Abstract
Background and Aims
Habitat fragmentation has transformed landscapes globally, leaving remnants embedded within a complex matrix that is rapidly becoming more developed. For many plant populations, the associated factors of decreased size and intensification of land use surrounding them are expected to increase pollen limitation (‘PL’), unless autonomous self-pollination provides reproductive assurance (‘RA’). Decreased pollinator visitation is often assumed to drive these patterns, but other, less studied mechanisms might include increased heterospecific pollen transfer or decreased conspecific pollen availability via florivory. I investigate how PL and RA and their potential underlying mechanisms vary with population size and land use intensity surrounding populations in the biennial Sabatia angularis (Gentianaceae).
Methods
I estimated the capacity for seed production via autonomous self-pollination (i.e. autofertility). Over 2 years in 22 S. angularis populations across a fragmented landscape, I performed emasculation and pollen supplementation experiments measuring RA and PL, and quantified visitation rates of potential pollinators and a pollen consumer, conspecific pollen loads and rates of heterospecific pollen deposition.
Key results
Autofertility based on fruit mass was 93 % under PL but only 51.6 % relative to maximal conditions. PL and RA were significant on average across populations in the first year of study. Variation in RA was significantly influenced by the interaction between population size and land use intensity, which in turn rendered PL independent of these factors. Visitation and heterospecific pollen deposition rates were greatest in small populations and declined with population size, while conspecific pollen loads were greatest in intermediate sized populations.
Conclusions
Increased reliance on RA is predicted in small S. angularis populations surrounded by intense development, which can explain elevated selfing rates in fragmented populations of plant species more generally. Results from this study point toward forces such as heterospecific pollen transfer, self-pollen limitation or resource availability influencing the need and ability to rely on RA.
Keywords: Autofertility, autonomous selfing, florivory, habitat fragmentation, heterospecific pollen transfer, land use intensity, plant reproduction, pollen deposition, pollen limitation, pollinator visitation, reproductive assurance, Sabatia angularis
INTRODUCTION
Globally, intensification of human land use from conversion of native habitats to agriculture, housing and other forms of development is rapidly changing the landscape (Foley et al., 2005; Hooke et al., 2012). A major concern for wild plant populations is how these changes will impact plant–pollinator interactions and subsequent reproduction (Rathcke and Jules, 1993; Kearns et al., 1998; González-Varo et al., 2013). Given that nearly 90 % of angiosperms rely on biotic pollination for successful seed production (Ollerton et al., 2011), understanding how pollination dynamics change with local- and landscape-level impacts of land use is critical for the conservation of remnant plant populations.
For many plant species, the degree to which seed production is limited by compatible pollen receipt, i.e. pollen limitation (‘PL’), is expected to increase in response to both local-and landscape-level metrics of human land use, including reduced population size and increased fragmentation or land use intensity (Ashman et al., 2004; Knight et al., 2005). In fact, one meta-analysis concluded that PL is probably the ‘most proximate cause of reproductive impairment in fragmented habitats’ (Aguilar et al., 2006), which, if sustained, could lead to lower recruitment and even population growth (Ashman et al., 2004). Yet, in comparison with studies of plant population size on pollinator visitation or mean seed production, few have explicitly measured the effect of population size on PL in wild populations (reviewed in Knight et al., 2005). Further, despite the fact that fragmented populations are often nestled in a complex landscape matrix, the impact of surrounding land use and its interaction with population size on pollination dynamics remains relatively unexplored (Jules and Shahani, 2003; Hadley and Betts, 2012).
For some wild plant species, PL may be abated through autonomous self-pollination (i.e. self-pollination in the absence of pollinators) providing reproductive assurance (‘RA’) (Schoen and Brown, 1991; Kalisz and Vogler, 2003; Eckert et al., 2006). Numerous plants are capable of autonomous self-pollination, and for some this ability boosts seed production, a minimum requirement of the RA hypothesis (Busch and Delph, 2012; Ruan and Teixeira da Silva, 2012). Less is known about the extent to which the level of RA varies in relation to ecological context (Kalisz et al., 2004; van Kleunen et al., 2007; Jacquemyn and Brys, 2008; Brys et al., 2011). Such investigations are necessary, because although self-compatible species are generally less likely to experience PL (Burd, 1994; Larson and Barrett, 2000), not all self-compatible species are capable of autonomous selfing, and this ability is not necessarily correlated with RA (Ruan and Teixeira da Silva, 2012). Moreover, examinations of the influence of the surrounding landscape matrix on RA are virtually non-existent (but see Brys and Jacquemyn, 2012) despite its links to pollinator and plant abundance. Increased RA with declines in population size and/or land use intensity would explain, in part, why reproduction in self-compatible species is less likely to be affected by fragmentation than self-incompatible species (Aguilar et al., 2006) and why selfing rates are elevated in many small, fragmented and/or disturbed populations (Aguilar et al., 2008; Eckert et al., 2010).
Pollen limitation and reliance on RA are symptomatic of receiving either insufficient amounts of conspecific pollen or excessive heterospecific pollen. Lower conspecific pollen loads are expected because of reduced pollinator visitation rates. Where fragmentation and habitat loss have led to local reductions in plant population size, populations may be rendered less attractive to foraging pollinators (Sih and Baltus, 1987; Jennersten, 1988; Kunin and Shmida, 1997). Compounding this problem, greater development surrounding fragments can lead to decreases in floral resources and/or pollinator nesting sites, reducing pollinator abundance and richness (Carvell et al., 2006; Kremen et al., 2007; Clough et al., 2014; Baude et al., 2016). However, fragmentation may also alter antagonistic interactions (Chávez-Pesqueira et al., 2015) that could also influence conspecific pollen loads. In particular, a relatively untested hypothesis is that reduced conspecific pollen loads arise because of increased pollen consumption by florivores in small populations or those most impacted by land use (Malo et al., 2001). In fact, the impact of pollen consumers on PL and RA more broadly is largely unknown (Hargreaves et al., 2009).
Little is also known about the effects of human land use on heterospecific pollen transfer. However, habitat fragmentation or loss with subsequent changes in population size and the landscape matrix can influence pollinator composition and plant–pollinator network structure (reviewed in Hagen et al., 2012). In particular, studies have found that habitat loss and intense land use lead to the loss of efficient pollinators and increased pollinator generalization (Larsen et al., 2005; Theodorou et al., 2017). Consequently, increased heterospecific pollen transfer might arise with decreased population size and/or increased land use intensity. Heterospecific pollen transfer can lead to reduced fecundity by physically clogging stigmas or interfering with conspecific pollen performance (Morales and Traveset, 2008). Recently, the influence of heterospecific pollen transfer on selfing has also been recognized (Arceo-Gómez and Ashman, 2014; Brys et al., 2016). Because autonomous self-pollination could alleviate heterospecific pollen effects if it occuried prior to heterospecific pollen arrival or dilute the effects if it increased the relative conspecific pollen load, greater reliance on autonomous self-pollen (i.e. RA) may evolve where heterospecific pollen transfer is greater (Goodwillie and Ness, 2013; Brys et al., 2016).
Here, I investigate how PL and RA and their potential underlying mechanisms vary with population size and surrounding land use intensity across wild populations of the self-compatible biennial Sabatia angularis. Previous work identified the importance of population size on reproductive patterns in this species, demonstrating that small populations experience reproductive Allee effects (Spigler and Chang, 2008) and increased selfing rates (Spigler et al., 2010), but the underlying mechanisms are unknown and the role of surrounding land use intensity has not yet been explored. I present a series of studies conducted over 2 years and 22 S. angularis populations occurring in remnant serpentine barren grassland patches across a complex landscape. I first confirm that plants can autonomously self-pollinate and quantify the fraction of seed production that can be achieved through autonomous self-fertilization (i.e. autofertility). I then ask the following questions. (1) Is seed production across wild populations pollen limited, and does autonomous selfing provide RA? (2) To what extents do population size, surrounding land use intensity, measured as the proportion of developed land within 0.5 km of populations, or their interaction influence PL and/or RA? To address the underlying mechanisms, I further asked, how do (3) visitation rates by potential pollinators and a pollen consumer and (4) conspecific and heterospecific pollen deposition vary with population size and/or surrounding land use intensity?
