Key Points
Question
Is the adjuvanted herpes zoster subunit vaccine likely to be cost-effective?
Findings
In this modeling study based on randomized clinical trial data, at the proposed price of $280 per 2-dose course, the adjuvanted herpes zoster subunit vaccine was more effective and less expensive than the live attenuated herpes zoster vaccine at all ages and had an incremental cost-effectiveness ratio from $20 038 to $30 084 per quality-adjusted life-year compared with no vaccination. The incremental cost-effectiveness ratio was sensitive to the vaccine price and certain combinations of low adherence rate with a second dose and low efficacy of a single dose of the adjuvanted herpes zoster subunit vaccine.
Meaning
At a price of $280 per series, the adjuvanted herpes zoster subunit vaccine would cost less than the live attenuated herpes zoster vaccine and has a high probability of offering good value.
Abstract
Importance
The live attenuated herpes zoster vaccine (ZVL) is recommended for immunocompetent adults 60 years or older, but the efficacy wanes with age and over time. A new adjuvanted herpes zoster subunit vaccine (HZ/su) has higher efficacy but might be more expensive. The choice of vaccines depends on their relative values.
Objective
To assess the cost-effectiveness of HZ/su.
Design, Setting, and Participants
Markov decision model with transition probabilities based on the US medical literature. Participants were immunocompetent adults 60 years or older. Data were derived from participant groups ranging in number from less than 100 to more than 30 000 depending on the variable assessed. The study dates were July 1 to 31, 2017.
Exposures
No vaccination, ZVL (single dose), and HZ/su (2-dose series) vaccine administered at different ages.
Main Outcomes and Measures
Total costs and quality-adjusted life-years (QALYs) were estimated.
Results
Based on randomized clinical trial data, at a price of $280 per series ($140 per dose), HZ/su was more effective and less expensive than ZVL at all ages. The incremental cost-effectiveness ratios compared with no vaccination ranged from $20 038 to $30 084 per QALY, depending on vaccination age. The finding was insensitive to variations in most model inputs other than the vaccine price and certain combinations of low adherence rate with a second dose and low efficacy of a single dose of HZ/su. At the current ZVL price ($213 per dose), HZ/su had lower overall costs than ZVL up to a price of $350 per 2-dose series. In probabilistic sensitivity analysis, HZ/su had 73% probability of being cost-effective for 60-year-olds at $50 000 per QALY.
Conclusions and Relevance
Under conservative assumptions, at a price of $280 per series ($140 per dose), HZ/su would cost less than ZVL and has a high probability of offering good value.
This modeling study based on randomized clinical trial data assesses the cost-effectiveness of the adjuvanted herpes zoster subunit vaccine.
Introduction
In 2006, the US Food and Drug Administration approved the live attenuated herpes zoster vaccine (ZVL) for prevention of postherpetic neuralgia (PHN). In randomized controlled trials (RCTs), ZVL reduced the incidence of PHN among people 60 years or older, and the Advisory Committee on Immunization Practices (ACIP) recommended vaccination in this age group. However, ZVL does not prevent all herpes zoster (HZ), particularly among the elderly. Moreover, the efficacy wanes completely after approximately 10 years.
To address these shortcomings, an adjuvanted HZ subunit vaccine (HZ/su) was recently developed. In RCTs, HZ/su reduced HZ incidence by 97% among people 50 years or older and was highly effective even after age 70 years. The HZ/su is expected to be approved by the Food and Drug Administration soon, but widespread acceptance and coverage by insurance plans will require a recommendation from the ACIP, which must decide whether to prefer HZ/su over ZVL. One important issue will be cost-effectiveness.
In June 2017, the ACIP met to examine the cost-effectiveness of the 2 vaccines. They considered one model from GlaxoSmithKline (GSK), which manufactures HZ/su, and another model developed by Merck, which manufactures ZVL. Both found HZ/su to be cost saving relative to ZVL, and the incremental cost-effectiveness ratios (ICERs) of HZ/su compared with no vaccination ranged from $12 000 per quality-adjusted life-year (QALY) for the GSK model to $74 000 per QALY for the Merck model. The range was influenced by assumptions of the modelers, who were employed by the competing manufacturers.
Once HZ/su is approved, physicians and patients will have to choose between the vaccines. The HZ/su appears to be more effective, especially among elderly patients, and is likely to be more expensive. The HZ/su also requires 2 doses, a potential barrier to vaccination, which would affect the cost and the efficacy. To help decision makers, including the ACIP, payers, physicians, and patients, to choose the vaccine that offers the best value, we compared the cost-effectiveness of HZ/su with that of ZVL using an independent model with no pharmaceutical funding.
Methods
Study Design
We modified a validated Markov decision model (eFigure 1 in the Supplement) to incorporate HZ/su and compare the cost-effectiveness of the following 3 strategies: (1) no vaccination, (2) vaccination with ZVL, and (3) vaccination with HZ/su. The ZVL strategy included 1 dose, and the HZ/su strategy included 2 doses administered 2 months apart. We did not consider booster strategies because the ACIP does not currently recommend them. Because the cost-effectiveness of ZVL varies by vaccination age, we conducted analyses separately for 60-year-olds, 70-year-olds, and 80-year-olds. After vaccination, patients are followed up in 1-year Markov cycles. Each year, they may experience HZ and attendant complications (PHN, monocular blindness, monaural deafness, and hospitalization), recovery, or death, before ending in a mutually exclusive health state for the next cycle. The model terminates once everyone dies or reaches age 120 years.
This study was modeled using data extracted from the literature, with no participant data involved. Therefore, institutional review board approval was not needed.
Compared with unvaccinated patients, vaccinated counterparts experience lower disease incidence and complication rates in proportion to each vaccine’s efficacy. The model inputs were drawn from the US medical literature. The search was updated from a previous study. We searched PubMed through January 2015, with search terms such as incidence, herpes zoster complications, prevalence of post-herpetic neuralgia, utility, and cost of herpes zoster relevant to each model input. The study dates were July 1 to 31, 2017. Outputs included cost and QALYs. The ICER was calculated as incremental costs divided by incremental QALYs between adjacent strategies after removing dominated strategies. Because there is no standard willingness-to-pay (WTP) threshold for cost-effectiveness in the United States, we chose $50 000 per QALY as the decision threshold and explored thresholds up to $100 000 per QALY.
