.72 | (0.58; 0.87) | 76 | 0.43 | (0.34; 0.55) | 78.8 | (63.2; 88.3) |
| 1981–1985 | 112 | 0.72 | (0.58; 0.87) | 99 | 0.49 | (0.40; 0.61) | 75.3 | (61.4; 84.8) |
| 1986–1990 | 91 | 0.55 | (0.44; 0.69) | 96 | 0.49 | (0.39; 0.61) | 71.8 | (55.5; 83.0) |
| 1991–1995 | 124 | 0.72 | (0.59; 0.87) | 104 | 0.50 | (0.41; 0.62) | 67.3 | (54.2; 77.4) |
| 1996–2000 | 128 | 0.72 | (0.59; 0.86) | 108 | 0.46 | (0.38; 0.57) | 70.2 | (59.3; 78.8) |
| 2001–2005 | 170 | 0.91 | (0.77; 1.07) | 104 | 0.44 | (0.35; 0.54) | 81.4 | (70.3; 88.6) |
| 2006–2010 | 193 | 0.98 | (0.84; 1.14) | 126 | 0.48 | (0.39; 0.58) | 80.4 | (70.7; 87.1) |
| 2011–2015 | 209 | 0.91 | (0.78; 1.05) | 146 | 0.50 | (0.42; 0.60) | 61.6 | (41.9; 76.4) |
Abbreviations: CI, confidence interval; RSP, relative survival proportion.
Figure 2.
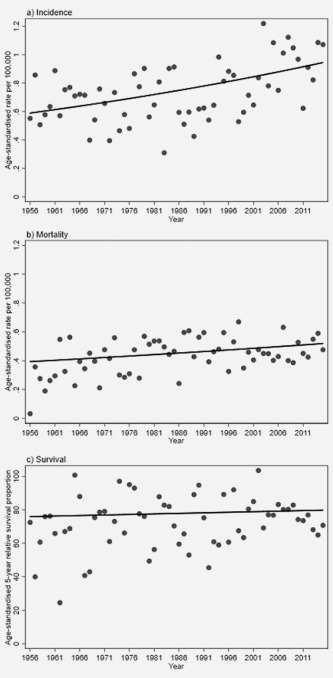
Best‐fitting joinpoint regression line for the age‐standardized (a) incidence, (b) mortality and (c) 5‐year relative survival of invasive SCC diagnosed in Norway during 1956–2016. Dots represent the observed yearly rates/proportions.
Table 3.
Trend analyses of age‐standardized incidence, mortality and survival rates of penile SCC in Norway
| Rate | Stratum | Period | # JP | JP (95% CI) | AAPC | (95% CI) | p‐Value |
|---|---|---|---|---|---|---|---|
| Incidence | Overall | 1956–2015 | 0 | 0.80 | (0.46; 1.15) | <0.01 | |
| Age <=64 | 1956–2015 | 0 | 1.47 | (0.90; 2.05) | <0.01 | ||
| Age 65–74 | 1956–2015 | 3 | 0.83 | (−2.60; 4.37) | 0.64 | ||
| 1956–1971 | 1971 (1958; 1986) | 4.64a | (−0.87; 10.47) | 0.10 | |||
| 1971–1991 | 1991 (1975; 1994) | −4.02a | (−7.05; −0.89) | 0.01 | |||
| 1991–1996 | 1996 (1991; 2010) | 21.61a | (−14.30; 72.55) | 0.27 | |||
| 1996–2015 | −1.84a | (−4.29; 0.68) | 0.15 | ||||
| Age >=75 | 1956–2015 | 0 | 0.26 | (−0.27; 0.80) | 0.33 | ||
| Mortality | Overall | 1956–2015 | 0 | 0.47 | (0.09; 0.85) | 0.01 | |
| Survival | Overall | 1956–2015 | 0 | 0.08 | (−0.19; 0.36) | 0.55 | |
| Localized tumors | 1956–2015 | 0 | 1.48 | (−0.20; 3.19) | 0.08 | ||
| Regional spread | 1956–2015 | 0 | 3.99 | (−1.21; 9.46) | 0.12 |
Abbreviations: # JP, number of joinpoints; CI, confidence interval; AAPC, average annual percentage change.