MATERIALS AND METHODS
Study system
Sabatia angularis (L.) Pursh (Gentianaceae) is a herbaceous biennial found in a variety of open habitats (glades, old fields, roadsides and grasslands) throughout eastern North America. Plants produce showy, pink flowers from July–August that are visited by a suite of generalist pollinators for a pollen reward and develop into many seeded, dry dehiscent capsules in the autumn. Sabatia angularis is self-compatible and on average is mixed mating (multilocus outcrossing rate tm = 0.78 ± 0.12 s.d.), but primary selfing rates (1– tm) can reach nearly 50 % (Spigler et al., 2010, 2017). Population size explains a large proportion of the variation in selfing (R2 = 0.53), with elevated selfing rates in small populations (Spigler et al., 2010). The predominant mode of self-pollination is unknown. Pollinator-mediated selfing is possible given that plants can simultaneously present >100 open flowers (mean 14 ± 13.6 s.d. flowers, n = 602 plants), but autonomous selfing is also possible when male and female phases of the protandrous flowers overlap. The mechanism of autonomous selfing is unknown, as is the degree of autofertility and the extent to which it provides RA in this species.
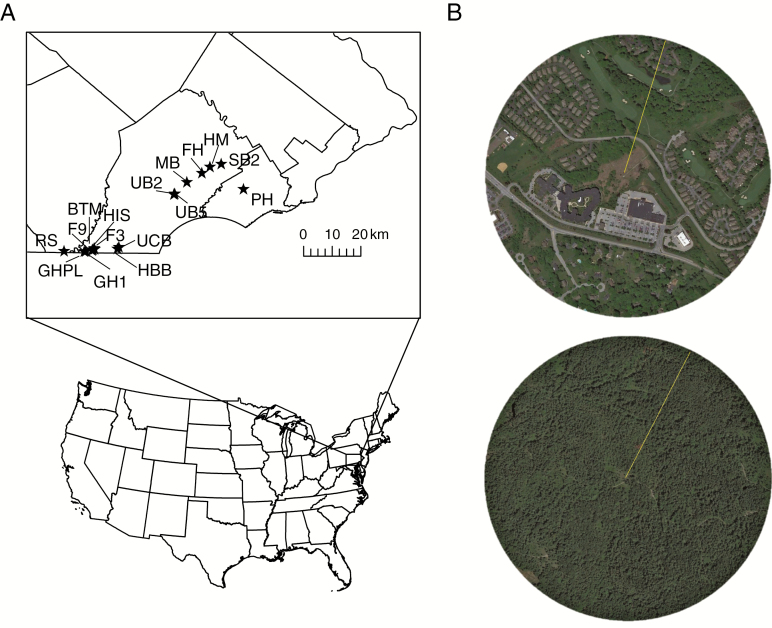
This study took place across S. angularis populations in the rare serpentine grasslands of south-eastern Pennsylvania, USA (Fig. 1). These unique habitats have undergone dramatic declines in the last century (Latham, 1993). Urban development, mining and changes in historical fire and grazing regimes have all led to habitat loss, with extirpation of entire sites and reduced extent of remaining sites. Historically situated in a matrix of mixed hardwood forest, these imperiled habitats are now embedded in a patchwork of agricultural, residential and industrial development. Consequently, remnant grasslands vary in the land composition surrounding them, and S. angularis populations occurring in these grasslands vary greatly in size. The present study was conducted over 2 years. Because of its biennial life cycle, S. angularis adults are not present every year in all sites. In 2014, I identified and included nine S. angularis populations. In 2015, 13 populations were included, six at sites used in 2014 and seven representing new serpentine sites. Thus, a total of 22 populations across both years were investigated (see Supplementary Data Table S1 for population details).
Fig. 1.
Map of Sabatia angularis study sites. (A) Regional map showing the location of study sites across counties in south-eastern Pennsylvania, USA. (B) Google Earth images (Map data: Google 2016) illustrating the extremes of surrounding land use intensity within a 0.5 km radius of populations at two sites (HM, top, and BTM, bottom). See Supplementary Data Table S1 for a complete list of sites.
Characterizing population size and surrounding land use intensity
Population size was determined either by directly counting all flowering plants or, in the largest populations, from transects. In the latter case, population size was extrapolated based on transect-based estimates of plant density and grassland area, calculated in ArcGIS 10.2.2 using GPS waypoints taken along each grassland perimeter.
The composition of land use surrounding each population was quantified using ArcGIS 10.2.2 and data gathered from the 2014 Cropscape (USDA National Agricultural Statistics Service) data set at a spatial resolution of 30 m and projected to NAD 83 UTM zone 18. I defined and quantified land use intensity as the cumulative proportion area of high-, medium- and low-intensity and open developed spaces, representing various residential and industrial uses, within 0.5 and 1 km radii from the estimated centre of each site. Because these were highly correlated (r = 0.98, P < 0.001), 0.5 km was used.
Evaluating autofertility
Autofertility is defined as the fraction of seed production that can potentially be achieved through autonomous self-pollination, calculated by comparing fruit or seed production of flowers protected from pollinators with that of either open-pollinated or pollen-supplemented flowers (Eckert et al., 2010). I used both approaches to obtain a range of autofertility values. First, in 2014, autofertility was determined in the field in a sub-set of populations (F9, HM and UB2). In each, 15 pairs of similarly sized plants were selected, for a total of 90 plants. One randomly chosen plant in each pair was enclosed in a mesh pollinator exclusion bag and the other was left open to pollinators. Up to 15 flower buds per plant were marked with non-toxic paint on the subtending pedicel (mean 9.6 ± 3.0 s.d. flower buds per plant) and fruits were collected upon ripening. Mass (mg) of each fruit was used as a surrogate for seeds per fruit, based on previous work demonstrating that fruit mass is a highly significant, linear predictor of seed mass (Spigler and Chang, 2009), which itself is a highly significant, linear predictor of seed number (Spigler and Chang, 2008). I then conducted a separate study to quantify autofertility relative to maximum seed production under controlled conditions. In Temple University’s Plant Growth Facility, plants were reared from open-pollinated seeds collected from population UB5. Once plants began to flower in the pollinator-free chamber, on each of 15 plants five pairs of freshly open flowers at terminal positions were selected; one flower in each pair was designated as untreated and left unpollinated while the other was hand pollinated using outcross pollen (single donor). Fruiting success was scored for each flower, and individual fruit mass was determined.
Evaluation of PL and RA
A series of field experiments were conducted in 2014 and 2015 to test for PL and RA. In 2014, an average of 37 focal plants per population were randomly selected (± 12.1 s.d., range: 10–49, depending on population size). On each plant, after 20 % of flowers had opened on a given plant (to standardize the timing of treatments across plants), individual flowers were assigned to one of the following treatments (up to two replicates per treatment per plant): outcross pollen supplemented (S treatment) or emasculated in the bud stage (E treatment). Similar stage flowers were identified and left intact to serve as corresponding controls (CS and CE, respectively). Pollen for supplemental pollinations was taken from haphazardly chosen plants. Due to high rates of natural pollen removal in some populations, pollen from another population was occasionally used. Previous work has repeatedly demonstrated that crosses between S. angularis populations have no impact on its fruit or seed set (Dudash, 1990; Spigler et al., 2017) and so should not influence the results. Focal flowers were uniquely labelled with non-toxic paint on the subtending pedicel. The date each treatment was performed was recorded and I scored whether each flower set fruit. Mature fruits were collected and individual fruit mass (mg) was determined. In 2015, I randomly selected an average of 25 plants per population (±13.9 s.d., range: 4–49, depending on population size). The replication of treatments and corresponding controls within a plant was increased (up to five replicates) and, as a consequence, many but not all plants were used for either PL or RA, but not both. Ultimately, 15 plants per treatment per population were included on average (range: 3–25). I recorded the same data as in 2014. A total of 3101 flowers were included across years and populations.
Two important considerations should be noted. First, evaluation of PL at the plant level is considered best because it removes the potential influence of resource reallocation among flowers receiving different treatments. However, this effect was not seen in a meta-analysis when seeds per fruit was used as the variable of interest (Knight et al., 2006) as is used here. Secondly, floral emasculation could negatively affect outcross pollen receipt, e.g. by reducing floral longevity, or damage flowers, causing reduced seed production and overestimation of RA (e.g. Dart and Eckert, 2013). However, emasculation does not truncate floral longevity in S. angularis (Spigler, 2017), and no damage to floral parts of emasculated flowers was observed. Emasculation could reduce visitation, but I do not expect this effect as only one to a few flowers were emasculated per plant. This assumption bore true in studies of a similarly pollen-rewarding Gentian, Centaurium erythraea, even when entire plants were emasculated (Brys et al., 2011). Importantly, any unintended consequences of emasculation on visitation are distributed across all populations. To test whether emasculation negatively impacts the capacity for seed production, I added another flower treatment in a sub-set of populations in 2014 (n = 5 populations, mean 18 ± 2.8 s.d. plants per population): emasculated in the bud and then supplemented with outcross pollen once receptive (ES treatment). Comparison of seed production between ES and S treatments tests whether emasculation reduces the capacity for seed production.