We conducted the study from the societal perspective, including direct medical costs and productivity losses. Costs were adjusted for inflation using the Consumer Price Index for Medical Care and expressed in 2016 US dollars. The costs and QALYs were discounted at 3% per year. Analyses were performed using a computer program (TreeAge Pro 2017; TreeAge Software).
Model Inputs and Assumptions
Base-case point estimates and ranges for sensitivity analyses were recorded. These are listed in Table 1. Data were derived from studies of fewer than 100 patients to more than 30 000 patients depending on the variable assessed.
Table 1. Model Inputs for Epidemiology, Vaccine Efficacy and Adverse Effects, and Utility and Costsa.
| Variable | Baseline Value | Sensitivity Analysis | Source | |
|---|---|---|---|---|
| One-Way Range | PSA Distribution | |||
| Epidemiology Model Inputs | ||||
| HZ incidence per 1000 person-years | Insinga et al, 2005; Leung et al, 2011 | |||
| Male age, y | ||||
| 60-69 | 8.9 | 8.4-9.3 | Beta | |
| 70-79 | 11.3 | 10.7-11.9 | b | |
| ≥80 | 12.2 | 11.2-13.3 | b | |
| Female age, y | ||||
| 60-69 | 12.5 | 12.0-12.9 | Beta | |
| 70-79 | 15.1 | 14.4-15.7 | b | |
| ≥80 | 16.5 | 15.6-17.6 | b | |
| Complications, % | ||||
| PHN given HZ | Oxman et al, 2005 | |||
| Age 60-69 y | 0.069 | 0.042-0.096 | Beta | |
| Age ≥70 to 79 y | 0.185 | 0.142-0.228 | b | |
| PHN from 6-12 mo | 0.215 | 0.188-0.247 | Beta | Bouhassira et al, 2012 |
| PHN ≥12 mo | Helgason et al,2000 | |||
| Age <70 y | 0.31 | 0.06-0.56 | Beta | |
| Age ≥70 y | 0.52 | 0.34-0.70 | b | |
| Any ophthalmic complications | 0.022 | 0.012-0.032 | Beta | Rothberg et al, 2007 |
| Monocular blindness given ophthalmic complications | 0.039 | 0.011-0.067 | Beta | Rothberg et al, 2007 |
| HZ oticus | 0.002 | 0.000-0.005 | Beta | Rothberg et al, 2007 |
| Monaural deafness given HZ oticus | 0.069 | 0.013-0.120 | Beta | Rothberg et al, 2007 |
| Hospitalization given HZ | Jackson et al, 2008 | |||
| Age 60-69 y | 0.013 | 0.005-0.021 | Beta | |
| Age 70-79 y | 0.018 | 0.011-0.026 | b | |
| Age ≥80 y | 0.055 | 0.042-0.068 | b | |
| Death due to HZ, per 1 million cases | National Center for Health Statistics, 1999-2015 | |||
| Age 60-69 y | 2.22 | 1.72-2.72 | Beta | |
| Age 70-79 y | 6.18 | 5.32-7.03 | b | |
| Age 80-89 y | 23.96 | 21.88-26.03 | b | |
| Age ≥90 y | 152.13 | 143.76-160.50 | b | |
| Duration of hospitalization, mean, d | 4.8 | 4.6-5.4 | Normal | Agency for Healthcare Research and Quality, 2017 |
| Vaccine Efficacy and Adverse Event Inputs | ||||
| Live attenuated HZ vaccine | ||||
| Efficacy function for HZ incidence | Morrison et al, 2015; Oxman et al, 2005; Schmader et al, 2012 | |||
| Intercept | 0.648 | 0.568-0.727 | Normal | |
| Slope, annual waning rate | 0.054 | 0.037-0.072 | Normal | |
| Likelihood ratio by age, y | Rohan, 2005 | |||
| 60-64 | 1.797 | NA | NA | |
| 65-69 | 1.582 | NA | NA | |
| 70-74 | 0.742 | NA | NA | |
| 75-79 | 0.541 | NA | NA | |
| 80-84 | 0.232 | NA | NA | |
| ≥85 | 0.080 | NA | NA | |
| Efficacy function for BOI | Morrison et al, 2015; Oxman et al, 2005; Schmader et al, 2012 | |||
| Intercept | 0.708 | 0.576-0.840 | Normal | |
| Slope, annual waning rate | 0.044 | 0.019-0.068 | Normal | |
| Efficacy function for PHN incidence | Morrison et al, 2015; Oxman et al, 2005; Schmader et al, 2012 | |||
| Efficacy for the first 5 y | 0.632 | 0.422-0.765 | Normal | |
| Slope from year 6, annual waning rate | 0.1 | NA | NA | |
| Adverse effect | Oxman et al, 2005 | |||
| Local reaction | 0.34 | 0.33-0.36 | Beta | |
| Grade 3 reaction | 0.003 | 0.000-0.006 | Beta | |
| Serious reaction | 0.007 | 0.001-0.013 | Beta | |
| Adjuvanted subunit HZ vaccine | ||||
| Efficacy function for 2 doses | ||||
| Intercept | Normal | Lal et al, 2015; Cunningham et al, 2016 | ||
| Age 60-69 y | 1.049 | 0.965-1.054 | ||
| Age ≥70 y | 1.008 | 0.963-1.04 | ||
| Slope, annual waning rate | 0.054 | 0.037-0.072 | Normal | Assumption |
| Adverse effect for 2 doses | Lal et al, 2015 | |||
| Local reaction | 0.791 | 0.778-0.802 | Beta | |
| Grade 3 reaction | 0.085 | 0.077-0.094 | Beta | |
| Serious reaction | 0.001 | 0.000-0.009 | Beta | |
| Efficacy function for 1 dose | ||||
| Intercept | Beta | Leidner, 2017 | ||
| Age 60-69 y | 0.927 | 0.648-1.017 | ||
| Age ≥70 y | 0.722 | 0.276-0.918 | ||
| Slope, annual waning rate | 0.109 | 0.054-0.163 | Normal | Assumption |
| Adverse effect for 1 dose | ||||
| Local reaction | 0.395 | 0.389-0.401 | Beta | Assumption |
| Grade 3 reaction | 0.059 | 0.052-0.066 | Beta | Lal et al, 2015 |
| Serious reaction | 0.001 | 0.000-0.005 | Beta | Assumption |
| Adherence to 2 doses | 0.562 | 0.436-0.630 | Beta | Nelson et al, 2009 |
| Utility and Cost Inputs | ||||
| Monocular blindness | 0.920 | 0.885-0.948 | Beta | Rothberg et al, 2007 |
| Monaural deafness | 0.970 | 0.958-0.982 | Beta | Rothberg et al, 2007 |
| Postherpetic neuralgia after 6 mo | 0.670 | 0.618-0.722 | Beta | Edmunds et al, 2001; Hornberger and Robertus, 2006 |