Annual percentage change.
The penile SCC age‐standardized mortality rate for the most recent 5‐year period (2011–2015) was 0.50 per 100,000 person‐years (95% CI: 0.42, 0.60). The mortality rates were quite stable over the 60‐year period investigated (Table 2). With the exception of the first 5‐year period (1956–1960), which had a relatively low mortality rate, the difference in mortality between any 5‐year period was modest and the confidence intervals were overlapping between the periods. The best‐fitting trend model did not include any joinpoints (Fig. 2 b, Table 3). It estimated a significantly increasing trend in age‐standardized mortality from penile SCC for the 1956–2015 period, with an AAPC of 0.47% (95% CI: 0.09, 0.85).
The age‐standardized 5‐year relative survival proportion for penile SCC fluctuated through most of the period investigated (Table 2). It was similar during the first period (1956–1960: 66.1% (95% CI: 48.6; 78.9)) and last period (2011–2015: 61.6% (95% CI: 41.9; 76.4)). The highest survival, exceeding 80%, was observed during 2001–2010 (Table 2). The trend in 5‐year relative survival over the whole 60‐year study period was increasing, but fell short of significance (AAPC: 0.08% (95% CI: −0.19, 0.36) Fig. 2 c, Table 3). The survival was higher for patients with localized tumors than for patients with regional spread (Table 4). However, the overall time‐trend in survival did not increase significantly over the study period for patients with localized tumors (AAPC: 1.48% (95% CI: −0.20, 3.19)), nor for patients with tumors showing regional spread (AAPC: 3.99% (95% CI: −1.21, 9.46), Table 3).
Table 4.
Age‐standardized 5‐year relative survival proportion (RSP) by tumor stage for penile SCC in Norway during 1956–2015
| Tumor stage | ||||||
|---|---|---|---|---|---|---|
| Localized | Regional | |||||
| Period | Cases (n) | RSP (%, 95% CI) | Cases (n) | RPS (%, 95% CI) | ||
| 1956–1960 | 53 | 88.0 | (58.5; 97.0) | 21 | 13.5 | (3.4; 30.7) |
| 1961–1965 | 73 | 73.3 | (54.4; 85.3) | 16 | 49.7 | (23.0; 71.7) |
| 1966–1970 | 64 | 71.7 | (51.1; 84.8) | 17 | 37.6 | (16.7; 58.6) |
| 1971–1975 | 62 | 79.6 | (55.3; 91.6) | 18 | 58.8 | (18.9; 84.4) |
| 1976–1980 | 74 | 86.7 | (64.0; 95.6) | 28 | 60.4 | (31.8; 80.1) |
| 1981–1985 | 93 | 83.7 | (65.6; 92.8) | 15 | 35.3 | (11.7; 60.3) |
| 1986–1990 | 75 | 79.5 | (60.8; 89.9) | 12 | 47.1 | (13.4; 75.4) |
| 1991–1995 | 95 | 77.5 | (61.5; 87.5) | 24 | 37.2 | (19.4; 55.1) |
| 1996–2000 | 73 | 80.4 | (65.9; 89.2) | 21 | 48.3 | (31.9; 63.0) |
| 2001–2005 | 96 | 95.0 | (60.5; 99.5) | 43 | 63.7 | (46.3; 76.8) |
| 2006–2010 | 105 | 94.5 | (68.2; 99.2) | 56 | 59.2 | (42.7; 72.4) |
| 2011–2015 | 139 | 77.7 | (53.7; 94.7) | 60 | 33.8 | (4.2; 75.7) |
Discussion
This is the first study to examine trends in penile SCC in Norway, and presents the national incidence, mortality and survival over the 60‐year period 1956–2015. We found that the incidence of penile SCC was increasing in Norway during this period. The mortality also increased moderately during the period, while the 5‐year relative survival did not change significantly.