Pollinator visitation
Pollinator observations were conducted in both years in all populations except populations F3 and PH in 2015. On a given day within each population, up to three 2 × 2 m plots centred on a randomly selected flowering S. angularis plant were observed. Within each plot, I counted flowering S. angularis individuals. During observation periods (mean 14 min ± 2.2 s.d., range: 10–20 min), I counted the number of visitors that entered the plot. All observations were made between 09.00 and 17.00 h. This process was repeated across multiple days. In total, a mean of 9.8 plots (± 6.4 s.d.) were observed per population, totalling 45.5 h (31 and 14.5 h in 2014 and 2015, respectively). Mean total visitation rate per plant per minute was calculated for each population as the cumulative number of visits divided by the cumulative number of S. angularis plants and observation minutes across plots. I divided visits into those made by putative pollinators vs. antagonists. Bees and less common flies, skippers and wasps were considered to be potential pollinators. Soft-winged beetles, however, were observed eating pollen, and are more likely to be antagonists.
Pollen deposition
In each population, stigmas were collected from up to 20 CE flowers (mean/population: 14.3 ± 3.9 s.d., median 15) to determine pollen deposition. I collected stigmas after fruits formed, which ensured full opportunities for pollen capture over the entire floral life span and did not influence ovule fertilization. In addition, because plants were chosen randomly and plants differed in their flowering phenology (Emel et al., 2017), stigmas are a random sample of pollen loads across the entire flowering season within each population. I visualized pollen grains on stigmas digested in 8 m NaOH under ×4 magnification on an epifluorescence microscope and counted the number of S. angularis pollen grains on one of the two stigma lobes, selected at random (Spigler and Chang, 2008). I also recorded heterospecific pollen grain deposition and calculated for each population the incidence and intensity of heterospecific pollen deposition, defined as the percentage of stigmas with heterospecific pollen present and the mean percentage heterospecific pollen in the total pollen load, respectively.
Statistical analyses
Evaluating autofertility.
I evaluated whether fruit set and fruit mass of bagged and open-pollinated flowers differed in the field. Fruit set was treated as a binomial response (number of fruits per number of flowers marked) and the effect of bagging evaluated using a generalized linear mixed model (proc glimmix, SAS® software Version 9.3); a general linear mixed model (proc mixed) was used to evaluate this effect on fruit mass. In both models, plant and population identity were included as random effects. For the pollinator-free growth house experiment, I only evaluated fruit mass because fruit set of focal flowers was 100 %. The effect of treatment (untreated vs. hand-pollinated) on fruit mass was evaluated using a general linear mixed model, with plant identity as a random effect. Replication of both treatments within plants in the growth house further allowed for testing of phenotypic variation in autofertility (random treatment × plant interaction). Autofertility was calculated as the ratio of bagged to open-pollinated fruit mass (field) or untreated to hand-pollinated fruit mass (growth house).
Testing for RA and PL.
I first tested whether emasculation, per se, negatively impacts fruit mass. A general linear mixed model (proc mixed) was used including treatment as a fixed effect, plant and population identities as random effects and treatment date as a covariate.
To evaluate whether PL and/or RA for seeds per fruit occurred and the extent to which these depended upon population size and land use intensity, I used a general linear mixed model (proc mixed). Analysis was restricted to seeds per fruit, estimated by fruit mass, because fruit set of focal flowers was 99 %, consistent with its monocarpic life history. I included pollination treatment, population size (log-transformed), proportion of developed land, year and all two- and three-way interaction terms as fixed variables in the model (higher order interactions were not considered). Interactions were retained where significant (P ≤ 0.05) or where they represented sub-sets of a significant higher order interaction. I also included treatment date of each flower, date by year interaction and the number of conspecifics within 1 m (‘local neighbourhood size’), which can also influence seed production (Spigler and Chang, 2008). Plant and population identities were random effects. Sites sampled across years were treated independently. Note that I adjusted dates of E and CE flowers to reflect the date that they were in female phase and, accordingly, to be comparable with S and CS dates. Using the ES treatments as a reference, I determined that the median time between bud emasculation and female phase was 3 d and added this to E and CE dates. Because I am interested in specific pairwise comparisons reflecting tests for PL (CS vs. S) and RA (CE vs. E) rather than omnibus tests, I used the lsmestimate statement in proc mixed to test a priori hypotheses. These tests can generate different results from Type III fixed effects for main treatment effects because, whereas Type III compares treatments setting all covariates to 0, comparisons of least squares means set covariates with their means (Littell et al., 1996). The dependent variable, fruit mass, was log-transformed, which allows for inferences about differences in the ratio fruit of mass between treatments (i.e. ratios of CS/S and CE/E that define PL and RA, respectively) when evaluating interactions between pollination treatment and covariates. I back-transformed the least squares mean estimates of the differences between treatments, log S/CS and log CE/E, to calculate mean PL = 1 – CS/S and RA = 1 – E/CE, respectively. I similarly back-transformed estimates of the lower and upper bounds of the differences to determine 95 % CI for PL and RA.
To probe further the significant three-way interaction between pollination treatment, population size (log-transformed) and proportion of developed land (see the Results) and to determine whether it is specific to either PL or RA, I divided the data into two (CS vs. S and CE vs. E) and repeated the above analysis. As a tool to visualize the interactions, I created 3-D surfaces plotting either mean RA or PL per population as a function of population size (log-transformed) and proportion of developed land.
Evaluating population-level patterns of pollinator visitation and pollen deposition.
A general linear mixed model (proc glimmix) was used to evaluate the fixed effects of population size (log-transformed), proportion of developed land, year, visitor type (beetles vs. potential pollinators) and all two-way interactions on visitation rate. Because of the number of factors relative to the number of populations, interactions were removed from the model if they were not significant (P > 0.05) but main effects were retained regardless of their significance in this and all other population-level analyses. Population identity was included as a random effect to account for multiple visitor type observations per population. Visitor type was included as a grouping factor and I tested for heterogeneity of variances based on likelihood ratio scores (Littell et al., 1996) because I suspected that variances were not equal among visitor types.
It was also evaluated whether S. angularis pollen deposition rate was predicted by visitation rate, year and their interaction. I performed three separate analyses using either total visitor, potential pollinator or beetle visitation rate as the predictor variable (α ≤ 0.017 to correct for multiple tests). Unequal variances among years was accounted and tested for based on preliminary evaluation of the data.
The influence of population size (log-transformed), proportion of developed land, year and all two-way and three-way interactions on mean S. angularis pollen loads and the frequency of heterospecific pollen deposition (incidence and intensity) was evaluated using general linear models (proc glm). Examination of scatterplots revealed a potentially non-monotonic relationship between conspecific pollen deposition and population size; therefore, I included a quadratic term for population size. In all analyses, residuals were checked for their conformity to model assumptions.
RESULTS
Autofertility
Sabatia angularis plants are highly capable of autonomous self-pollination. In fact, fruit set of bagged flowers (0.82 ± 0.058 s.e.) and control flowers (0.85 ± 0.06 s.e.) in the field was statistically equivalent (F1,79.88 = 0.14, P = 0.71), as was bagged (27 ± 2 mg s.e.) and control (29 ± 2 mg s.e.) fruit mass (F1,114 = 0.66, P = 0.42). Under controlled growth conditions, fruit mass for hand-pollinated flowers (63 ± 4 mg s.e.) was significantly greater than that for untreated flowers (33 ± 4 mg s.e.) (F1,14.1 = 61.47, P < 0.001). These translate to 93 % autofertility in the field but only 51.6 % relative to maximum seed production. The growth house study also revealed significant phenotypic variation in autofertility (i.e. significant plant × treatment interaction: Z = 1.99, P = 0.02).
Pollination treatments
Emasculation did not damage seed set capacity of flowers. Fruit mass was equivalent between hand-pollinated, emasculated flowers and hand-pollinated, intact flowers (F1,123 = 1.31, P = 0.25).