| Local reaction | 0.730 | 0.680-0.780 | Beta | Edmunds et al, 2001 |
| Grade 3 reaction | 0.470 | 0.410-0.530 | Beta | Edmunds et al, 2001 |
| Short-term morbidities, QALYs | ||||
| Acute HZ | Oxman et al, 2005; Coplan et al, 2004 | |||
| Age 60-69 y | 0.013 | 0.005-0.021 | Gamma | |
| Age ≥70 y | 0.022 | 0.014-0.029 | b | |
| Hospitalization | 0.013 | 0.013-0.015 | Gamma | Length of stay |
| Serious reaction | 0.008 | 0.003-0.016 | Gamma | Agency for Healthcare Research and Quality, 2017 |
| Direct medical costs, $ per case | ||||
| Acute HZ | 412 | 317-605 | Gamma | Rothberg et al, 2007 |
| PHN | 812 | 693-966 | Gamma | Rothberg et al, 2007 |
| Ophthalmic complications | 16 147 | 11 843-19 016 | Gamma | Rothberg et al, 2007 |
| HZ oticus | 512 | 143-1004 | Gamma | Rothberg et al, 2007 |
| Hospitalization for HZ | 8656 | 8184-8793 | Gamma | Agency for Healthcare Research and Quality, |
| Serious reaction | 6710 | 5010-8220 | Gamma | Agency for Healthcare Research and Quality, 2017 |
| Indirect cost, $ per HZ case | Bureau of Labor Statistics, 2017; Drolet et al, 2012 | |||
| Age 60-64 y | 4915 | 3544-6285 | Gamma | |
| Age ≥65 y | 4632 | 3340-5924 | b | |
| Vaccine price, $ per dosec | ||||
| Live attenuated herpes zoster vaccine | 212.67 | 100-300 | NA | Centers for Disease Control and Prevention, 2017 |
| Adjuvanted herpes zoster subunit vaccine | 140 | 100-250 | NA | Leidner, 2017 |
| Time lost due to vaccination with second dose, hd | 2 | 0-4 | Normal | Assumption |
| Travel cost of vaccination with second dose, $ | 5 | 0-10 | Gamma | Assumption |
| Vaccine administration costs, $ | 26 | 15-35 | NA | Centers for Medicare & Medicaid Services, not dated |
Abbreviations: BOI, burden of illness; HZ, herpes zoster; NA, not applicable; PHN, postherpetic neuralgia; PSA, probabilistic sensitivity analysis; QALYs, quality-adjusted life-years.
Costs are given in 2016 US dollars.
For the model inputs with age-specific values, the distribution was first defined for the lowest age group, which was considered as the reference. Distributions for remaining age groups were determined by multiplying relative likelihood ratios among these ages and the reference age by the reference distribution. Because the value was drawn randomly from the distribution in PSA, this definition of distributions ensured that the probabilistic values of different age groups had the appropriate relative magnitudes compared with one another as when they were deterministic.
Although the vaccine price is variable, it is determined by the manufacturer and not uncertain. Therefore, we did not define a probabilistic distribution for the vaccine price. However, we ran a number of PSAs at various HZ/su prices to show the variation of the probability of being cost-effective of each strategy by the vaccine price.
This time loss was multiplied by the age-specific wage rate and the percentage of labor participation, resulting in an actual productivity loss due to vaccination with the second dose of $30, $9, and $4 per dose for people aged 60, 70, and 80 years, respectively.
Epidemiologic Parameters
Our estimate of HZ incidence has been described previously. Patients with previous HZ episodes had the same risk for subsequent episodes of HZ as those without previous HZ episodes. Age-specific complication rates and background mortality were drawn from our previous model.
Vaccine-Related Parameters
The efficacy of ZVL was modeled from the Shingles Prevention Study, the Short-Term Persistence Substudy, and the Long-Term Persistence Substudy. We used different functions to capture the long-term efficacy of ZVL against HZ incidence, PHN incidence, and burden of illness (BOI) using the following respective equations: y = 0.6478 − (0.0544 × year), y = 1.218 − (0.1 × year), and y = 0.7083 − (0.0437 × year). We developed an equation to interrelate the efficacy against HZ incidence and the additional efficacy against PHN and BOI. The efficacy was further adjusted for vaccination age.
The efficacy of HZ/su was reported in 2 RCTs. Apart from the efficacy against HZ incidence, HZ/su had no additional efficacy against PHN incidence or BOI. Therefore, we used a single efficacy function. The efficacy was higher at age 60 to 69 years compared with 70 years or older. The efficacy declined over 4 years, with a slope of −3.6% per year, which did not differ significantly from the efficacy decline for ZVL of −5.4% per year; moreover, the wide 95% CI for HZ/su (−10.7% to 3.4%) completely encompassed the 95% CI for ZVL (−7.2% to −3.7%). Therefore, we assumed that initial efficacy differed by vaccination age and would decline at a rate identical to that of ZVL. The efficacy functions for HZ/su were y = 1.049 − (0.0544 × year) (age 60-69 years) and y = 1.008 − (0.0544 × year) (age ≥70 years). Therefore, the duration of HZ/su efficacy (the period that the efficacy exceeded 0%) was 19.3 years for vaccination at age 60 to 69 years and 18.5 years for vaccination at 70 years or older. Different durations were examined in sensitivity analyses.