The current incidence of penile SCC in Norway is low, at 0.91 per 100,000 during 2011–2015, which is similar to the incidence in the USA,32 and slightly lower than in Denmark3 and the Netherlands.5 The trend in incidence of penile cancer has been reported as increasing over time in Denmark during 1978–2008,3 and in England during 1979–2009.2 In contrast, the incidence was decreasing in Denmark during 1943–1990,33 in Finland during 1971–199534 and in the USA during 1973–2002/2003.1, 6 Moreover, no change in the incidence trend for penile cancer was found in the Netherlands during 1989–2006,5 in Denmark during 1997–2008,35 in Norway during 1964–200336 or in Australia during 1982–2005.21 Hence, the majority of studies on the incidence of penile cancer did not observe an increase over time. Note, however, that direct comparison of trends between studies is limited by differences in case definitions, data quality, analytical approach and study period. Common to the studies that do report an increase over time is an extensive study period that also includes relatively recent data.
The causes for the observed increasing incidence of penile SCC in Norway are not certain. We are not aware of any changes over time in how penile cancer has been registered in Norway. Moreover, undiagnosed or misclassified cases are likely to be very few, and their frequency is not likely to have changed with time. Temporal changes in risk factors for penile SCC could potentially explain the increasing incidence. Childhood circumcision has a strong protective effect against penile cancer.15 Circumcision rates in Norway are low and the practice is largely confined to Muslim and Jewish communities. Immigration from Muslim countries has increased during recent decades, thus the number of circumcised men is likely to have increased rather than decreased in Norway over the period analyzed here. It is thus unlikely that changes in circumcision rates explain the increasing incidence. Smoking is also associated with increased risk of penile cancer,19 but temporal change in smoking habits is highly unlikely to explain the increase in incidence. The smoking prevalence among men in Norway has been declining in old birth cohorts since the 1950s and in all male birth cohorts since the 1970s, and the lung cancer incidence is currently declining among Norwegian men.37 A positive association between personal/penile hygiene and the risk of penile cancer has also been reported.38 Access to sanitary facilities has improved or been ubiquitous during the lifespan of the men in the cohorts analyzed here, thus the increased incidence of penile SCC cannot reasonably be attributed to changes in hygienic practices at the population level.
Oncogenic HPV infection is a major risk factor for penile SCC, and increased HPV exposure over time in the population, through changes in sexual behavior, remains the most plausible explanation for the observed increased incidence in Norway. Changes in sexual behavior at the population level has been documented in several populations, with a general decrease in age at first intercourse, and an increase in the lifetime number of sexual partners in a long‐term perspective39, 40, 41 Similar changes have also taken place in Norway, both among males and females. The documented change in sexual behavior in Norway is not recent, but seems to have been on‐going already among cohorts born in the 1940s, or even before.42 It is highly likely that the changes in sexual habits have resulted in increased transmission of HPV in the Norwegian population, also among the older men in our cohort. Internationally, consistent incidence increases have also been observed for carcinomas of the anus,43, 44 oropharynx45, 46 and cervical adenocarcinoma.4, 47 For all these carcinomas, a high proportion of cases can be attributed to oncogenic HPV,48, 49, 50 leaving increased HPV exposure a plausible explanation for the increasing incidence. A relatively lower proportion of penile SCC is associated with oncogenic HPV,17 thus any association between population changes in HPV exposure and cancer rates is expected to be weaker for penile SCC than for most of the other HPV‐related carcinomas. Interestingly, there is also mounting evidence of an increasing incidence of vulvar SCC.51, 52, 53 Like penile SCC, vulvar SCC has a relatively low proportion of tumors attributable to HPV infection and is relatively rare.54 The natural history of penile and vulvar cancer is not well documented, and there may be risk factors we are unaware of that also could explain the observed increases in incidence over time. Both locations lack a transformation zone that is present in the cervix, anus and oropharynx, where HPV easily can infect the basal cells and thus continue its life cycle and progress to malignant transformation via precursor lesions.