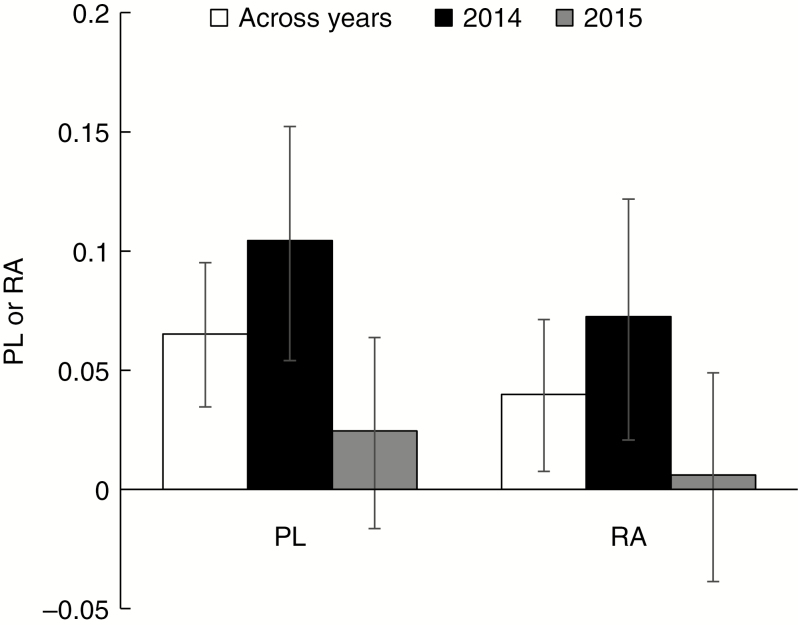
Pollination treatment influenced fruit mass, but the effect varied across years and there was a significant three-way interaction between pollination treatment, population size and developed land (Table 1). Pre-planned comparisons of least squares means revealed that PL was significant on average across years, populations and all covariates (t = 4.04, P < 0.0001), and its mean was 6.5 % (Fig. 2). However, the significance of PL across years was mainly driven by the significant difference between CS and S fruit mass in 2014 (t = 3.93, P < 0.0001), when PL was 10.4 % on average. PL was only 2.4 % on average in 2015 and not significant (t = 1.12, P = 0.26) (Fig. 2). Pre-planned comparisons further revealed an overall difference between E and CE fruit mass across years (t = –2.4, P = 0.017), again driven by a significant difference in 2014 (t = –2.54, P = 0.011) but not 2015 (t = –4.8, P = 0.63) (Fig. 2). Thus, selfing provided RA. Its effective value, however, was relatively low: 4.0 % on average and 7.2 % in 2014 compared with only 0.6 % in 2015.
Table 1.
Fixed effects from a general linear mixed model evaluating pollination treatment, population size, land use intensity and covariates on seeds per fruit (fruit mass)
| Effect | Numerator d.f. | Denominator d.f | F | P |
|---|---|---|---|---|
| Pollination treatment | 3 | 2319 | 1.26 | 0.29 |
| Population size (log) | 1 | 18 | 8.56 | 0.009 |
| Developed land (%) | 1 | 16.3 | 1.75 | 0.20 |
| Local neighbourhood size | 1 | 565 | 5.9 | 0.016 |
| Year | 1 | 2234 | 61 | <0.0001 |
| Date | 1 | 2418 | 365.36 | <0.0001 |
| Date × year | 1 | 2421 | 67.43 | <0.0001 |
| Pollination treatment × size | 3 | 2344 | 0.79 | 0.50 |
| Pollination treatment × development | 3 | 2346 | 3.75 | 0.011 |
| Pollination treatment × year | 3 | 2371 | 6.2 | 0.0003 |
| Population size × land | 1 | 16 | 2.37 | 0.14 |
| Pollination treatment × size × development | 3 | 2316 | 3.7 | 0.011 |
Fig. 2.
Mean pollen limitation (PL) and reproductive assurance (RA) based on seeds per fruit, estimated as fruit mass (mg), on average across and within years. Back-transformed least squares means for PL and RA and their 95 % confidence interval are shown.
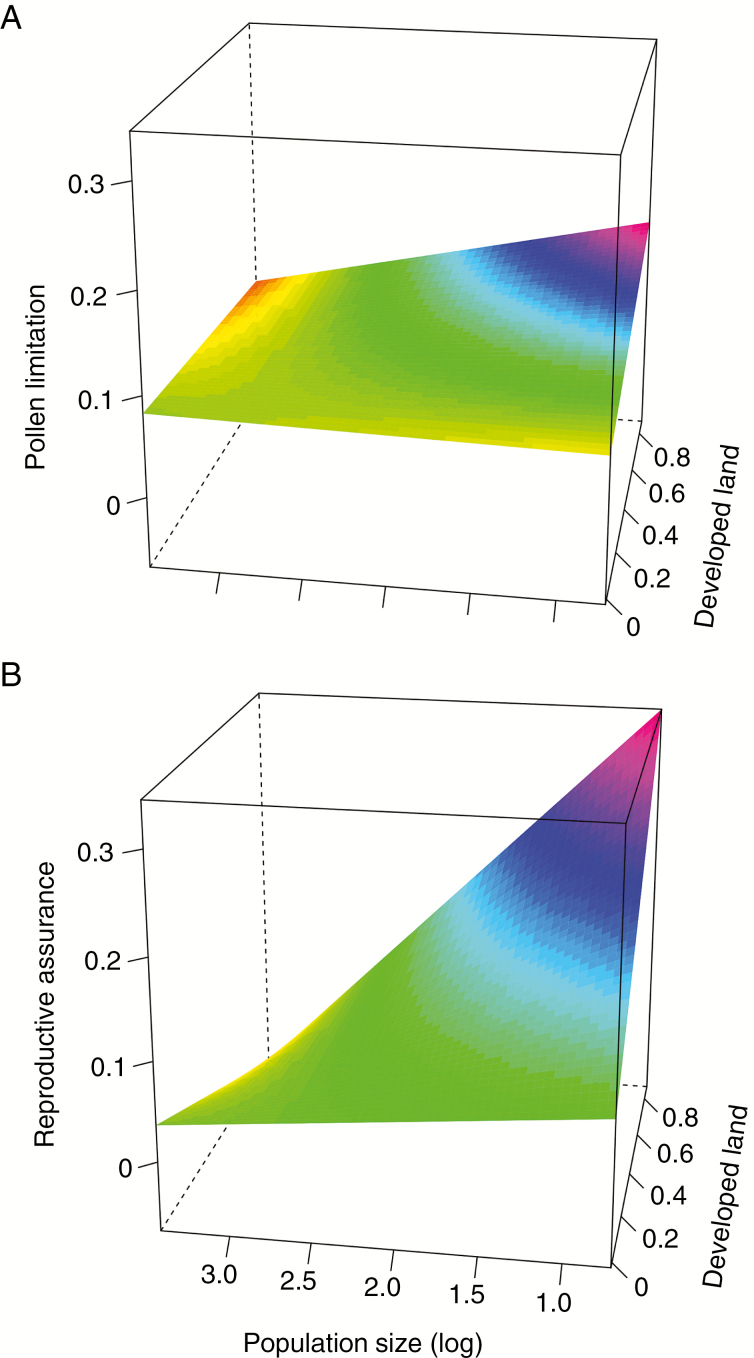
Evaluation of separate PL and RA models revealed that the three-way interaction between treatment, population size and development was highly significant for RA (F1,876 = 10.2, P = 0.0015) but not significant for PL (F1,1020 = 0.14, P = 0.71) (Fig. 3) (see Supplementary Data Table S2 for complete model results). Although caution must be used in extrapolating surfaces beyond the range of the data (Supplementary Data Figure S1), Fig. 3B illustrates that RA increases with declining population size under intense land use but that this effect dissipates at lower levels of development.
Fig. 3.
Three-dimensional surface plots illustrating the interactive effects of population size (log-transformed) and proportion of developed land surrounding each population on (A) pollen limitation and (B) reproductive assurance. Note that these figures represented predicted surfaces with the reference year set to 2014. Predicted values are calculated across the entire surface (all combinations of population size and development values) based on the statistical models that use mean population parameters as the response. The distribution of data points used to create this surface is shown in Supplementary Data Fig. S1.
Additional factors influenced fruit mass (Table 1). Fruit mass increased with population size but did not vary with development on average. A significant random population effect (Z = 2.52, P = 0.006) further indicates variation among populations in fruit mass beyond that explained by population size. Fruit mass was significantly higher in 2014 (34.5 ± 2.66 mg s.e.) compared with 2015 (26.3 ± 2.34 mg s.e.) and declined over the season within years, with a steeper decline in 2015 (significant date × year interaction). In addition, local neighbourhood size had a significant negative impact on fruit mass.