The HZ/su requires 2 doses. In practice, some people will receive only 1 dose. Absent published data, we based single-dose efficacy on manufacturer’s information presented at the ACIP meeting. Because there are no data, to date, on the waning rate of single-dose HZ/su, we assumed that the efficacy waned twice as fast for single-dose HZ/su as for 2 doses. All assumptions were evaluated in sensitivity analyses. The eAppendix in the Supplement describes the derivation of all efficacy functions.
Although 95% of RCT participants received both doses of HZ/su, the proportion of persons who complete the series in practice will likely be smaller. Based on the adult hepatitis A vaccine series, which also contains 2 injections, we assumed 56.2% adherence.
Quality-Adjusted Life-years
The utility estimates have been described previously. Local and grade 3 reactions were estimated to last one day, with utility equal to mild and severe pain, respectively. Utility of PHN beyond 6 months was calculated, assuming that 77% of patients have mild pain and 23% of patients have severe pain. Serious reactions were estimated to require 3 days in the hospital (the mean length of stay for a drug allergy), with a utility of zero. Because the frequencies of vaccine reactions after the first and second doses were the same, we assumed the probabilities of vaccine reactions for 1 dose to be half of 2 doses. We adjusted all utilities for age.
Costs
Cost of ZVL was based on the private sector price of the Centers for Disease Control and Prevention. Because HZ/su is not yet licensed, we assumed a base-case price of $280 for a 2-dose regimen and varied it in sensitivity analysis. This assumption came from the GSK model as presented at the ACIP meeting. Administration cost was set at Medicare’s national reimbursement rate. We assumed that the first dose of either vaccine was administered during a wellness visit and incurred no travel or productivity costs, but the second HZ/su dose included these costs. We estimated travel cost equal to a round-trip bus ticket in Cleveland, Ohio, and productivity cost equal to 2 hours of time loss. Productivity loss due to HZ was based on a US study, adjusted for age-specific wage rate and workforce participation. Local reactions were assumed not to incur costs, whereas grade 3 reactions resulted in 1 lost workday. Cost of serious reactions was assumed to equal that of other allergic reactions requiring hospitalization. Other costs were drawn from a previous study, inflated to 2016 US dollars. We estimated length of stay and hospitalization costs from the 2014 Healthcare Cost and Utilization Project.
Sensitivity Analysis
Deterministic Sensitivity Analysis
We conducted 1-way sensitivity analyses to examine the association of input variables with cost-effectiveness. In addition, we explored values outside the ranges in Table 1 for prices of HZ/su, the waning rate and initial efficacy of a single HZ/su dose, and the adherence rate because these had the least data to support our estimates. We also tried excluding productivity loss. In 2-way sensitivity analysis, we examined the joint effect of price, the adherence rate of 2 HZ/su doses, initial efficacy, and the waning rate of 1 dose and 2 doses of HZ/su and ZVL. Finally, we conducted a 3-way analysis in which we varied the adherence rate, the waning rate, and the efficacy of 1 dose of HZ/su at the same time.
Probabilistic Sensitivity Analysis
We performed 10 000 iterations of Monte Carlo simulation to assess the effect of varying all model inputs simultaneously. Findings were presented as the cost-effectiveness acceptability curve, showing the probability of each strategy being cost-effective over a range of WTP values, assuming an HZ/su price of $280 per series. We also performed probabilistic sensitivity analysis for 10 different HZ/su prices within the range of $150 to $600 per series ($75-$300 per dose) and plotted the percentage of iterations that each strategy had an ICER not exceeding $50 000 per QALY as a function of HZ/su price.
Results
Base-Case Analysis
Table 2 lists the costs and QALYs gained for each strategy. At all ages, no vaccination was always the least expensive and least effective, while HZ/su was always the most effective and less expensive than ZVL (ie, ZVL was dominated). The HZ/su was highly cost-effective compared with no vaccination, with an ICER below $50 000 per QALY at all ages.
Table 2. Costs and Effectiveness of 3 Strategies by Vaccination Age in Base Casea.
| Strategy | Cost, $ | Incremental Cost, $ | QALYs | Incremental QALYs | ICER, $ per QALYb |
|---|---|---|---|---|---|
| Vaccination Age of 60 y | |||||
| No vaccination | 549 | NA | 12.8630 | NA | NA |
| HZ/su | 642 | 93 | 12.8661 | 0.0031 | 30 084 |
| ZVL | 696 | NA | 12.8652 | NA | Dominated |
| Vaccination Age of 70 y | |||||
| No vaccination | 388 | NA | 9.2799 | NA | NA |
| HZ/su | 520 | 131 | 9.2865 | 0.0066 | 20 038 |
| ZVL | 600 | NA | 9.2862 | NA | Dominated |
| Vaccination Age of 80 y | |||||
| No vaccination | 263 | NA | 5.8720 | NA | NA |
| HZ/su | 403 | 139 | 5.8784 | 0.0064 | 21 726 |
| ZVL | 509 | NA | 5.8781 | NA | Dominated |
Abbreviations: HZ/su, adjuvanted herpes zoster subunit vaccine; ICER, incremental cost-effectiveness ratio; NA, not applicable; QALY, quality-adjusted life-year; ZVL, live attenuated herpes zoster vaccine.
Costs are given in 2016 US dollars.
A dominated strategy means that it has a higher cost but lower QALYs than the other.
Sensitivity Analysis
One-Way Sensitivity Analysis
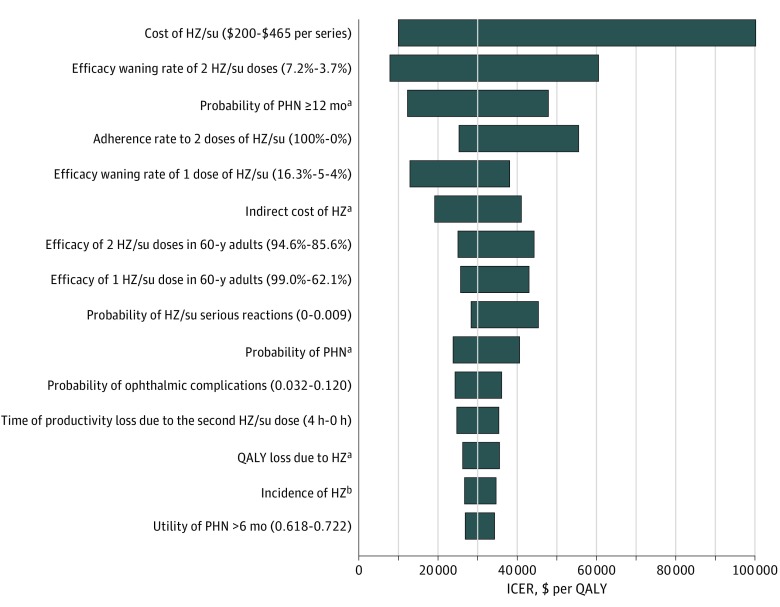
Figure 1 shows the model inputs that caused the ICER of HZ/su compared with no vaccination to change by at least 10% for people vaccinated at age 60 years. The price and efficacy waning rate of 2 doses of HZ/su and the probability of PHN 12 months or longer were the 3 most important factors. Compared with no vaccination, HZ/su would be cost saving up to a price of $160 ($80 per dose).