The HPV‐related subtypes of penile SCC generally present at lower ages than the non‐HPV‐related subtypes.55 Thus, the finding that the increasing incidence of penile SCC occurred only among the relatively younger men is consistent with an increase in the HPV‐related proportion of tumors. Moreover, HPV‐related carcinomas of the vulva and oropharynx also show a pattern of lower age at onset of HPV‐related histological types, and a more pronounced increase in incidence among relatively younger patients.56, 57
Few studies have examined time‐trends in penile cancer mortality and survival. Slight decreases in mortality were observed in the Netherlands during 1989–2006.5 A slight decrease in mortality from penile cancer was also observed in England for the period 1979–2009, despite a concurrent increase in incidence, resulting in increased survival.2 Survival for penile cancer has also increased during 1990–2007 in Northern Europe, but not in a wider selection of European regions.22 The same study found a decreasing survival trend for penile cancer in the USA. Although our survival trend point estimates were positive, we did not observe significant improvement in survival from penile SCC in Norway during 1956–2015. Hence, any change in diagnostics, treatment and patient/clinician awareness of symptoms during this period has not had a strong impact on patient survival. A recent study showed an increase in the use of penile sparing surgery over time, and that the survival of patients treated with penile sparing surgery was similar to patients who underwent full or partial penile amputation.58 Although a shift toward organ sparing management of penile SCC may not strongly affect survival, it may improve patient quality of life.11
This is the first study to investigate incidence, mortality and survival trends in penile SCC in Norway. However, a previous study36 described trends in penile cancer combined with other male genital organs (ICD‐10 C60 and C63) diagnosed during 1964–2003. Penile SCC constitutes the majority of these cases, and for the period of overlap between the studies, the results are generally consistent with the findings reported here. Bray et al.36 did not present formal tests for trend, but noted that the incidence and mortality appeared stable, and that the relative survival appeared rather stable but increasing during some parts of the period analyzed.36 The differences between the studies are likely due to different case definitions, calendar period examined and analytical approach.
The major strength of our study is the high accuracy of the cancer data, which covers virtually all cancer cases in the whole Norwegian population,24 and the extensive study period. The data also allow a comprehensive view of cancer trends, which in addition to incidence also addresses mortality and survival. Moreover, we employ an analysis that can identify several distinct temporal trends during the study period. The main limitation is the lack of detail in the tumor data. The histological diagnoses were not revised and reclassified according to the present WHO classification, thus we lack information on histological subtypes of penile SCC. Moreover, the tumors were not tested for HPV. Data on histological subtypes and HPV analyses would enable a more precise investigation into the potential association between HPV and the penile cancer trends. Sparse data in some of the strata addressed also limited the statistical analyses.
In conclusion, we have shown that the incidence of penile SCC has increased moderately in Norway during 1956–2015, and speculate that this increase may be related to increased HPV exposure at the population level, which may have increased the incidence of the proportion of penile SCC that are attributable to HPV. Because a substantial proportion of penile SCC is attributable to HPV, many incident cases could be prevented by prophylactic HPV vaccination, and vaccination could curb the increasing incidence trend. We also show that the mortality from penile SCC was increasing slightly during the 60‐year period, while survival did not change. This indicates that changes in diagnostics and treatment during the study period have not had a strong impact on the prognosis of penile SCC patients.
Author Contribution
MN, MO and BTH conceived the study. All authors designed the study. MO managed and analyzed the data. BTH drafted the manuscript. All authors critically reviewed manuscript drafts and approved the final version.
Disclosures
None.
Acknowledgements
We thank Inga Hatle for additional information on penile cancer registration practice, and Stein Aaserud for statistical advice.
