Key Points
Question
Does clinical evaluation and noninvasive cardiac testing improve outcomes in patients who present to the emergency department (ED) with acute chest pain compared with clinical evaluation alone?
Findings
In this secondary analysis of data from a randomized clinical trial, patients who underwent clinical evaluation without noninvasive testing had a shorter length of stay, less diagnostic testing, lower cumulative radiation exposure, and reduced cost; there was no difference in missed diagnosis of acute coronary syndromes, development of major adverse cardiac events, and return ED visits.
Meaning
Noninvasive testing to rule out acute coronary syndromes in low- and intermediate-risk patients who present to the ED with chest pain seems to provide no clinical benefit over clinical evaluation alone.
This secondary analysis of data from a randomized clinical trial examines differences in outcomes with clinical evaluation and noninvasive testing vs clinical evaluation alone.
Abstract
Importance
The incremental benefit of noninvasive testing in addition to clinical evaluation (history, physical examination, an electrocardiogram [ECG], and biomarker assessment) vs clinical evaluation alone for patients who present to the emergency department (ED) with acute chest pain is unknown.
Objective
To examine differences in outcomes with clinical evaluation and noninvasive testing (coronary computed tomographic angiography [CCTA] or stress testing) vs clinical evaluation alone.
Design, Setting, and Participants
This study was a retrospective analysis of data from the randomized multicenter Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography (ROMICAT-II) trial. Data for 1000 patients who presented with chest pain to the EDs at 9 hospitals in the United States were evaluated.
Interventions
Clinical evaluation plus noninvasive testing (CCTA or stress test) vs clinical evaluation alone.
Main Outcomes and Measures
Primary outcome was length of stay (LOS). Secondary outcomes included hospital admission, direct ED discharge, downstream testing, rates of invasive coronary angiography, revascularization, major adverse cardiac events (MACE), repeated ED visit or hospitalization for recurrent chest pain at 28 days, and cost. Safety end points were missed acute coronary syndrome (ACS) and cumulative radiation exposure during the index visit and follow-up period.
Results
Of the 1000 patients randomized, 118 patients (12%) (mean [SD] age, 53.2 [7.8]; 49 [42%] were female) did not undergo noninvasive testing, whereas 882 (88%) (mean [SD] age, 54.4 [8.14] years; 419 [48%] were female) received CCTA or stress testing. There was no difference in baseline characteristics or clinical presentation between groups. Patients who underwent clinical evaluation alone experienced a shorter LOS (20.3 vs 27.9 hours; P < .001), lower rates of diagnostic testing (P < .001) and angiography (2% vs 11%; P < .001), lower median costs ($2261.50 vs $2584.30; P = .009), and less cumulative radiation exposure (0 vs 9.9 mSv; P < .001) during the 28-day study period. Lack of testing was associated with a lower rate of diagnosis of ACS (0% vs 9%; P < .001) and less coronary angiography and percutaneous coronary intervention (PCI) during the index visit (0% vs 10%; P < .001, and 0% vs 4%; P = .02, respectively). There was no difference in rates of PCI (2% vs 5%; P = .15), coronary artery bypass surgery (0% vs 1%; P = .61), return ED visits (5.8% vs 2.8%; P = .08), or MACE (2% vs 1%; P = .24) in the 28-day follow-up period.
Conclusions and Relevance
In patients presenting to the ED with acute chest pain, negative biomarkers, and a nonischemic ECG result, noninvasive testing with CCTA or stress testing leads to longer LOS, more downstream testing, more radiation exposure, and greater cost without an improvement in clinical outcomes.
Trial Registration
clinicaltrials.gov Identifier: NCT01084239
Introduction
An acute coronary syndrome (ACS) is usually caused by the rupture, fissure, or erosion of an atherosclerotic plaque resulting in intraluminal coronary thrombosis and downstream myocardial injury or infarction (MI). Approximately 10 million patients present to emergency departments (EDs) in the United States annually with chest pain suggestive of an ACS, presenting a frequent challenge to ED physicians. The most appropriate testing strategy to exclude the diagnosis of ACS is controversial, and the stakes are high, given the potential consequences of missed diagnoses of ACS, both in terms of patient outcomes and malpractice litigation. Cardiac computed tomographic angiography (CCTA) is an advanced imaging modality with excellent negative predictive value for the diagnosis of coronary artery disease (CAD). Multiple large, multicenter, randomized clinical trials have demonstrated that CCTA is a safe and effective alternative to standard evaluation in the ED when implemented early in the evaluation of chest pain and is associated with reduced length of stay (LOS).