Pollinator visitation and pollen receipt
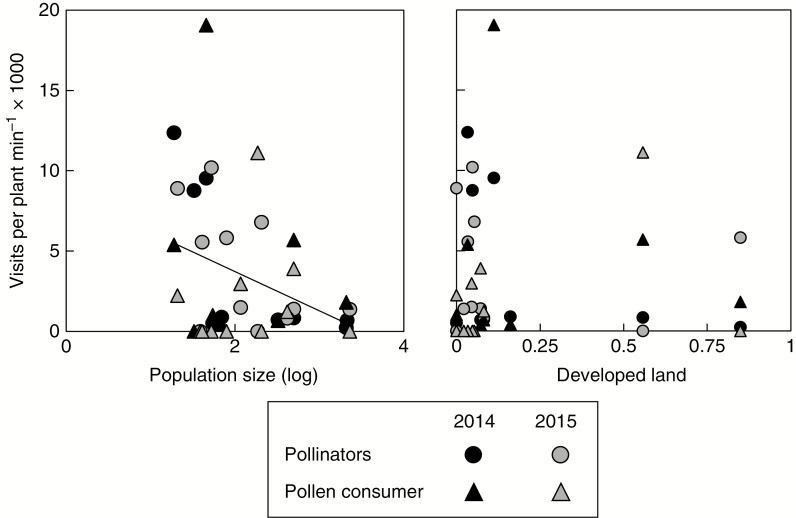
In 2014, approximately half of all visits were by bees and other potential mutualist pollinators (49.4 ± 29.3 % s.d.), with the remainder representing visits by soft-winged beetles. In 2015, beetles were less abundant, representing 32.0 % of visits. Mean visitation rate significantly declined with increasing population size (F1,15.9 = 9.02, P = 0.009) but was not influenced by land use intensity (F1,15.9 = 0, P = 0.95) (Fig. 4). Mean visitation rates were similar across years (F1,15.9 = 0.19, P = 0.67) and visitor types (F1,21.4 = 0.62, P = 0.44), but variance in beetle visitation was significantly greater compared with potential pollinators (χ2 = 11.84, P < 0.001). No pairwise interactions were significant (P > 0.25, not shown), although power to detect significant interactions in this (and all other) population-level analysis may be limited. Sites studied in both years best illustrate the relative impacts of population size vs. land use. For example, populations of alternating generations at HM were drastically different in size (Supplementary Data Table S1), and the visitation rate of potential pollinators, in particular, was substantially lower in 2014, when the population was large, compared with 2015 when the population was small (0.22 visits per plant min–1 × 10–3 vs. 5.8 visits per plant min–1 × 10–3, respectively). In contrast, visitation rates were similar across years at sites with relatively constant population sizes. Site MB had 33 individuals in 2014 and 53 in 2015, and total visitation rates were relatively high in both years: 8.8 and 10.2 visits per plant min–1 (× 10–3), respectively. In contrast, site UB2, with similarly large populations in both years, had relatively low total visitation rates in 2014 and 2015: 1.4 vs. 2.0 visits per plant–1 min (× 10–3), respectively.
Fig. 4.
Scatterplots showing the influence of population size (log-transformed) and surrounding land use intensity (proportion of developed land surrounding populations) on rates of visitation by potential pollinators vs. beetles, a potential antagonist (pollen consumer). Visitation rate is shown by year and visitor type according to the key. A regression line based on average visitation across years and pollination types represents the significant influence of population size on visitation rate.
Mean conspecific pollen receipt declined with increasing total visitation rate (estimate = –4.6 ± 1.96 s.e., F1,7.5 = 5.52, P = 0.049) and varied significantly across years (F1,12.91 = 22.76, P = 0.0004). Conspecific pollen loads were nearly twice as high in 2015 than in 2014 (453.0 ± 40.9 s.e. vs. 239.9 ± 17.8 s.e., respectively) and significantly more variable in 2015 (χ2 = 5.79, P = 0.02). Analyses by visitor type revealed that the overall negative effect of total visitation is due to a strong negative impact of beetle visitation (estimate = –8.7 ± 2.63 s.e., F1,7.7 = 10.87, P = 0.01). In fact, analysis of 2014 data alone reveals that beetle visitation rate explains 58.7 % of the variation in conspecific pollen deposition among populations (F = 9.93, P = 0.02). Visitation by bees and other potential pollinators did not predict mean conspecific pollen deposition (F1,9.4 = 0.41, P = 0.54). The year × visitation interaction was not significant in any of these analyses (P > 0.40).
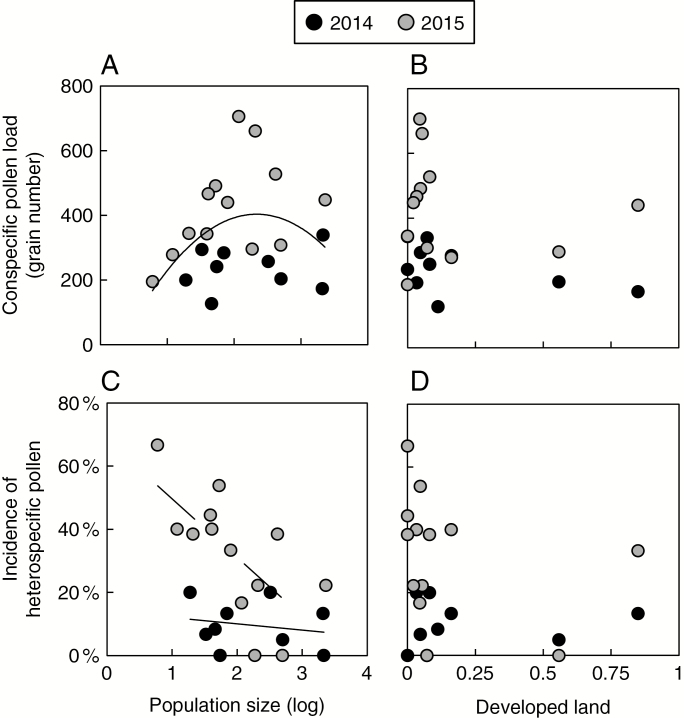
Mean S. angularis conspecific pollen receipt, nevertheless, significantly varied with population size (F1,17 = 8.11, P = 0.01), but this relationship was non-monotonic (quadratic term: F1,17 = 6.18, P = 0.02), with intermediate-sized populations exhibiting the highest mean pollen receipt on average (Fig. 5A). Mean pollen receipt declined with increasing development, but this relationship was not statistically significant (F1,17 = 3.04, P = 0.10) (Fig. 5B). No interactions among population size, land use and year were significant (0.13 ≤ P ≤ 0.93). As in the previous analysis, year significantly influenced pollen receipt (F1,17 = 18.48, P < 0.001).
Fig. 5.
Scatterplots showing the influence of population size (left panel, log-transformed) and proportion of developed land surrounding each population (right panel) on stigmatic pollen loads. Top panel: mean conspecific stigmatic pollen loads per population in relation to (A) population size and (B) proportion of developed land. Bottom panel: incidence (percentage) of stigmas with heterospecific pollen per population in relation to (C) population size and (D) proportion of developed land. Regression lines are shown where significant. Data for the 2 years are differentiated as shown in the key.
Heterospecific pollen was found on 20.1 % of stigmas examined. This frequency varied significantly among years (F = 10.20, P = 0.005) and was more than three times as high in 2015 than in 2014 (30.5 % vs. 9.1 %, respectively), consistent with the pattern of yearly variation in conspecific pollen loads. The percentage of flowers with heterospecific pollen within a population ranged from 0 to 67 %, and declined significantly with increasing population size (F = 4.75, P = 0.04), but not development (F = 0.63, P = 0.44) (Fig. 5C, D). The effect of population size was stronger in 2015, indicated by a significant population size × year interaction (F = 5.17, P = .04). The final model, including population size, development, year and the size × year interaction, explained 64 % of the variation in the incidence of heterospecific pollen deposition. The intensity of heterospecific pollen in the total pollen load was low among individuals, ranging only from 0 to 8.0 % among individuals (mean 0.30 ± 1.23 % s.d.). Mean intensity per population ranged only from 0 to 1.04 %, but similarly declined with population size (F = 6.52, P = 0.02). However, the simultaneous F-test for the final model including the main effects of population size, development and year was not significant (F = 2.7, P = 0.07).