References
- 1. Barnholtz‐Sloan JS, Maldonado JL, Pow‐Sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol 2007;25:361–7. [DOI] [PubMed] [Google Scholar]
- 2. Arya M, Li R, Pegler K, et al. Long‐term trends in incidence, survival and mortality of primary penile cancer in England. Cancer Causes Control 2013;24:2169–76. [DOI] [PubMed] [Google Scholar]
- 3. Baldur‐Felskov B, Hannibal CG, Munk C, et al. Increased incidence of penile cancer and high‐grade penile intraepithelial neoplasia in Denmark 1978–2008: a nationwide population‐based study. Cancer Causes Control 2012;23:273–80. [DOI] [PubMed] [Google Scholar]
- 4. Parkin DM, Bray F. The burden of HPV‐related cancers. Vaccine 2006;24:11–25. [DOI] [PubMed] [Google Scholar]
- 5. Graafland NM, Verhoeven RH, Coebergh JW, et al. Incidence trends and survival of penile squamous cell carcinoma in the Netherlands. Int J Cancer 2011;128:426–32. [DOI] [PubMed] [Google Scholar]
- 6. Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995–2003. Cancer Epidemiol Biomarkers Prev 2007;16:1833–9. [DOI] [PubMed] [Google Scholar]
- 7. Persson B, Sjodin JG, Holmberg L, et al. The National Penile Cancer Register in Sweden 2000–2003. Scand J Urol Nephrol 2007;41:278–82. [DOI] [PubMed] [Google Scholar]
- 8. Horenblas S, van Tinteren H. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival: analysis of tumor, nodes and metastasis classification system. J Urol 1994;151:1239–43. [DOI] [PubMed] [Google Scholar]
- 9. Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142–50. [DOI] [PubMed] [Google Scholar]
- 10. Misra S, Chaturvedi A, Misra NC. Penile carcinoma: a challenge for the developing world. Lancet Oncol 2004;5:240–7. [DOI] [PubMed] [Google Scholar]
- 11. Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol 2014;192:1105–10. [DOI] [PubMed] [Google Scholar]
- 12. Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology 2005;66:1292–5. [DOI] [PubMed] [Google Scholar]
- 13. Keeping ST, Tempest MJ, Stephens SJ, et al. Penile cancer treatment costs in England. BMC Public Health 2015;15:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dillner J, von Krogh G, Horenblas S, et al. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl 2000;189–93. [DOI] [PubMed] [Google Scholar]
- 15. Larke NL, Thomas SL, dos Santos Silva I, et al. Male circumcision and penile cancer: a systematic review and meta‐analysis. Cancer Causes Control 2011;22:1097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 2009;20:449–57. [DOI] [PubMed] [Google Scholar]
- 17. Miralles‐Guri C, Bruni L, Cubilla AL, et al. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol 2009;62:870–8. [DOI] [PubMed] [Google Scholar]
- 18. Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst 1993;85:19–24. [DOI] [PubMed] [Google Scholar]
- 19. Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer 2005;116:606–16. [DOI] [PubMed] [Google Scholar]
- 20. Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med 2004;164:2206–16. [DOI] [PubMed] [Google Scholar]
- 21. Grulich AE, Jin F, Conway EL, et al. Cancers attributable to human papillomavirus infection. Sex Health 2010;7:244–52. [DOI] [PubMed] [Google Scholar]
- 22. Verhoeven RH, Janssen‐Heijnen ML, Saum KU, et al. Population‐based survival of penile cancer patients in Europe and the United States of America: no improvement since 1990. Eur J Cancer 2013;49:1414–21. [DOI] [PubMed] [Google Scholar]
- 23. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 2011;364:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31. [DOI] [PubMed] [Google Scholar]
- 25. Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical report. Berlin: Springer Verlag, 1966. [Google Scholar]
- 26. Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. Bethesda, MD: End Results Evaluation Section: National Cancer Institute, 1959. [Google Scholar]
- 27. Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16. [DOI] [PubMed] [Google Scholar]
- 28. Brenner H, Arndt V, Gefeller O, et al. An alternative approach to age adjustment of cancer survival rates. Eur J Cancer 2004;40:2317–22. [DOI] [PubMed] [Google Scholar]
- 29. Brenner H, Gefeller O, Hakulinen T. Period analysis for 'up‐to‐date' cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer 2004;40:326–35. [DOI] [PubMed] [Google Scholar]
- 30. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 31. Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med 2009;28:3670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hernandez BY, Barnholtz‐Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer 2008;113:2883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frisch M, Friis S, Kjaer SK, et al. Falling incidence of penis cancer in an uncircumcised population (Denmark 1943‐90). BMJ 1995;311:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pukkala E, Weiderpass E. Socio‐economic differences in incidence rates of cancers of the male genital organs in Finland, 1971–95. Int J Cancer 2002;102:643–8. [DOI] [PubMed] [Google Scholar]