As an anatomic evaluation of the coronary arteries, a completely normal CCTA result effectively rules out CAD and the substrate for ACS. However, although almost all patients with ACS have CAD, only a small minority of patients with CAD have ACS. Thus, the presence of CAD on CCTA in a patient with chest pain does not prove a causal relationship between the chest pain and an ACS. Likewise, the presence of ischemia on a stress test suggests the diagnosis of CAD but does not prove that an ACS is the cause of the chest pain. Given that diagnosis of an ACS in a patient with chest pain is based on the demonstration of myocardial injury or infarction on electrocardiography (ECG) and/or biomarkers and that anatomic (CCTA) and functional (stress) testing diagnose a different albeit predisposing condition (CAD), we hypothesized that noninvasive testing would provide no clinical benefit beyond clinical evaluation including history, physical examination, ECG, and biomarker analysis. Using data from the Rule Out Myocardial Ischemia/Infarction by Computer Assisted Tomography (ROMICAT-II) trial, we compared the effectiveness, safety, including radiation exposure and downstream testing, and cost of clinical evaluation and noninvasive cardiac testing with clinical evaluation alone.
Methods
Data Source
Deidentified data were obtained from the ROMICAT-II trial through the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) of the National Heart Lung and Blood Institute under a data use agreement. The Washington University Human Research Protection Office granted this study an exemption from institutional review board oversight. The study was approved by the institutional review board at each participating site, and all participants provided written informed consent.
Study Design
ROMICAT-II was a randomized, multicenter clinical trial consisting of 1000 patients seen at 9 hospitals in the United States between April 23, 2010, and January 30, 2012. The trial was designed to evaluate use of CCTA as a first diagnostic test as early as possible compared with standard ED evaluation of acute chest pain suggestive of ACS. The study design, inclusion, and exclusion criteria, and primary results were reported previously. Eligible patients were 40 to 74 years old and presented to the ED during weekday, daytime hours with symptoms suggestive of ACS but without ischemic ECG changes or a positive troponin test result on initial laboratory evaluation. All patients had chest pain or an anginal equivalent of at least 5 minutes duration within 24 hours of ED presentation, were in sinus rhythm, and warranted further risk stratification to rule out ACS as determined by an attending physician in the ED. Major exclusion criteria included new diagnostic ischemic changes on the initial ECG, initial troponin level in excess of the 99th percentile of the local assay, history of known CAD, impaired renal function (creatinine level >1.5 mg/dL [12.6 μmol/L]), hemodynamic or clinical instability, known allergy to iodinated contrast agents, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) greater than 40, or symptomatic asthma. (See the Trial Protocol in the online Supplement.)
All patients were randomized either to CCTA as part of the initial evaluation or to the standard ED evaluation strategy, as dictated by local attending physicians in the ED. CCTA was performed with at least 64-slice computed tomographic technology; both retrospectively ECG-gated and prospectively ECG-triggered CCTA protocols were permitted. The use of tube modulation to lower radiation exposure was strongly encouraged. Nine patients randomized to CCTA underwent no testing during the index visit. The standard ED evaluation strategy was performed at the discretion of the local clinicians and included no further diagnostic testing (n = 109) or functional testing (exercise treadmill test, exercise or pharmacological nuclear imaging, stress echocardiography). Patients were contacted by phone within 72 hours if discharged within 24 hours of ED presentation to evaluate for possible missed ACS. Patients were followed up for 28 days after discharge from the ED or hospital by phone interview and questioned regarding repeated ED visits or hospitalizations for recurrent chest pain and diagnostic testing/interventions; reported events were verified with medical records.
This analysis focused on comparing differences between patients who received clinical evaluation and noninvasive testing in either intervention arm and patients who received clinical evaluation with no noninvasive testing during the index ED evaluation. Noninvasive testing was defined as CCTA, treadmill exercise stress test, stress echocardiography, or a myocardial perfusion single-photon emission computed tomography (SPECT) study.
End Points
The primary end point was LOS, defined as the time from ED presentation to the time of the discharge order. Secondary effectiveness end points included rates of direct ED discharge (defined as the proportion of patients discharged from the ED without being admitted to an observation unit or hospital), hospital admission, and diagnostic testing (defined as any of CCTA, exercise treadmill test, nuclear stress test, or stress echocardiography). Additional secondary end points included rates of invasive coronary angiography, percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG), major adverse cardiac events (MACE), defined as death, myocardial infarction, unstable angina, or urgent coronary revascularization within 28 days, and repeated ED visit or hospitalization for recurrent chest pain at 28 days. In a subset of 649 patients (69 with clinical evaluation alone and 580 with noninvasive testing), health care costs during the index care episode were assessed from hospital cost-accounting systems and physician billing records and were adjusted to 2011 dollars. Mean costs for patient care, diagnostic testing, and interventions during the index care episode were used to estimate the costs during follow-up. Safety end points included missed ACS (unexpected cardiovascular event within 72 hours after hospital discharge in patients with a hospital stay of <24 hours) and cumulative radiation exposure during the index visit and follow-up period. Radiation exposure from testing was calculated in millisieverts for CCTA, nuclear perfusion imaging and invasive coronary angiography using standard methods; a conversion coefficient of 0.014 for the chest was used for CCTA scans.