DISCUSSION
Reproductive assurance in the most vulnerable populations may ameliorate pollen limitation
This study demonstrates that S. angularis is autofertile and illustrates how population size and land use can interact to determine reliance on self-pollination in a way that is consistent with the RA hypothesis (Busch and Delph, 2012). Specifically, the highest levels of RA are predicted where S. angularis populations are both small and nestled within a highly developed matrix. This finding is consistent with two similar studies of the biennial Centaurium erythraea, which showed that RA strongly declined with population size (Brys et al., 2011) and in natural vs. highly developed areas (Brys and Jacquemyn, 2012). However, the current study is novel in revealing an interaction between population size and development. That is, for some species, small size or large habitat loss alone may minimally impact reliance on self-pollination, whereas having both leads to significant impacts. Indeed, autonomous selfing in S. angularis has the potential to provide between nearly complete seed production per fruit relative to pollen-limited conditions in the field and approximately half of maximum seed production. Also, while its effective value in terms of providing RA was low on average, RA reached as high as 37 % in population F3 in 2015, comprised of only six flowering plants. Even low levels of RA can impact the mating system. RA in C. verna, for example, which was similar to the average RA in S. angularis seen here, significantly increased the selfing rate (Kalisz et al., 2004). Thus, the patterns shown in this study can help explain elevated selfing rates in small, fragmented S. angularis populations (Spigler et al., 2010) as well as offer a mechanistic explanation for the widespread pattern of increased selfing rates seen across small, fragmented and/or disturbed populations of self-compatible species more generally (Aguilar et al., 2008; Eckert et al., 2010).
Autofertility and reliance on RA are consistent with both the low mean levels of PL seen across S. angularis populations and diminished variation in PL in relation to population size and development (compare Fig. 3A with B). PL was only 6.5 % on average across populations and years. This estimate is notably similar to the average 7 % PL found in another study for species with levels of autofertility on a par with S. angularis (González and Pérez, 2010). PL did not vary in relation to population size or land use intensity across S. angularis populations in this study, even in 2014 when PL was significant. In contrast, the handful of other studies that experimentally quantified PL in relation to population size or fragmentation found support for the predicted increase in PL in small or fragmented populations (reviewed in Knight et al., 2005). A key difference between S. angularis and focal species of those previous studies, however, is the self-compatibility of S. angularis and its ability to rely on autonomous self-pollination. Studies of land use impacts on PL are even rarer. Ekroos et al. (2015) similarly did not find variation in PL in relation to landscape-level metrics of land use in a study of the partially self-incompatible sticky catchfly, Lychnis viscaria. Interestingly, they suggested that land use might more readily impact the degree of variability in PL within populations rather than differences in mean PL.
Which mechanisms are likely to drive patterns of RA and PL?
Pollen limitation and RA are primarily predicted to arise in small populations and/or those surrounded by intense development due to reduced pollinator visitation and subsequent reductions in conspecific pollen receipt (Knight et al., 2005). Yet, in contrast to these general expectations, visitation by potential pollinators to S. angularis declined with increasing S. angularis population size and did not vary predictably with the amount of surrounding developed land. This suggests that intra-specific competition, rather than facilitation, for pollinators occurs where S. angularis is more abundant, consistent with earlier work demonstrating increased PL in large experimental S. angularis populations (Spigler and Chang, 2009). While less common, the pattern of decreased visitation in larger plant populations has been found in a few other studies (Mustajärvi et al., 2001; Campbell and Husband, 2007; Ward et al., 2013). The absence of a relationship between visitation rates and surrounding land use intensity was surprising. Instead, I found low rates in more intensely developed areas, as expected, but highly variable rates in undeveloped areas. Other factors could be obscuring the impacts of development. Insects may respond instead to the proportion of surrounding agricultural land or landscape complexity per se (Steffan-Dewenter and Kuhn, 2003; Taki et al., 2010; Ekroos et al., 2013; Clough et al., 2014) or to different spatial scales (Westphal et al., 2006). However, effects of land use metrics including agriculture as well as well as consideration of larger spatial scales similarly fail to predict visitation to S. angularis (data not shown). The influence of population size but not landscape-scale features may instead truly reflect that at least some pollinators respond more to characteristics at the local scale (Winfree et al., 2009; Williams and Winfree, 2013).
Moreover, mean pollinator visitation was a poor predictor of mean S. angularis pollen deposition. This disconnect between visitation rate and pollen deposition is well documented (King et al., 2013). First, pollen receipt is a function of both pollinator and pollen (mate) availability. Visitation will increase pollen deposition only if pollen is available, whereas even low visitation rates could result in sufficient deposition provided pollen is abundant. Reduced mate availability in small populations combined with increased competition for pollinator visitation in the largest populations would explain the unimodal relationship between mean S. angularis pollen deposition and population size shown here. Secondly, not all floral visitors are efficient pollinators (Ne’eman et al., 2010). Differential responses of pollinator species to fragmentation and land use can alter pollinator composition or other aspects of plant–pollinator interaction networks influencing pollination efficiency (Sjödin et al., 2008; Ekroos et al., 2013; Spiesman and Inouye, 2013; Theodorou et al., 2017), making the composition of the visitors more important for determining pollen deposition than the visitation rate per se. Thirdly, I show that changes in interactions with antagonists influencing pollen deposition may also be at play. Soft-winged beetles negatively influence S. angularis stigmatic pollen loads. Because beetle visitation also increased with decreasing population size, their consumptive effects in small populations may have negated any benefits of increased pollinator visitation and exacerbated the issue of mate availability, contributing to PL and the need for RA. The possible impact of pollen consumption on stigmatic pollen loads and PL is also reflected in the differences in these parameters across years in the study. Stigmatic pollen loads in 2014 were nearly half of that in 2015, despite similar mean pollinator visitation rates across years. The beetle visitation rate, however, was 2-fold higher in 2014 than 2015, and in 2014 beetles represented a greater percentage of floral visitors, potentially contributing to lower conspecific pollen loads and significant PL in 2014.
Notably, mean pollen deposition did not vary predictably with land use or the interaction between land use and population size, and thus does not appear to explain variation in RA across S. angularis populations. Indeed, this is probably because unlike PL, RA also depends on pollen removal and self-pollen availability. Pollen deposition and removal need not be correlated (Wilson and Thomson, 1991). For example, Wilson and Thomson (1991) found that pollen deposition and receipt were decoupled in the similarly protandrous Impatiens capensis. In fact, among I. capensis patches, they found that the one with higher pollen removal had next to no pollen deposited, while another patch had relatively lower pollen removal but high pollen deposition. Such differences can have a major impact on the ability of plants to rely on selfing for RA, as low rates of pollen deposition coupled with high rates of pollen removal leave plants not only outcross pollen limited but self-pollen limited. Pollen consumption could similarly limit self-pollen availability. Consequently one hypothesis is that high levels of pollen consumption could lead to selection for greater autonomous selfing before consumers visit to provide RA (van Kleunen et al., 2007). Differences in pollen removal rates among populations varying in population size and land use, perhaps due to shifts in pollinator composition, could potentially explain the pattern of RA seen across S. angularis populations. Future work examining changes in pollinator community and rates of pollen removal in relation to population size and land use can help inform on these dynamics.
More recently, the importance of heterospecific pollen transfer in shaping pollination dynamics has gained traction (Morales and Traveset, 2008; Ashman and Arceo-Gómez, 2013), particularly with respect to its role in reliance on autonomous self-pollination (Brys et al., 2016). Heterospecific pollen transfer could be greater in small populations or those surrounded by intense development. For example, changes in pollinator foraging patterns with the relative abundance of plant species have been shown in a few studies (Kephart, 1983; Feinsinger et al., 1991; Stout et al., 1998), while distance to nature reserves was a critical factor determining this pollination parameter in the bee-pollinated herb Dianella revoluta (Duncan et al., 2004). I demonstrate that the frequency of heterospecific pollen transfer in S. angularis, with respect to both its incidence and intensity, increased with decreasing plant population size as predicted. Although the intensity of heterospecific pollen transfer, i.e. the percentage of heterospecific pollen in the total stigmatic pollen load, is low, reaching no more than 9 % on a given flower and an average of approx. 1 % in any population, it is consistent with the mode across species (Ashman and Arceo-Gómez, 2013). On the other hand, this study reveals that the incidence of heterospecific pollen transfer in S. angularis can be high. Given its negative consequences for fruit and seed set (Morales and Traveset, 2008), the higher frequency of heterospecific pollen transfer in small populations could represent another dimension of a reproductive Allee effect, (inter)acting with reduced conspecific pollen loads to reduce mean fecundity (Waal et al., 2015). However, because heterospecific pollen transfer did not vary with surrounding land use and only weakly varied with population size in 2014, it is unlikely to explain the interactive effects of population size and land use on RA in S. angularis.