Figure 1. Tornado Diagram of the Incremental Cost-effectiveness Ratio (ICER) of the Adjuvanted Herpes Zoster Subunit Vaccine (HZ/su) at Vaccination Age of 60 Years.
Ranges are in parentheses, with the left values leading to the leftmost ICERs. BOI indicates burden of illness; HZ, herpes zoster; PHN, postherpetic neuralgia; QALY, quality-adjusted life-year; and ZVL, live attenuated herpes zoster vaccine. ICER values are given in 2016 US dollars.
aRanges for these age-specific parameters are listed in Table 1, with the highest values leading to the leftmost ICERs.
bIncidences in both men and women were varied at the same time with ranges listed in Table 1.
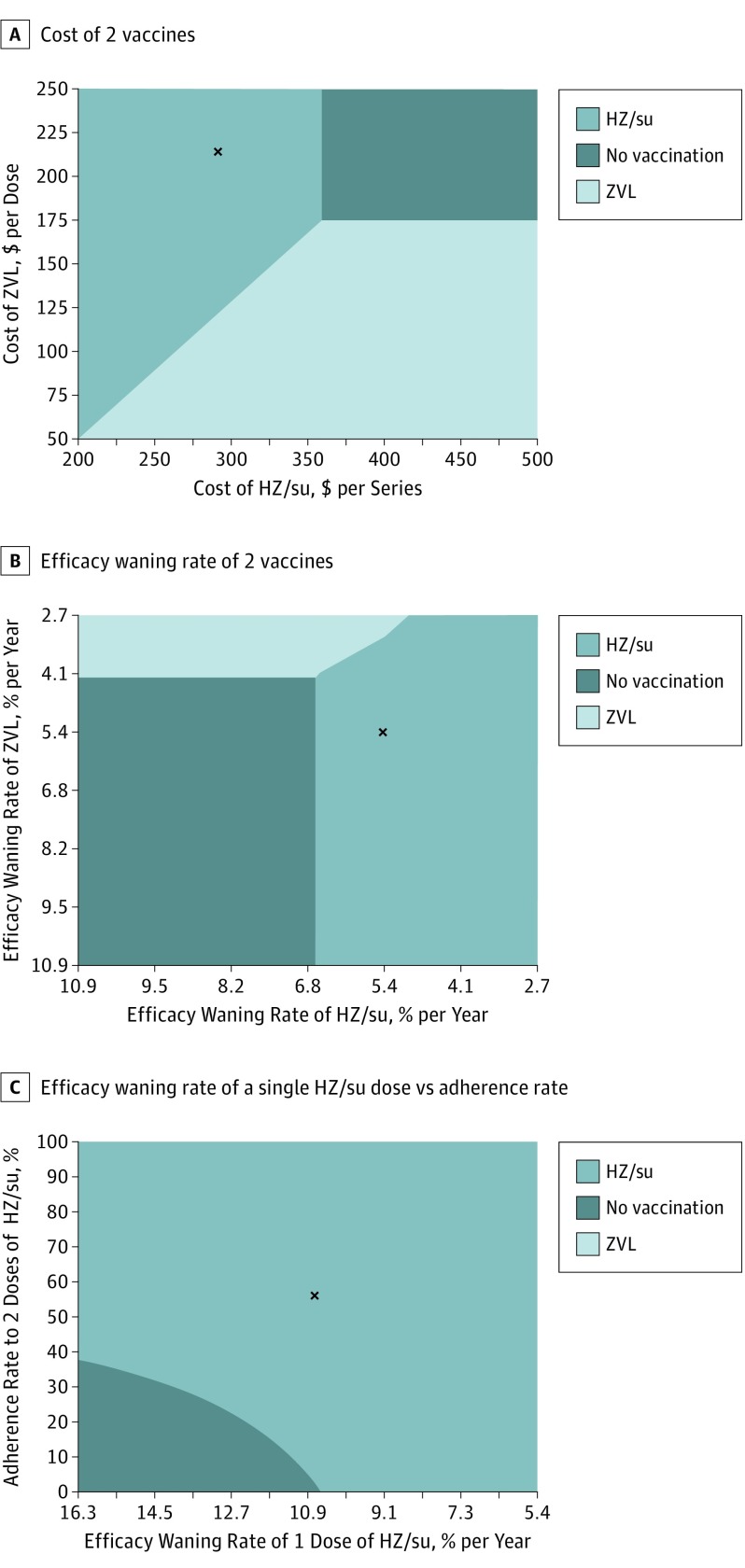
Two-Way Sensitivity Analysis
Under all circumstances, HZ/su was always more effective than ZVL. The cost-effectiveness of HZ/su was sensitive to the price of HZ/su but insensitive to the price of ZVL. At the current price of ZVL ($213 per dose), HZ/su would be less costly than ZVL up to a price of $350 per series and cost-effective (compared with no vaccination) up to a price of $359 per series, assuming a WTP threshold of $50 000 per QALY (Figure 2A). Conversely, at the proposed price of $280 per series, the price of ZVL would have to fall below $113 or below $68 to make HZ/su not cost-effective at $50 000 per QALY and $100 000 per QALY, respectively. Alternatively, if GSK increased the price of HZ/su by 50%, ZVL would become cost-effective if it cost less than $175 per dose.
Figure 2. Two-Way Sensitivity Analysis at Vaccination Age of 60 Years With a Willingness-to-Pay Threshold of $50 000 per Quality-Adjusted Life-year.