Statistical Analysis
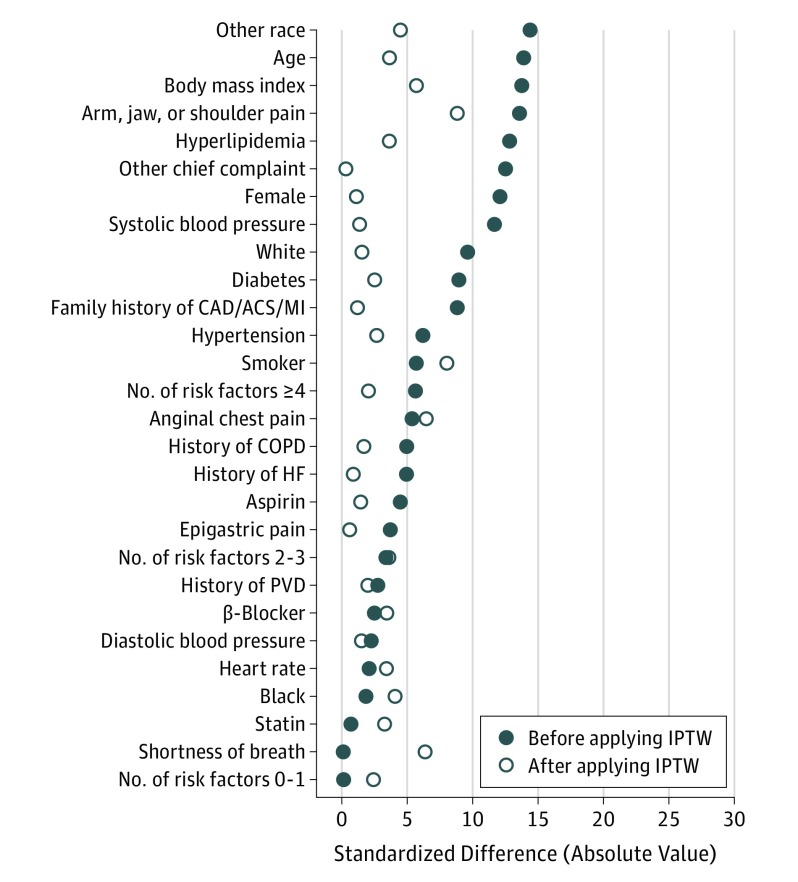
Comparisons between groups were conducted using 2-sample t test and Fisher exact test for continuous and categorical variables, respectively. Ordinal variables and variables with nonnormal distributions were summarized by the median (first quartile, third quartile) and compared using the Mann-Whitney U test. P < .05 was considered statistically significant. Length of stay was also examined via failure curves, which were compared with the log-rank test. Given the nonrandomized derivation of the study groups, outcomes were also compared between tested and nontested groups using inverse probability of treatment weighting (IPTW). Use of IPTW creates a pseudopopulation in which covariates are independent of treatment (ie, are balanced between treatment groups). The probability for testing assignment was determined from a logistic regression model containing the following independent variables: age; sex; race; hypertension; diabetes; hyperlipidemia; smoking status; family history of CAD, ACS, and MI; number of risk factors; aspirin use; β-blocker use; statin use; chief complaint; history of heart failure; history of peripheral vascular disease; history of chronic obstructive lung disease; heart rate; systolic blood pressure (BP); diastolic BP; and BMI. The inverse of the probability of group assignment was then used to create a weight for each patient. To lessen influential weights, weights were stabilized via methods of Robins and Robins et al. Standardized differences were calculated to examine covariate balance before and after IPTW. Standardized differences before and after IPTW are presented in Figure 1. All covariates had standardized differences of less than 0.10 after IPTW. For dichotomous outcomes and LOS, weighted logistic and linear regression models were created, respectively, and robust standard errors were used to compare groups. For dichotomous outcomes in which the nontested group did not have any events, models were not estimable and weighted χ2 test results were reported instead. All other continuous outcomes with skewed distributions were compared using a weighted rank-based test.
Figure 1. Standardized Differences in Baseline Characteristics Between Patients Receiving Clinical Evaluation and Noninvasive Testing vs Clinical Evaluation Alone.
Standardized differences in baseline characteristics between patients receiving clinical evaluation and noninvasive testing vs clinical evaluation alone before and after inverse probability of treatment weighting (IPTW). CAD/ACS/MI indicates coronary artery disease/acute coronary syndrome/myocardial infarction; COPD, chronic obstructive pulmonary disease; HF, heart failure; PVD, peripheral vascular disease.