Finally, although not directly measured in these studies, several lines of evidence suggest that variation in resource availability could be influencing RA and PL patterns. Pollen can limit seed production only where resources are sufficient to enable plants to mature all fertilized ovules. Otherwise, where resources are limiting, PL may be relatively low or absent, even if not all ovules are fertilized (Ashman et al., 2004). For example, while one might conclude that higher pollen deposition in 2015 relieved PL, resource scarcity is more likely to be responsible. Mean seeds per fruit in 2015 was actually substantially lower than in 2014, even for the supplemental pollination treatment, and the decline in seeds per fruit across the season was greater, indicating that plants were incapable of translating greater pollen loads into greater seed production in 2015. Previous work also highlights the importance of resource availability for PL in S. angularis, demonstrating higher PL for large plants, which presumably have greater access to resources, than for small plants (Dudash, 1993). In a similar manner, even if autonomous self-pollination increases stigmatic pollen loads, RA may be limited by resource availability. Could population variation in per capita resource availability have influenced the pattern of RA seen among S. angularis populations? One clue lies in the impact of local neighbourhood size on fruit mass. This and previous work demonstrate reduced seed production with increased local neighbourhood size, suggesting competition for resources (Spigler and Chang, 2008). Post-hoc analysis indicates that fruit mass is also significantly impacted by a three-way interaction between local neighborhood size, population size and land use intensity, which further suggests that the intensity of competition varies with population size and land use intensity (data not shown). Further study can explicitly evaluate how variation in per capita resource availability interacts with pollination dynamics to determine both the need and ability to rely on RA.
Conclusions
The results of this study highlight the influence of local- and landscape-level effects of changing land use on critical pollination parameters. In particular, this study demonstrates how land use intensity surrounding grassland habitats can interact with population size to influence reliance on RA and, potentially, the mating system of plant populations. Moreover, the study illustrates how patterns of variation may deviate among pollination parameters. In fact, all of the parameters measured here – pollinator visitation rates, stigmatic pollen loads, PL and RA – followed different patterns in relation to population size and land use intensity and across years, underscoring the complexity of forces in shaping these patterns. Studies that experimentally manipulate both pollen and resource availability and consider pollinator composition and pollen removal rates can help to disentangle these forces.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aoband consist of the following. Table S1: study site and population information. Table S2: results for fixed effects in separate PL and RA analyses. Figure S1: distribution of data points used to create the 3-D surface plots illustrating the interactive effects of population size and proportion of developed land on PL and RA. Figure S2: scatterplots showing the influence of population size and land use intensity on rates of visitation by potential pollinators vs. antagonists.
ACKNOWLEDGEMENTS
The author thanks M. Le Sage, A. Collins, J. Wyatt, S. Olshevski, J. Hart, H. Dashnaw and A. Woodard for assistance in the field and plant facility; S. Kalisz and T.-L. Ashman for helpful comments; and J. Tao at SAS Technical Support. J. Karron and two anonymous reviewers provided valuable feedback on an earlier version of this manuscript. The author also thanks Natural Lands Trust, The Nature Conservancy, PA DCNR, Hershey’s Mill, Nottingham County Park, Lancaster County Conservancy, North Hills Civic Association, Tyler Arboretum and the Hanna family for permission to work on their lands, and R. Latham, B. Foreacre, J. Gregg and Friends of the State Line Serpentine Barrens for logistical support. This work was supported by funding from Temple University.
LITERATURE CITED
- Aguilar R, Ashworth L, Galetto L, Aizen MA. 2006. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta‐analysis. Ecology Letters 9: 968–980. [DOI] [PubMed] [Google Scholar]
- Aguilar R, Quesada M, Ashworth L, Herrerias‐Diego Y, Lobo J. 2008. Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Molecular Ecology 17: 5177–5188. [DOI] [PubMed] [Google Scholar]
- Arceo-Gómez G, Ashman T-L. 2014. Coflowering community context influences female fitness and alters the adaptive value of flower longevity in Mimulus guttatus. American Naturalist 183: E50–E63. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Arceo-Gómez G. 2013. Toward a predictive understanding of the fitness costs of heterospecific pollen receipt and its importance in co-flowering communities. American Journal of Botany 100: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Knight TM, Steets JA et al. . 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85: 2408–2421. [Google Scholar]
- Baude M, Kunin WE, Boatman ND et al. . 2016. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys R, de Crop E, Hoffmann M, Jacquemyn H. 2011. Importance of autonomous selfing is inversely related to population size and pollinator availability in a monocarpic plant. American Journal of Botany 98: 1834–1840. [DOI] [PubMed] [Google Scholar]
- Brys R, Jacquemyn H. 2012. Effects of human‐mediated pollinator impoverishment on floral traits and mating patterns in a short‐lived herb: an experimental approach. Functional Ecology 26: 189–197. [Google Scholar]
- Brys R, van Cauwenberghe J, Jacquemyn H. 2016. The importance of autonomous selfing in preventing hybridization in three closely related plant species. Journal of Ecology 104: 601–610. [Google Scholar]
- Burd M. 1994. Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review 60: 83–139. [Google Scholar]
- Busch JW, Delph LF. 2012. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany 109: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LG, Husband BC. 2007. Small populations are mate‐poor but pollinator‐rich in a rare, self‐incompatible plant, Hymenoxys herbacea (Asteraceae). New Phytologist 174: 915–925. [DOI] [PubMed] [Google Scholar]
- Carvell C, Roy DB, Smart SM, Pywell RF, Preston CD, Goulson D. 2006. Declines in forage availability for bumblebees at a national scale. Biological Conservation 132: 481–489. [Google Scholar]
- Chávez-Pesqueira M, Carmona D, Suárez-Montes P, Núñez-Farfán J, Aguilar R. 2015. Synthesizing habitat fragmentation effects on plant–antagonist interactions in a phylogenetic context. Biological Conservation 192: 304–314. [Google Scholar]
- Clough Y, Ekroos J, Báldi A et al. . 2014. Density of insect-pollinated grassland plants decreases with increasing surrounding land-use intensity. Ecology Letters 17: 1168–1177. [DOI] [PubMed] [Google Scholar]
- Dart S, Eckert CG. 2013. Experimental manipulation of flowers to determine the functional modes and fitness consequences of self‐fertilization: unexpected outcome reveals key assumptions. Functional Ecology 27: 362–373. [Google Scholar]
- Dudash MR. 1990. Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae) – a comparison in 3 environments. Evolution 44: 1129–1139. [DOI] [PubMed] [Google Scholar]
- Dudash MR. 1993. Variation in pollen limitation among individuals of Sabatia angularis (Gentianaceae). Ecology 74: 959–962. [Google Scholar]
- Duncan DH, Nicotra AB, Wood JT, Cunningham SA. 2004. Plant isolation reduces outcross pollen receipt in a partially self‐compatible herb. Journal of Ecology 92: 977–985. [Google Scholar]
- Eckert CG, Kalisz S, Geber MA et al. . 2010. Plant mating systems in a changing world. Trends in Ecology and Evolution 25: 35–43. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Dart S. 2006. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 183–203. [Google Scholar]
- Ekroos J, Rundlöf M, Smith HG. 2013. Trait-dependent responses of flower-visiting insects to distance to semi-natural grasslands and landscape heterogeneity. Landscape Ecology 28: 1283–1292. [Google Scholar]
- Ekroos J, Jakobsson A, Wideen J, Herbertsson L, Rundlöf M, Smith HG. 2015. Effects of landscape composition and configuration on pollination in a native herb: a field experiment. Oecologia 179: 509–518. [DOI] [PubMed] [Google Scholar]
- Emel SL, Franks SJ, Spigler RB. 2017. Phenotypic selection varies with pollination intensity across populations of Sabatia angularis. New Phytologist 215: 813–824. [DOI] [PubMed] [Google Scholar]
- Feinsinger P, Tiebout HM, Young BE. 1991. Do tropical bird‐pollinated plants exhibit density‐dependent interactions? Field experiments. Ecology 72: 1953–1963. [Google Scholar]
- Foley JA, DeFries R, Asner GP et al. . 2005. Global consequences of land use. Science 309: 570–574. [DOI] [PubMed] [Google Scholar]