A-C, Letter x denotes base case. HZ/su indicates adjuvanted herpes zoster subunit vaccine; ZVL, live attenuated herpes zoster vaccine. Costs are given in 2016 US dollars.
The cost-effectiveness of HZ/su was insensitive to the waning rate of either vaccine (Figure 2B). Conversely, results were more sensitive to the joint variations of the adherence and the waning rate. At base-case adherence (56.2%), HZ/su would be cost-effective regardless of the waning rate (Figure 2C) or initial efficacy of a single dose (eFigure 2 in the Supplement). However, when the adherence was less than 37.6%, HZ/su would not be cost-effective if the waning rate was greater than 10.9% per year or initial efficacy was less than 72.8%.
Three-Way Sensitivity Analysis
If the adherence to the second dose of HZ/su was greater than 56.8%, results were insensitive to the combined variation of the efficacy and the waning rate of single-dose HZ/su (eFigure 2 in the Supplement). However, at adherence rates below 40%, most combinations of faster waning rate and lower initial efficacy made HZ/su not cost-effective at either threshold. Vaccination with ZVL was never cost-effective at age 60 years.
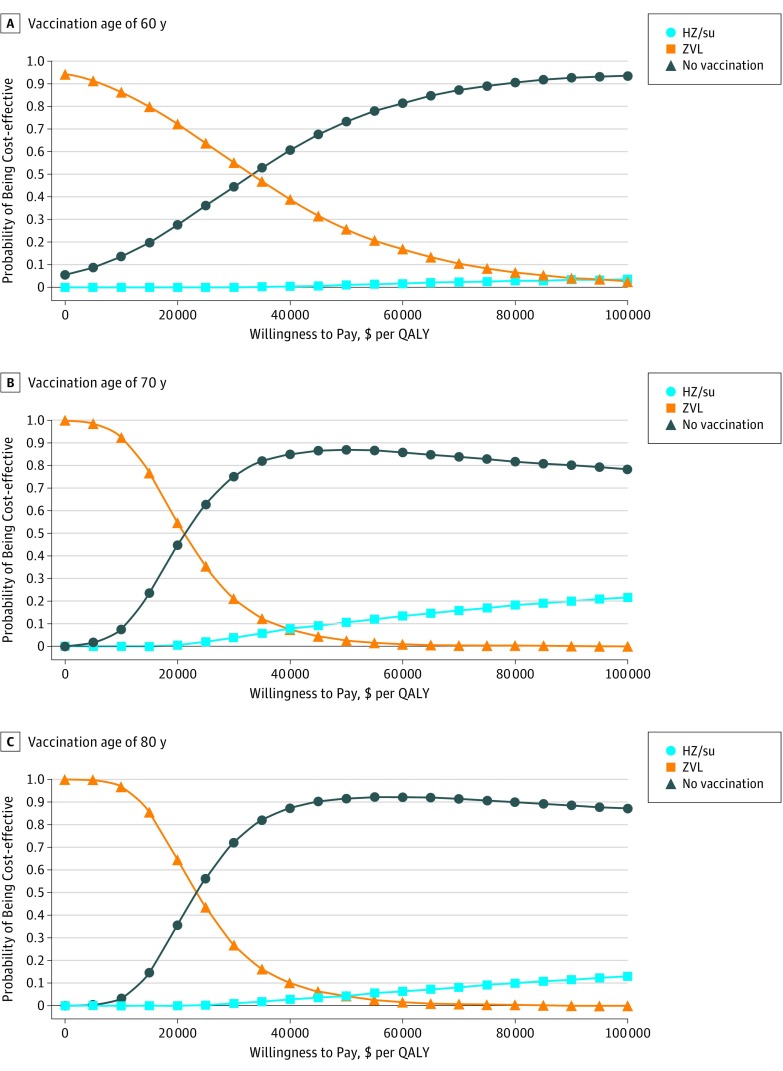
Probabilistic Sensitivity Analysis
Figure 3 shows the probability of each strategy being cost-effective at various WTP thresholds. At a price of $280 per series, HZ/su had between 73% and 91% probability of being cost-effective at $50 000 per QALY and between 78% and 93% probability of being cost-effective at $100 000 per QALY, depending on vaccination age. In contrast, at age 60 years, ZVL had less than 5% chance of being cost-effective at either threshold. Additional sensitivity analyses are described in the eAppendix (including eFigures 3-8) in the Supplement.
Figure 3. Probability of Each Strategy Being Cost-effective at Different Willingness-to-Pay Thresholds.
A-C, Probabilistic sensitivity analysis. HZ/su indicates adjuvanted herpes zoster subunit vaccine; QALY, quality-adjusted life-year; and ZVL, live attenuated herpes zoster vaccine. Costs are given in 2016 US dollars.
Discussion
In this modeling study based on RCT data, we found that the new HZ/su was highly cost-effective compared with no vaccination for people 60 years or older. At a proposed price of $280 per series, HZ/su was both more effective and less expensive than the current ZVL at all ages. The finding was robust in sensitivity analysis. Across conceivable ranges of the efficacy duration, vaccine price, and probability of having PHN 12 months or longer, HZ/su was never more expensive than ZVL. Therefore, all discussion of the cost-effectiveness is limited to comparison of HZ/su with no vaccination. In the base case, HZ/su cost less than $50 000 per QALY at all ages. In one-way sensitivity analysis, HZ/su sometimes had an ICER greater than $50 000 per QALY but never greater than $100 000 per QALY, unless the price exceeded $465 per series. What made the vaccine so cost-effective was its high efficacy, which is initially at least 90% even among people 70 years or older, and this improvement could command a substantial premium over ZVL. Alternatively, if HZ/su cost less than $160 per series, it would actually save money compared with not vaccinating.
At the June 2017 meeting, most of the ACIP work group leaned toward stating a preference for HZ/su over ZVL, which could allow for monopoly pricing. To understand how this might affect price and ultimately cost-effectiveness, the case of ZVL is instructive. Original estimates of the cost-effectiveness of ZVL ranged from $27 000 to $112 000 per QALY, which the ACIP determined was at the high end of the acceptable range. After their recommendation, the price of ZVL increased at a rate faster than the Consumer Price Index for Medical Care, rising from $145.35 in 2007 to $212.67 in 2017. At the June 2017 ACIP meeting, both models presented found that the cost-effectiveness of ZVL now exceeds $120 000 per QALY, which is likely no longer in the acceptable range. If HZ/su was to rise at a similar rate, reductions in the price of ZVL could make it competitive. For that reason, it is important that the ACIP make its preferred recommendation conditional on maintaining the price, with a commitment to revisit the decision periodically.