All analyses were conducted in SAS statistical software (version 9.4; SAS Institute Inc) and R “survey” package.
Results
Of the 1000 patients in ROMICAT-II, 882 (88%) received some form of noninvasive cardiac testing during the index ED evaluation (mean [SD] age, 54.4 [8.14] years; 419 [48%] were female), while 118 (12%) (mean [SD] age, 53.2 [7.8]; 49 [42%] were female) underwent clinical evaluation with no additional testing beyond ECG and biomarker assessment. Table 1 includes the baseline characteristics of the ROMICAT-II patients who underwent clinical evaluation and noninvasive testing or clinical evaluation alone. There was no significant difference between groups with respect to age, sex, or race. The incidence of hypertension, diabetes, and hyperlipidemia as well as smoking history, family history of CAD, and the number of CAD risk factors were similar between groups. The groups did not differ in resting heart rate, systolic or diastolic BP, or BMI. The patients who underwent clinical evaluation alone were more commonly diagnosed as having noncardiac chest pain compared with the noninvasive testing group (107 [91%] vs 764 [87%]), and less commonly diagnosed with ACS (0 [0%] vs 75 [9%]).
Table 1. Patient Characteristics by Evaluation Group.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| Clinical Evaluation Only (n = 118) |
Clinical Evaluation and Noninvasive Testing (n = 882) |
||
| Age, mean (SD), y | 53.2 (7.79) | 54.4 (8.14) | .14 |
| Female | 49 (42) | 419 (48) | .24 |
| Race | .23 | ||
| White | 73 (62) | 586 (66) | |
| African American | 34 (29) | 247 (28) | |
| Other | 11 (9) | 49 (6) | |
| Hypertension | 67 (57) | 474 (54) | .56 |
| Diabetes | 17 (14) | 156 (18) | .44 |
| Hyperlipidemia | 47 (40) | 407 (46) | .20 |
| Former or current smoking | 61 (52) | 431 (49) | .62 |
| First-degree relative with CAD/ACS/MI | 28 (24) | 243 (28) | .44 |
| Risk factors | .90 | ||
| 0-1 | 44 (37) | 329 (37) | |
| 2-3 | 64 (54) | 464 (53) | |
| ≥4 | 10 (8) | 89 (10) | |
| Medications | |||
| Aspirin | 25 (21) | 203 (23) | .73 |
| β-Blockers | 21 (18) | 149 (17) | .79 |
| Statins | 35 (30) | 259 (29) | >.99 |
| Chief complaint | .44 | ||
| Anginal chest pain | 104 (88) | 792 (90) | |
| Epigastric pain | 2 (2) | 11 (1) | |
| Arm/jaw/shoulder pain | 1 (1) | 23 (3) | |
| Shortness of breath | 2 (2) | 15 (2) | |
| Other | 9 (8) | 41 (5) | |
| Medical history | |||
| Congestive heart failure | 1 (1) | 12 (1) | >.99 |
| Peripheral vascular disease | 2 (1) | 12 (1) | .68 |
| Chronic lung disease or COPD | 2 (2) | 21 (2) | >.99 |
| Resting heart rate, mean (SD) | 77.5 (15.44) | 77.2 (14.24) | .87 |
| Systolic blood pressure, mm Hg, mean (SD) | 141.6 (21.82) | 144.2 (22.84) | .23 |
| Diastolic blood pressure, mm Hg, mean (SD) | 82.9 (14.10) | 83.1 (13.21) | .85 |
| BMI, mean (SD) | 28.56 (5.21) | 29.31 (5.03) | .13 |
| Primary discharge diagnosis | <.001 | ||
| Noncardiac chest pain | 107 (91) | 764 (87) | |
| Noncoronary cardiac chest pain | 3 (3) | 12 (1) | |
| Cardiac chest pain not meeting ACS criteria | 8 (7) | 31 (4) | |
| Acute coronary syndrome | 0 | 75 (9) | <.001 |
| MI | 0 | 23 (3) | .10 |
| Unstable angina | 0 | 52 (6) | .002 |
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Primary and Secondary Effectiveness End Points
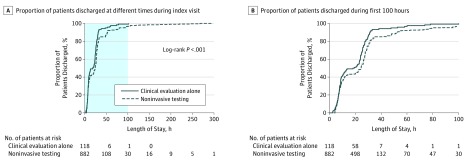
Unadjusted and adjusted outcomes are displayed in Table 2. In the adjusted analysis, patients who underwent clinical evaluation alone had significantly reduced LOS compared with those who underwent noninvasive testing (20.3 vs 27.9 hours; P < .001). Figure 2 shows the cumulative distribution of discharged patients in the clinical evaluation alone group vs the clinical evaluation plus noninvasive testing group (log-rank P < .001). For the secondary end points, there was no difference found in rate of direct ED discharge (30% vs 30%; P = .98) or hospital admission (25% vs 23%; P = .67).