- 35. Kjaer SK, Baldur‐Felskov B, Hannibal CG, et al. Questionable evidence of increasing incidence of invasive penile cancer in Denmark: authors reply. Cancer Causes Control 2012;23:661–2. [DOI] [PubMed] [Google Scholar]
- 36. Bray F, Klint A, Gislum M, et al. Trends in survival of patients diagnosed with male genital cancers in the Nordic countries 1964–2003 followed up until the end of 2006. Acta Oncol 2010;49:644–54. [DOI] [PubMed] [Google Scholar]
- 37. Lund I, Lund KE. Lifetime smoking habits among Norwegian men and women born between 1890 and 1994: a cohort analysis using cross‐sectional data. BMJ Open 2014;4:e005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brinton LA, Li JY, Rong SD, et al. Risk factors for penile cancer: results from a case–control study in China. Int J Cancer 1991;47:504–9. [DOI] [PubMed] [Google Scholar]
- 39. Bajos N, Bozon M, Beltzer N, et al. Changes in sexual behaviours: from secular trends to public health policies. AIDS 2010;24:1185–91. [DOI] [PubMed] [Google Scholar]
- 40. Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013;382:1781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu G, Hariri S, Bradley H, et al. Trends and patterns of sexual behaviors among adolescents and adults aged 14 to 59 years, United States. Sex Transm Dis 2015;42:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stigum H, Samuelsen SO, Traeen B. Analysis of first coitus. Arch Sex Behav 2010;39:907–14. [DOI] [PubMed] [Google Scholar]
- 43. Shiels MS, Kreimer AR, Coghill AE, et al. Anal cancer incidence in the United States, 1977–2011: distinct patterns by histology and behavior. Cancer Epidemiol Biomarkers Prev 2015;24:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soeberg MJ, Rogers K, Currow DC, et al. Trends in incidence and survival for anal cancer in New South Wales, Australia, 1972–2009. Cancer Epidemiol 2015;39:842–7. [DOI] [PubMed] [Google Scholar]
- 45. Nygard M, Aagnes B, Bray F, et al. Population‐based evidence of increased survival in human papillomavirus‐related head and neck cancer. Eur J Cancer 2012;48:1341–6. [DOI] [PubMed] [Google Scholar]
- 46. Braakhuis BJ, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol 2014;50:670–5. [DOI] [PubMed] [Google Scholar]
- 47. Lönnberg S, Hansen BT, Haldorsen T, et al. Cervical cancer prevented by screening: long‐term incidence trends by morphology in Norway. Int J Cancer 2015;137:1758–64. [DOI] [PubMed] [Google Scholar]
- 48. Li N, Franceschi S, Howell‐Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2011;128:927–35. [DOI] [PubMed] [Google Scholar]
- 49. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta‐analysis. Lancet Oncol 2014;15:1319–31. [DOI] [PubMed] [Google Scholar]
- 50. Alemany L, Saunier M, Alvarado‐Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer 2015;136:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schuurman MS, van den Einden LC, Massuger LF, et al. Trends in incidence and survival of Dutch women with vulvar squamous cell carcinoma. Eur J Cancer 2013;49:3872–80. [DOI] [PubMed] [Google Scholar]
- 52. Lai J, Elleray R, Nordin A, et al. Vulval cancer incidence, mortality and survival in England: age‐related trends. BJOG 2014;121:728–38. [DOI] [PubMed] [Google Scholar]
- 53. Meltzer‐Gunnes CJ, Smastuen MC, Kristensen GB, et al. Vulvar carcinoma in Norway: a 50‐year perspective on trends in incidence, treatment and survival. Gynecol Oncol 2017;145:543–8. [DOI] [PubMed] [Google Scholar]
- 54. de Sanjose S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 2013;49:3450–61. [DOI] [PubMed] [Google Scholar]
- 55. Sanchez DF, Canete S, Fernandez‐Nestosa MJ, et al. HPV‐ and non‐HPV‐related subtypes of penile squamous cell carcinoma (SCC): morphological features and differential diagnosis according to the new WHO classification (2015). Semin Diagn Pathol 2015;32:198–221. [DOI] [PubMed] [Google Scholar]
- 56. Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus‐related and ‐unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26:612–9. [DOI] [PubMed] [Google Scholar]
- 57. Hampl M, Deckers‐Figiel S, Hampl JA, et al. New aspects of vulvar cancer: changes in localization and age of onset. Gynecol Oncol 2008;109:340–5. [DOI] [PubMed] [Google Scholar]
- 58. Djajadiningrat RS, van Werkhoven E, Meinhardt W, et al. Penile sparing surgery for penile cancer‐does it affect survival? J Urol 2014;192:120–5. [DOI] [PubMed] [Google Scholar]