In addition to maintaining competition, the ACIP working group identified several reasons not to express a preference for HZ/su. There was concern that the 2-dose regimen would create another barrier to vaccination. Our model is reassuring in this regard. Because a single dose appears to offer high levels of protection (90% for 60-year-olds), HZ/su was the cost-effective option even if only 5% of patients received 2 doses. One caveat is that the efficacy estimate for a single dose was based on unpublished data provided by the manufacturer. GlaxoSmithKline performed post hoc analyses without a priori sample size calculations. These included data from the 2-month window between doses and from the 5% of individuals who did not receive the second dose. This represents approximately 29 311 patients but with a mean follow-up of less than 90 days. The 95% CIs were wide, especially for patients 70 years or older. If the efficacy is lower than expected, the adherence will need to be high to maintain cost-effectiveness: for example, if the efficacy of the first dose in patients at age 60 years was only 62% (the lower end of the 95% CI), the adherence to the second dose would need to exceed 47% for HZ/su to be cost-effective at $50 000 per QALY. Future studies should examine the effectiveness and the waning rate of a single dose even as physicians should strongly encourage patients to complete the series. If HZ/su is preferred, there will be more grade 3 local reactions. Even so, the cost of HZ is so high that reactions had little effect on the cost-effectiveness of HZ/su. Last, relying on one manufacturer, the supply of Hz/su would be less assured. This is a legitimate concern; however, unlike the case with influenza, an interruption in vaccine supply to prevent HZ would not constitute a public health crisis.
If the ACIP expresses no preference for HZ/su over ZVL, there will be many cases of avoidable HZ and PHN among vaccinated patients who could have received a more effective vaccine. Moreover, physicians, patients, or insurers may be tempted to choose the less expensive option, not realizing how much less effective it is. In that case, independent analyses, such as this one, will provide important information for relevant stakeholders. Because our study showed that ZVL could be cost-effective compared with HZ/su if Merck lowered the price substantially, we think that patients and insurers would benefit from the competition between 2 vaccines. One-way analysis also demonstrated that most uncertainties around the efficacy duration, serious reactions, and adherence rate would not make HZ/su less cost-effective than ZVL. Future studies to better define these values would provide more precise ICER estimates but would be unlikely to change our conclusions.
Limitations
Our study has several limitations. First, the long-term efficacy of HZ/su is unknown but did not appear to be an important determinant in sensitivity analyses. Second, the efficacy and duration for a single dose of HZ/su are unknown, as is the adherence to a second dose. We based our efficacy estimates on data from the ACIP June 2017 meeting and made assumptions about vaccine duration. The adherence rate was extrapolated from that of hepatitis A vaccine, which is rarely administered in the elderly. Because sensitivity analysis showed that combinations of these could potentially be important determinants, well-designed studies should assess them. Third, we did not model the possibility of a ZVL booster, although it might be relevant given ZVL’s limited efficacy duration. Fourth, some cost estimates were based on studies from the 1990s, inflated to current US dollars.
Conclusions
At a projected price of $280 per series, HZ/su was both more effective and less expensive than ZVL for adults 60 years or older. These findings were insensitive to most model inputs, particularly those about which there is considerable uncertainty, so long as the adherence to the second dose exceeds 50%. We made reasonable assumptions about the vaccine’s efficacy duration and price, but our results would change if the vaccine price was to rise markedly in the future, if a single dose is much less effective than GSK reported, or if the adherence to the second dose is extremely low. Because of its superior efficacy, HZ/su was cost saving compared with ZVL and cost-effective compared with no vaccination in most scenarios and should be recommended by the ACIP. An ACIP recommendation stating a preference for HZ/su over ZVL could lead to future price increases, which would render the vaccine no longer cost-effective. Therefore, a recommendation linked to periodic reassessment of cost-effectiveness based on the vaccine price might help to mitigate the effect of the recommendation on vaccine affordability.
eFigure 1. Markov Model
eFigure 2. Three-Way Sensitivity Analysis at Vaccination Age of 60 Years With a Willingness-to-Pay Threshold of $50 000/QALY
eFigure 3. Two-Way Sensitivity Analysis of Adherence Rate and Cost of HZ/su
eFigure 4. Two-Way Sensitivity Analysis of Efficacy of a Single Dose and Cost of HZ/su
eFigure 5. Two-Way Sensitivity Analysis of Efficacy of Two Doses and Cost of HZ/su
eFigure 6. Two-Way Sensitivity Analysis of Adherence Rate and Efficacy of Two Doses of HZ/su
eFigure 7. Scatter Plot of Incremental Cost-effectiveness Ratios of the Adjuvanted Subunit Vaccine (HZ/su) (a) and Live Attenuated Vaccine (ZVL) (b) vs No Vaccination From Probabilistic Sensitivity Analysis in Person Vaccinated at 60 Years
eFigure 8. The Probability of Being Cost-effective at $50 000/QALY of Each Strategy vs HZ/su Prices in People Vaccinated at 60 Years
eAppendix. Supplemental Appendix
eTable 1. Vaccine Efficacy by Years Post Vaccination by Age at Vaccination
eTable 2. Costs and Effectiveness of the Three Strategies at Different Prices of the HZ/su for People Vaccinated at Age 60 Years
References
- 1.Morrison VA, Johnson GR, Schmader KE, et al. ; Shingles Prevention Study Group . Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oxman MN, Levin MJ, Johnson GR, et al. ; Shingles Prevention Study Group . A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. [DOI] [PubMed] [Google Scholar]
- 3.Schmader KE, Levin MJ, Gnann JW Jr, et al. . Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012;54(7):922-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmader KE, Oxman MN, Levin MJ, et al. ; Shingles Prevention Study Group . Persistence of the efficacy of zoster vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin Infect Dis. 2012;55(10):1320-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-2096. [DOI] [PubMed] [Google Scholar]
- 6.Leidner AJ. Overview of two economic models that assess the cost-effectiveness of herpes zoster vaccinations. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/zoster-04-leidner.pdf. Published June 21, 2017. Accessed July 14, 2017.