Table 2. Clinical and Safety Outcomes by Treatment Groupa.
| Variable | Unadjusted Analysis | P Value | Adjusted (IPTW) Analysisb | P Value | ||
|---|---|---|---|---|---|---|
| Clinical Evaluation Only (n = 118) |
Clinical Evaluation and Noninvasive Testing (n = 882) |
Clinical Evaluation Only (n = 118) |
Clinical Evaluation and Noninvasive Testing (n = 882) |
|||
| Clinical Outcomes, No. (%) | ||||||
| LOS, mean, h | 19.6 (SD, 16.01) | 27.0 (SD, 34.51) | <.001 | 20.3 (SE, 1.94) | 27.9 (SE, 1.16) | <.001 |
| LOS, median (IQR), h | 18.8 (7.6-26.4) | 24.2 (7.8-29.7) | .02 | 21.2 (7.5-26.4) | 24.1 (7.8-29.6) | .02 |
| Direct ED discharge | 34 (29) | 261 (30) | .91 | (30) | (30) | .98 |
| Hospital admission | 28 (24) | 204 (23) | .91 | (25) | (23) | .67 |
| Diagnostic testing at index | <.001 | <.001 | ||||
| 0 | 118 (100) | 0 | (100) | (0) | ||
| 1 | 0 | 713 (81) | (0) | (81) | ||
| 2 | 0 | 132 (15) | (0) | (15) | ||
| ≥3 | 0 | 37 (4) | (0) | (4) | ||
| Diagnostic testing at index or follow-up | <.001 | <.001 | ||||
| 0 | 98 (83) | 0 | (83) | (0) | ||
| 1 | 19 (16) | 690 (78) | (16) | (78) | ||
| 2 | 1 (1) | 149 (17) | (1) | (17) | ||
| ≥3 | 0 | 43 (5) | (0) | (5) | ||
| Exercise treadmill test during index visit | 0 | 159 (18) | <.001 | (0) | (18) | <.001 |
| SPECT during index visit | 0 | 174 (20) | <.001 | (0) | (20) | <.001 |
| Stress echocardiography during index visit | 0 | 122 (14) | <.001 | (0) | (14) | <.001 |
| CCTA during index visit | 0 | 474 (54) | <.001 | (0) | (54) | <.001 |
| Invasive coronary angiography | ||||||
| During index visit | 0 | 90 (10) | <.001 | (0) | (10) | <.001 |
| During index or follow-up | 2 (2) | 97 (11) | <.001 | (2) | (11) | .008 |
| PCI | ||||||
| During index hospitalization | 0 | 38 (4) | .02 | (0) | (4) | .01 |
| During follow-up but not during index visit | 2 (2) | 4 (0) | .15 | (2) | (0) | .13 |
| During index or follow-up | 2 (2) | 42 (5) | .15 | (2) | (5) | .16 |
| CABG | ||||||
| During index hospitalization | 0 | 9 (1) | .61 | (0) | (1) | .23 |
| During index or follow-up | 0 | 9 (1) | .61 | (0) | (1) | .23 |
| Return ED visits for chest pain within 28 d | 8 (7) | 25 (3) | .047 | (6) | (3) | .08 |
| Major adverse cardiac events | 2 (2) | 6 (1) | .24 | (2) | (1) | .27 |
| Subgroupc | (n = 69) | (n = 580) | (n = 69) | (n = 580) | ||
| ED costs at index visit, median (IQR), $ | 1405.4 (974.7-2261.5) | 1942.4 (1543.8-3033.1) | <.001 | 1467.3 (1037.9-2261.5) | 1944.8 (1543.2-3060.4) | <.001 |
| Total costs at index visit, median (IQR), $ | 1920.9 (1269.3-2812.5) | 2475.4 (1643.4-4006.9) | <.001 | 2136.8 (1285.9-2989.2) | 2460.2 (1644.6-3980.3) | <.001 |
| Total costs, median (IQR), $ | 2182.2 (1345.8-3223.6) | 2586.0 (1653.9-4118.5) | .002 | 2261.5 (1400.5-3223.6) | 2584.3 (1650.4-4108.8) | .009 |
| Safety Outcomes | ||||||
| Missed ACS within 72 h, No. | 0 | 0 | NA | 0 | 0 | NA |
| Median radiation dose, index visit, (IQR), mSv | 0.0 (0.0-0.0) | 9.7 (0.0-15.3) | <.001 | 0.0 (0.0-0.0) | 9.6 (0.0-15.2) | <.001 |
| Median cumulative radiation dose, (IQR), mSv | 0.0 (0.0-0.0) | 9.9 (0.0-15.5) | <.001 | 0.0 (0.0-0.0) | 9.9 (0.0-15.4) | <.001 |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CCTA, coronary computed tomographic angiography; ED, emergency department; IPTW, inverse probability of treatment weighting; IQR, interquartile range; LOS, length of stay; NA, not applicable; PCI, percutaneous coronary intervention; SPECT, single-photon emission computed tomography myocardial perfusion imaging.