- González AV, Pérez F. 2010. Pollen limitation and reproductive assurance in the flora of the coastal Atacama Desert. International Journal of Plant Sciences 171: 607–614. [Google Scholar]
- González-Varo JP, Biesmeijer JC, Bommarco R et al. . 2013. Combined effects of global change pressures on animal-mediated pollination. Trends in Ecology and Evolution 28: 524–530. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Ness JM. 2013. Interactions of hybridization and mating systems: a case study in Leptosiphon (Polemoniaceae). American Journal of Botany 100: 1002–1013. [DOI] [PubMed] [Google Scholar]
- Hadley AS, Betts MG. 2012. The effects of landscape fragmentation on pollination dynamics: absence of evidence not evidence of absence. Biological Reviews 87: 526–544. [DOI] [PubMed] [Google Scholar]
- Hagen M, Kissling WD, Rasmussen C et al. . 2012. Biodiversity, species interactions and ecological networks in a fragmented world. Advances in Ecological Research 46: 89–210. [Google Scholar]
- Hargreaves AL, Harder LD, Johnson SD. 2009. Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biological Reviews 84: 259–276. [DOI] [PubMed] [Google Scholar]
- Hooke RL, Martín-Duque JF, Pedraza J. 2012. Land transformation by humans: a review. GSA Today 22: 4–10. [Google Scholar]
- Jacquemyn H, Brys R. 2008. Density-dependent mating and reproductive assurance in the temperate forest herb Paris quadrifolia (Trilliaceae). American Journal of Botany 95: 294–298. [DOI] [PubMed] [Google Scholar]
- Jennersten O. 1988. Pollination in Dianthus deltoides (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conservation Biology 2: 359–366. [Google Scholar]
- Jules ES, Shahani P. 2003. A broader ecological context to habitat fragmentation: why matrix habitat is more important than we thought. Journal of Vegetation Science 14: 459–464. [Google Scholar]
- Kalisz S, Vogler DW. 2003. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology 84: 2928–2942. [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. 2004. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature 430: 884–887. [DOI] [PubMed] [Google Scholar]
- Kearns C, Inouye D, Waser NM. 1998. Endangered mutualisms: the conservation of plant–pollinator interactions. Annual Review of Ecology and Systematics 29: 83–112. [Google Scholar]
- Kephart SR. 1983. The partitioning of pollinators among three species of Asclepias. Ecology 64: 120–133. [Google Scholar]
- King C, Ballantyne G, Willmer PG. 2013. Why flower visitation is a poor proxy for pollination: measuring single-visit pollen deposition, with implications for pollination networks and conservation. Methods in Ecology and Evolution 4: 811–818. [Google Scholar]
- van Kleunen M, Fischer M, Johnson SD. 2007. Reproductive assurance through self‐fertilization does not vary with population size in the alien invasive plant Datura stramonium. Oikos 116: 1400–1412. [Google Scholar]
- Knight TM, Steets JA, Vamosi JC et al. . 2005. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution, and Systematics 36: 467–497. [Google Scholar]
- Knight TM, Steets JA, Ashman T-L. 2006. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. American Journal of Botany 93: 271–277. [DOI] [PubMed] [Google Scholar]
- Kremen C, Williams NM, Aizen MA et al. . 2007. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land‐use change. Ecology Letters 10: 299–314. [DOI] [PubMed] [Google Scholar]
- Kunin WE, Shmida A. 1997. Plant reproductive traits as a function of local, regional and global abundance. Conservation Biology 11: 183–192. [Google Scholar]
- Larsen TH, Williams NM, Kremen C. 2005. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecology Letters 8: 538–547. [DOI] [PubMed] [Google Scholar]
- Larson BM, Barrett SC. 2000. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society 69: 503–520. [Google Scholar]
- Latham R. 1993. The serpentine barrens of temperate eastern North America: critical issues in the management of rare species and communities. Bartonia 57: 61–74. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. 1996. SAS system for mixed models. Cary, NC: SAS Institute Inc. [Google Scholar]
- Malo JE, Leirana-Alcocer J, Parra-Tabla V. 2001. Population fragmentation, florivory, and the effects of flower morphology alterations on the pollination success of Myrmecophila tibicinis (Orchidaceae). Biotropica 33: 529–534. [Google Scholar]
- Morales CL, Traveset A. 2008. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Critical Reviews in Plant Sciences 27: 221–238. [Google Scholar]
- Mustajärvi K, Siikamäki P, Rytkönen S, Lammi A. 2001. Consequences of plant population size and density for plant–pollinator interactions and plant performance. Journal of Ecology 89: 80–87. [Google Scholar]
- Ne’eman G, Jürgens A, Newstrom-Lloyd L, Potts SG, Dafni A. 2010. A framework for comparing pollinator performance: effectiveness and efficiency. Biological Reviews 85: 435–451. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals?Oikos 120: 321–326. [Google Scholar]
- Rathcke BJ, Jules ES. 1993. Habitat fragmentation and plant pollinator interactions. Current Science 65: 273–277. [Google Scholar]
- Ruan C-J, Teixeira da Silva JA. 2012. Evolutionary assurance vs. mixed mating. Critical Reviews in Plant Sciences 31: 290–302. [Google Scholar]
- Schoen DJ, Brown AHD. 1991. Whole- and part-flower self-pollination in Glycine clandestina and G. argyrea and the evolution of autogamy. Evolution 45: 1651–1664. [DOI] [PubMed] [Google Scholar]
- Sih A, Baltus M-S. 1987. Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology 68: 1679–1690. [DOI] [PubMed] [Google Scholar]
- Sjödin NE, Bengtsson J, Ekbom B. 2008. The influence of grazing intensity and landscape composition on the diversity and abundance of flower‐visiting insects. Journal of Applied Ecology 45: 763–772. [Google Scholar]
- Spiesman BJ, Inouye BD. 2013. Habitat loss alters the architecture of plant–pollinator interaction networks. Ecology 94: 2688–2696. [DOI] [PubMed] [Google Scholar]
- Spigler RB. 2017. Plasticity of floral longevity and floral display in the self-compatible biennial Sabatia angularis (Gentianaceae): untangling the role of multiple components of pollination. Annals of Botany 119: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigler RB, Chang S-M. 2008. Effects of plant abundance on reproductive success in the biennial Sabatia angularis (Gentianaceae): spatial scale matters. Journal of Ecology 96: 323–333. [Google Scholar]
- Spigler RB, Chang S-M. 2009. Pollen limitation and reproduction varies with population size in experimental populations of Sabatia angularis (Gentianaceae). Botany 87: 330–338. [Google Scholar]
- Spigler RB, Hamrick JL, Chang S-M. 2010. Increased inbreeding but not homozygosity in small populations of Sabatia angularis (Gentianaceae). Plant Systematics and Evolution 284: 131–140. [Google Scholar]
- Spigler RB, Theodorou K, Chang SM. 2017. Inbreeding depression and drift load in small populations at demographic disequilibrium. Evolution 71: 81–94. [DOI] [PubMed] [Google Scholar]
- Steffan-Dewenter I, Kuhn A. 2003. Honeybee foraging in differentially structured landscapes. Proceedings of the Royal Society B: Biological Sciences 270: 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Allen JA, Goulson D. 1998. The influence of relative plant density and floral morphological complexity on the behaviour of bumblebees. Oecologia 117: 543–550. [DOI] [PubMed] [Google Scholar]
- Taki H, Okabe K, Yamaura Y et al. . 2010. Effects of landscape metrics on Apis and non-Apis pollinators and seed set in common buckwheat. Basic and Applied Ecology 11: 594–602. [Google Scholar]
- Theodorou P, Albig K, Radzevičiūtė R et al. . 2017. The structure of flower visitor networks in relation to pollination across an agricultural to urban gradient. Functional Ecology 31: 838–847. [Google Scholar]
- Waal C, Anderson B, Ellis AG. 2015. Relative density and dispersion pattern of two southern African Asteraceae affect fecundity through heterospecific interference and mate availability, not pollinator visitation rate. Journal of Ecology 103: 513–525. [Google Scholar]
- Ward M, Johnson SD, Zalucki MP. 2013. When bigger is not better: intraspecific competition for pollination increases with population size in invasive milkweeds. Oecologia 171: 883–891. [DOI] [PubMed] [Google Scholar]
- Westphal C, Steffan-Dewenter I, Tscharntke T. 2006. Bumblebees experience landscapes at different spatial scales: possible implications for coexistence. Oecologia 149: 289–300. [DOI] [PubMed] [Google Scholar]
- Williams NM, Winfree R. 2013. Local habitat characteristics but not landscape urbanization drive pollinator visitation and native plant pollination in forest remnants. Biological Conservation 160: 10–18. [Google Scholar]
- Wilson P, Thomson JD. 1991. Heterogeneity among floral visitors leads to discordance between removal and deposition of pollen. Ecology 72: 1503–1505. [Google Scholar]
- Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. 2009. A meta‐analysis of bees’ responses to anthropogenic disturbance. Ecology 90: 2068–2076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.