- 7.Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163(7):489-497. [DOI] [PubMed] [Google Scholar]
- 8.Le P, Rothberg MB. Determining the optimal vaccination schedule for herpes zoster: a cost-effectiveness analysis. J Gen Intern Med. 2017;32(2):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis. 2007;44(10):1280-1288. [DOI] [PubMed] [Google Scholar]
- 10.Bureau of Labor Statistics Consumer Price Index for All Urban Consumers: medical care. http://Data.bls.gov/cgi-bin/surveymost. Accessed November 20, 2014.
- 11.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20(8):748-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52(3):332-340. [DOI] [PubMed] [Google Scholar]
- 13.Jackson LA, Reynolds MA, Harpaz R. Hospitalizations to treat herpes zoster in older adults: causes and validated rates. Clin Infect Dis. 2008;47(6):754-759. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics, Centers for Disease Control and Prevention Compressed mortality file 1999-2012 on CDC WONDER online database, released October 2014. Data are from the compressed mortality file 1999-2012 Series 20 No. 2R, 2014, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. http://Wonder.cdc.gov/cmf-icd10.html. Accessed November 12, 2014.
- 15.Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145(5):317-325. [DOI] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). https://www.ahrq.gov/research/data/hcup/index.html. Page last reviewed November 2017. Accessed April 30, 2016.
- 17.Rohan P. FDA clinical briefing document for Merck & Co, Inc: zoster vaccine live (Oka/Merck): Zostavax. https://www.fda.gov/OHRMS/DOCKETS/ac/05/briefing/5-4198B2_1.pdf. Vaccines and Related Biological Products Advisory Committee meeting date December 15, 2005. Accessed October 30, 2014.
- 18.Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019-1032. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JC, Bittner RC, Bounds L, et al. . Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a Vaccine Safety Datalink study. Am J Public Health. 2009;99(suppl 2):S389-S397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine. 2001;19(23-24):3076-3090. [DOI] [PubMed] [Google Scholar]
- 21.Coplan PM, Schmader K, Nikas A, et al. . Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the Brief Pain Inventory. J Pain. 2004;5(6):344-356. [DOI] [PubMed] [Google Scholar]
- 22.Drolet M, Levin MJ, Schmader KE, et al. . Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine. 2012;30(12):2047-2050. [DOI] [PubMed] [Google Scholar]
- 23.Bureau of Labor Statistics Usual weekly earnings of wage and salary workers archived news releases: 2014. usual weekly earnings of wage and salary workers: fourth quarter. https://www.bls.gov/bls/news-release/wkyeng.htm. Accessed March 27, 2015.
- 24.Centers for Disease Control and Prevention Adult vaccine price list. http://Www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/. Prices last reviewed/updated September 1, 2017. Accessed September 12, 2014.
- 25.Centers for Medicare & Medicaid Services Physician fee schedule search tool. http://Www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=0&T=0&HT=0&CT=3&H1=90471&M=5. Accessed September 12, 2014.
- 26.Bouhassira D, Chassany O, Gaillat J, et al. . Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain. 2012;153(2):342-349. [DOI] [PubMed] [Google Scholar]
- 27.Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ. 2000;321(7264):794-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391-400. [DOI] [PubMed] [Google Scholar]
- 30.Prosser LA, O’Brien MA, Molinari NA, et al. . Non-traditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics. 2008;26(2):163-178. [DOI] [PubMed] [Google Scholar]
- 31.Whitney CG, Zhou F, Singleton J, Schuchat A; Centers for Disease Control and Prevention (CDC) . Benefits from immunization during the Vaccines for Children program era: United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63(16):352-355. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F, Bisgard KM, Yusuf HR, Deuson RR, Bath SK, Murphy TV. Impact of universal Haemophilus influenzae type b vaccination starting at 2 months of age in the United States: an economic analysis. Pediatrics. 2002;110(4):653-661. [DOI] [PubMed] [Google Scholar]
- 33.Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ. 2011;14(5):639-645. [DOI] [PubMed] [Google Scholar]
- 34.Bureau of Labor Statistics May 2016 national occupational employment and wage estimates: United States. http://Www.bls.gov/oes/current/oes_nat.htm. Last modified date March 31, 2017. Accessed July 10, 2017.
- 35.Bureau of Labor Statistics Labor force statistics from the Current Population Survey: employment status of the civilian noninstitutional population by age, sex, and race. http://www.bls.gov/web/empsit/cpseea13.htm. Updated 2015. Accessed February 11, 2015.
- 36.Dooling K. Considerations for the use of herpes zoster vaccines. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/zoster-05-dooling.pdf. Presented June 21, 2017. Accessed July 14, 2017.
- 37.Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) . Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1-30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Markov Model
eFigure 2. Three-Way Sensitivity Analysis at Vaccination Age of 60 Years With a Willingness-to-Pay Threshold of $50 000/QALY
eFigure 3. Two-Way Sensitivity Analysis of Adherence Rate and Cost of HZ/su
eFigure 4. Two-Way Sensitivity Analysis of Efficacy of a Single Dose and Cost of HZ/su
eFigure 5. Two-Way Sensitivity Analysis of Efficacy of Two Doses and Cost of HZ/su
eFigure 6. Two-Way Sensitivity Analysis of Adherence Rate and Efficacy of Two Doses of HZ/su
eFigure 7. Scatter Plot of Incremental Cost-effectiveness Ratios of the Adjuvanted Subunit Vaccine (HZ/su) (a) and Live Attenuated Vaccine (ZVL) (b) vs No Vaccination From Probabilistic Sensitivity Analysis in Person Vaccinated at 60 Years
eFigure 8. The Probability of Being Cost-effective at $50 000/QALY of Each Strategy vs HZ/su Prices in People Vaccinated at 60 Years
eAppendix. Supplemental Appendix
eTable 1. Vaccine Efficacy by Years Post Vaccination by Age at Vaccination
eTable 2. Costs and Effectiveness of the Three Strategies at Different Prices of the HZ/su for People Vaccinated at Age 60 Years