All values are given as number (percentage) unless otherwise indicated.
Summary statistics derived from the pseudopopulation created after applying IPTW weights.
Costs among the subset of 649 patients (69 with clinical evaluation alone and 580 with noninvasive testing) with cost data available, adjusted to 2011 US dollars.
Figure 2. Length of Stay in the Hospital of Patients Receiving Clinical Evaluation and Noninvasive Testing vs Those Receiving Clinical Evaluation Alone.
A, The proportion of patients discharged at different times from the index visit. The shaded area represents the scale depicted in panel B. B, The proportion of patients discharged during the first 100 hours on the x-axis to highlight the separation between the curves.
During the index visit, the noninvasive testing group included 713 patients (81%) who received 1 diagnostic test, 132 patients (15%) who received 2 diagnostic tests, and 37 patients (4%) who received 3 or more tests; by definition, the 118 patients (100%) in the clinical evaluation alone group received no diagnostic tests during the index encounter (P < .001). During the follow-up period, 690 patients (78%) in the noninvasive testing group received 1 diagnostic test, 149 (17%) received 2 diagnostic tests, and 43 (5%) received 3 or more tests. During follow-up of the clinical evaluation alone group, 98 patients (83%) underwent no tests, 19 (16%) underwent 1 test, and 1 (1%) underwent 2 tests, while no patients received 3 or more tests (P < .001). The noninvasive testing group had a higher proportion of patients who underwent coronary angiography during the index visit (10% vs 0%; P < .001) and follow-up (11% vs 2%; P = .008). More patients who underwent noninvasive testing had PCI during the index visit (4% vs 0%; P = .02) with no difference in PCI following discharge (0% vs 2%, P = .15). There was no difference in rates of CABG at the index visit or follow-up (1% vs 0%; P = .23). In the unadjusted analysis, patients who underwent no testing had more return visits to the ED for chest pain within 28 days (8 [7%] vs 25 [3%]; P = .047), whereas after adjustment there was no difference (5.8% vs 2.8%, P = .08).
After adjustment, costs were significantly lower for the clinical evaluation cohort, including median ED costs at the index visit ($1467.30 vs $1944.80; P < .001), median total costs at the index visit ($2136.80 vs $2460.20; P < .001), and median total costs for the entire study period ($2261.50 vs $2584.30; P = .009).
Safety End Points
The noninvasive testing group had greater adjusted radiation exposure during the index visit (median, 9.6 mSv vs 0.0 mSv; P < .001). At follow-up, radiation exposure remained significantly higher among patients who underwent noninvasive testing during the index visit (9.9 mSv vs 0.0 mSv; P < .001). There were no cases of missed ACS in either group and no difference in MACE (6 [1%] vs 2 [2%]; P = .24).
Discussion
In this retrospective analysis of the prospective, randomized ROMICAT-II trial, patients who presented to the ED with acute chest pain and underwent only a clinical evaluation had a shorter LOS and lower radiation exposure without any increase in adverse outcomes compared with patients who underwent noninvasive testing in addition to clinical evaluation. These patients were discharged more than 7.5 hours earlier than patients who underwent noninvasive tests and avoided radiation exposure from cardiac testing. Most important, there were no cases of missed ACS and no difference in MACE between the 2 groups during the 28-day follow-up. Although more cases of ACS were diagnosed in patients who underwent noninvasive testing, our current understanding of the pathophysiologic origins of ACS would suggest that the noninvasive test itself did not contribute to making that diagnosis.
Despite the disconnect between the proximate cause of ACS and the information returned from noninvasive anatomic or functional testing, the 2007 American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend that patients receive noninvasive testing prior to discharge or within a 3-day follow-up period. This is similar to the 2015 European Society of Cardiology (ESC) guidelines, which note that stress testing should be performed in patients with negative biomarkers and nonischemic ECGs before proceeding with an invasive strategy. The 2010 National Institute for Health and Care Excellence (NICE) guidelines recommend that ischemia testing be considered before discharge in patients who have not received coronary angiography. However, noninvasive testing has not been shown to reduce MI in patients who present with acute chest pain. A recent large retrospective analysis that compared 293 788 patients who received no initial noninvasive test with 127 986 patients who underwent noninvasive testing, found no difference in the risk of MI at day 7 or day 190.
While the goal of the guidelines is to reduce the rate of missed ACS, reliance on noninvasive anatomic or functional testing to do so is misguided. Neither CCTA nor stress testing can diagnose ACS, which is a clinical diagnosis made by medical history, ECG findings, and biomarkers. Furthermore, the widespread adoption of troponin testing has very likely reduced the rate of missed MI since the guidelines were written; this trend is likely to continue with the introduction of high-sensitivity troponin testing. New algorithms incorporating high-sensitivity troponin assays have negative predictive values as high as 99.5% for events at index and at 30-day follow up, and 2-year survival rates for patients ruled out for MI approaching or equaling 100%. Even though high-sensitivity assays are not yet widely available in the United States, other tools are available to reduce imaging in low-risk patients who present to the ED with acute chest pain. A recent trial randomized low-risk ED patients with chest pain to evaluation with the HEART score (an algorithm to assess risk) and troponin measurements at 0 and 3 hours vs usual ED care. Patients with low-risk HEART scores, negative troponin test results, and nonischemic ECG were discharged from the ED, resulting in decreased length of stay, less cardiac testing, and no difference in MACE at 30 days.
It is important to emphasize that the goal of the ED evaluation is not to make a diagnosis of CAD, but rather to rule out the diagnosis of ACS. Our results and those of others suggest that noninvasive testing does not achieve this goal in the ED setting. Clearly, the presence of CAD demonstrated on CCTA or inferred from ischemia on stress testing has implications for long-term outcomes, but that diagnosis is not acutely life-threatening and is best managed through shared decision-making with a primary care physician or cardiologist who has or will develop a long-term relationship with the patient. Growing support for a move away from noninvasive testing has prompted calls for adjustment of the guidelines and clinical trials examining patients randomized to standard clinical evaluation or no imaging. When one considers that an estimated $10 billion is spent annually in the United States on the evaluation of chest pain in EDs, reduced reliance on cardiac imaging represents significant potential cost savings. Our data show that patients receiving clinical evaluation alone had significantly lower costs compared with the noninvasive testing group, with a median difference of approximately $500 for the ED course and approximately $300 for the entire study period. This is similar in scale to findings from a cost analysis of the HEART trial, which showed a median cost reduction of $253 among low-risk patients, mainly driven by reduced cardiac diagnostic testing.
The absence of benefit from noninvasive testing is coupled with an increase in potential harm from radiation exposure. While the initial ROMICAT-II trial endorsed the use of CCTA for routine use in the ED, it is still a high-radiation procedure, with an estimated dose of 12 mSv in the PROTECTION I study. New protocols and modifications, such as electrocardiographically controlled tube current modulation (ECTCM), can significantly reduce the radiation dose in some patients. However, even with ECTCM, the lifetime cancer risks from CCTA in a 60-year-old woman and 60-year-old man are estimated at 1 in 715 and 1 in 1911, respectively. Because the patients in the ROMICAT-II population were on average 6 years younger than this, their lifetime cancer risk would be even higher. The routine use of CCTA, as has been advocated by some, in the 10 million patients who present to the ED with chest pain would be expected to result in thousands of new cases of cancer. The risk of cancer, in addition to increased health care costs, demands that the benefits of noninvasive testing associated with radiation be demonstrated in randomized clinical trials.
Limitations
Our study has several limitations. First, the 2 groups analyzed were not randomized. Although their baseline characteristics showed no significant differences, unmeasured differences between the groups may exist. We attempted to minimize the potential bias in our results by performing the adjusted IPTW analysis, which demonstrated similar overall outcomes. Second, the relatively short follow-up of 28 days does not allow for the assessment of outcomes over a longer time. Finally, the results apply only to patients evaluated during weekday, daytime hours and may not apply to patients who present at other times. If anything, we expect that if patients had been enrolled at other times, those receiving noninvasive testing would have had even longer delays waiting for tests to be performed and interpreted, which would have magnified the difference in length of stay between the groups.
Conclusions
In low- to intermediate-risk patients presenting to the ED with acute chest pain, noninvasive cardiac testing in addition to clinical evaluation leads to longer stay, more downstream testing, more radiation exposure, and greater cost without evidence of improving clinical outcomes.
Trial Protocol
References
- 1.Anderson JL, Adams CD, Antman EM, et al. ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction); American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine . ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1-e157. [DOI] [PubMed] [Google Scholar]
- 2.Owens PL, Barrett ML, Gibson TB, Andrews RM, Weinick RM, Mutter RL. Emergency department care in the United States: a profile of national data sources. Ann Emerg Med. 2010;56(2):150-165. [DOI] [PubMed] [Google Scholar]
- 3.Pope JH, Aufderheide TP, Ruthazer R, et al. . Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170. [DOI] [PubMed] [Google Scholar]
- 4.Katz DA, Williams GC, Brown RL, et al. . Emergency physicians’ fear of malpractice in evaluating patients with possible acute cardiac ischemia. Ann Emerg Med. 2005;46(6):525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline JA, Jones AE, Shapiro NI, et al. . Multicenter, randomized trial of quantitative pretest probability to reduce unnecessary medical radiation exposure in emergency department patients with chest pain and dyspnea. Circ Cardiovasc Imaging. 2014;7(1):66-73. [DOI] [PubMed] [Google Scholar]
- 6.Studdert DM, Mello MM, Sage WM, et al. . Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Dowe D, Jollis JG, et al. . Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724-1732. [DOI] [PubMed] [Google Scholar]
- 8.Miller JM, Rochitte CE, Dewey M, et al. . Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324-2336. [DOI] [PubMed] [Google Scholar]
- 9.Meijboom WB, Meijs MF, Schuijf JD, et al. . Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135-2144. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann U, Truong QA, Schoenfeld DA, et al. ; ROMICAT-II Investigators . Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann U, Bamberg F, Chae CU, et al. . Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53(18):1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein JA, Chinnaiyan KM, Abidov A, et al. ; CT-STAT Investigators . The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann U, Truong QA, Fleg JL, et al. ; ROMICAT II . Design of the Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography: a multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. Am Heart J. 2012;163(3):330-338, 338.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber TC, Carr JJ, Arai AE, et al. . Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119(7):1056-1065. [DOI] [PubMed] [Google Scholar]
- 15.Robins JM. Marginal structural models. 1997 Proceedings of the American Statistical Association, Section on Bayesian Statistical Science. Alexandria, VA: ASA; 1998:1-10. [Google Scholar]
- 16.Robins JM. Marginal structural models versus structural nested models as tools for causal inference In: Halloran ME, Berry D, eds. Statistical Models in Epidemiology, the Environment, and Clinical Trials. New York, NY: Springer-Verlag; 1999:95-134. [Google Scholar]
- 17.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550-560. [DOI] [PubMed] [Google Scholar]
- 18.Lumley T, Scott AJ Two-sample rank tests under complex sampling. Technical Report 2012-4. Auckland, New Zealand: Department of Statistics, University of Auckland; 2012.
- 19.Roffi M, Patrono C, Collet JP, et al. ; Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence Unstable Angina and NSTEMI: Early Management (CG94). London, England: NICE; 2010. [PubMed] [Google Scholar]
- 21.Foy AJ, Liu G, Davidson WR Jr, Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015;175(3):428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad V, Cheung M, Cifu A. Chest pain in the emergency department: the case against our current practice of routine noninvasive testing. Arch Intern Med. 2012;172(19):1506-1509. [DOI] [PubMed] [Google Scholar]
- 23.Chapman AR, Anand A, Boeddinghaus J, et al. . Comparison of the efficacy and safety of early rule-out pathways for acute myocardial infarction. Circulation. 2017;135(17):1586-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeddinghaus J, Nestelberger T, Twerenbold R, et al. . Direct comparison of 4 very early rule-out strategies for acute myocardial infarction using high-sensitivity cardiac troponin i. Circulation. 2017;135(17):1597-1611. [DOI] [PubMed] [Google Scholar]
- 25.Morrow DA. Clinician’s guide to early rule-out strategies with high-sensitivity cardiac troponin. Circulation. 2017;135(17):1612-1616. [DOI] [PubMed] [Google Scholar]
- 26.Mahler SA, Riley RF, Hiestand BC, et al. . The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med. 2012;367(4):375-376. [DOI] [PubMed] [Google Scholar]
- 28.Riley RF, Miller CD, Russell GB, et al. . Cost analysis of the History, ECG, Age, Risk factors, and initial Troponin (HEART) Pathway randomized control trial. Am J Emerg Med. 2017;35(1):77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausleiter J, Meyer T, Hermann F, et al. . Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507. [DOI] [PubMed] [Google Scholar]
- 30.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317-323. [DOI] [PubMed] [Google Scholar]
- 31.Woodard PK, McWilliams SR, Raptis DA, et al. . R-SCAN: cardiac CT angiography for acute chest pain. J Am Coll Radiol. 2017;14(9):1212-1214. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AJ, Cerqueira M, Hodgson JM, et al. ; American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; American Heart Association; American Society of Echocardiography; American Society of Nuclear Cardiology; North American Society for Cardiovascular Imaging; Society for Cardiovascular Angiography and Interventions; Society for Cardiovascular Magnetic Resonance . ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56(22):1864-1894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol