Abstract
Background and Aims
Two main types of triploid limes are produced worldwide. The ‘Tahiti’ lime type (Citrus latifolia) is predominant, while the ‘Tanepao’ type (C. aurantiifolia) is produced to a lesser extent. Both types result from natural interspecific hybridization involving a diploid gamete of C. aurantiifolia ‘Mexican’ lime type (itself a direct interspecific C. micrantha × C. medica hybrid). The meiotic behaviour of a doubled-diploid ‘Mexican’ lime, the interspecific micrantha/medica recombination and the resulting diploid gamete structures were analysed to investigate the possibility that ‘Tahiti’ and ‘Tanepao’ varieties are derived from natural interploid hybridization.
Methods
A population of 85 tetraploid hybrids was established between a doubled-diploid clementine and a doubled-diploid ‘Mexican’ lime and used to infer the genotypes of ‘Mexican’ lime diploid gametes. Meiotic behaviour was studied through combined segregation analysis of 35 simple sequenbce repeat (SSR) and single nucleotide polymorphismn (SNP) markers covering the nine citrus chromosomes and cytogenetic studies. It was supplemented by pollen viability assessment.
Key Results
Pollen viability of the doubled-diploid Mexican lime (64 %) was much higher than that of the diploid. On average, 65 % of the chromosomes paired as bivalents and 31.4 % as tetravalents. Parental heterozygosity restitution ranged from 83 to 99 %. Disomic inheritance with high preferential pairing values was deduced for three chromosomes. Intermediate inheritances, with disomic trend, were found for five chromosomes, and an intermediate inheritance was observed for one chromosome. The average effective interspecific recombination rate was low (1.2 cM Mb–1).
Conclusion
The doubled-diploid ‘Mexican’ lime had predominantly disomic segregation, producing interspecific diploid gamete structures with high C. medica/C. micrantha heterozygosity, compatible with the phylogenomic structures of triploid C. latifolia and C. aurantiifolia varieties. This disomic trend limits effective interspecific recombination and diversity of the diploid gamete population. Interploid reconstruction breeding using doubled-diploid lime as one parent is a promising approach for triploid lime diversification.
Keywords: Triploid limes, Citrus latifolia, Citrus aurantiifolia, phylogeny, tetraploid, meiosis, disomic inheritance, polyploidy breeding, cytogenetic, single nucleotide polymorphism, simple sequence repeats
INTRODUCTION
Diploidy is the general rule in citrus with a basic chromosome number of nine (x = 9) (Krug, 1943) and an estimated genome size of approx. 367 Mb (Terol et al., 2008). Only a few triploid and tetraploid genotypes have been found in citrus germplasm (Longley, 1925; Lee, 1988). Despite this scarcity of polyploid germplasm, it appears that polyploidization events are relatively frequent in citrus seedlings. Polyploidization is a major mechanism of angiosperm evolution (Soltis and Soltis, 1993; Wendel and Doyle, 2005), and many authors consider that most polyploids arise from unreduced (2n) gametes (Bretagnolle and Thompson, 1995; Ramsey and Schemske, 1998, 2002). However, in citrus, doubled-diploid plants were observed in seedlings of diploid apomictic genotypes (Lapin, 1937; Russo and Torrisi, 1951; Cameron and Frost, 1968). They arise from spontaneous duplication of chromosomes in nucellar cells, and their frequency depends on the genotypes and environment (Cameron and Frost, 1968; Aleza et al., 2011). The tetraploid ‘Giant Key’ lime originated from this mechanism (Curk et al., 2016). Unreduced female and male gametes have also been described in citrus (Esen and Soost, 1971; Ollitrault et al., 2008; Cuenca et al., 2015; Rouiss et al., 2017a, b), and they can lead to the creation of triploid and tetraploid hybrids. Various mechanisms can produce 2n gametes in citrus. Second division restitution (SDR) is predominant for mandarin 2n megagametophytes (Esen et al., 1979; Cuenca et al., 2011, 2015; Aleza et al., 2015), while first meiotic restitution (FDR) was described as the major mechanism for the production of 2n pollen in a clementine × sweet orange hybrid (Rouiss et al., 2017a) and a secondary mechanism in lemon megagametophytes (Rouiss et al., 2017b).
Lime is the only horticultural group of the Citrus genus that includes triploid and tetraploid natural germplasm in addition to diploid germplasm. Limes are cultivated under sub-tropical and inter-tropical climates, and lime consumption has increased dramatically since the 1980s (Duportal et al., 2013). Interestingly, the triploid ‘Tahiti’ lime is one of the least susceptible citrus varieties with regard to the main threat to citrus production in tropical and sub-tropical areas, Huanglongbing disease (HLB) caused by the phloem-limited bacterium Candidatus liberibacter spp. (Folimonova et al., 2009). However, lime production is founded on a very narrow genetic base including a few diploid and triploid cultivars, and varietal diversification is needed to promote sustainable lime production. At the triploid level, the seedless ‘Tahiti’ lime type is predominantly produced for the export market. The other major triploid variety, i.e. the ‘Tanepao’ lime type, produces seedy fruits and has only limited areas of production.
Cultivated lime varieties are based on complex interspecific genomic structures, like most cultivated citrus. Citrus is a large genus that includes several major cultivated species. It is believed to be native to South-east Asia (Webber et al., 1967), and it was probably first cultivated as a fruit crop >4000 years ago (Legge, 1865). Molecular markers and genomic studies have identified four taxa, i.e. Citrus reticulata Blanco, C. maxima (Burm.) Merr., C. medica L. and C. micrantha Wester, as the ancestors of all cultivated Citrus species (Nicolosi et al., 2000; Barkley et al., 2006; Ollitrault et al., 2012a; Garcia-Lor et al., 2013a; Curk et al., 2016). Differentiation between these ancestral taxa occurred through allopatric evolution, and then the so-called secondary species [C. sinensis (L.) Osb, sweet oranges; C. aurantium L., sour oranges; C. paradisi, grapefruits Macf; C. limon (L.) Burm., lemons] and particularly limes [C. aurantiifolia (Cristm.) Swing. and C. latifolia Tan.] were the result of reticulate evolution with a limited number of interspecific meiosis events.
While many citrus horticultural groups are diploid and result only from C. reticulata and C. maxima gene pools (Nicolosi et al., 2000; Barkley et al., 2006; Ollitrault et al., 2012a; Garcia-Lor et al., 2013a), the genomic structure of limes appears more complex. Indeed, it involves the four ancestral taxa (Curk et al., 2016). Various molecular analyses (Nicolosi et al., 2000; Ollitrault et al., 2012a; Garcia-Lor et al., 2013a; Curk et al., 2016) and cytogenetic studies (Carvalho et al., 2005) revealed that diploid ‘Mexican’ lime (C. aurantiifolia) results from direct natural hybridization between C. micrantha as female parent and C. medica as male parent. The tetraploid ‘Giant Key’ lime was selected by H. C. Barrett in Florida (US Horticultural Research Laboratory, Orlando) in a seedling of the diploid ‘Key’ lime classified as C. aurantiifolia by Tanaka (1961).
Recently, Curk et al. (2016) demonstrated the contribution of the four ancestral taxa to the C. latifolia triploid variety (‘Tahiti’ lime type) genome and proposed that it resulted from fertilization of a haploid ovule of C. limon by a diploid gamete of C. aurantiifolia. Lemon is a complex genome resulting from the hybridization of a citron (C. medica) and sour orange (a C. maxima × C. reticulata direct hybrid). The same authors proposed that C. aurantiifolia triploid varieties (‘Tanepao’ lime like) with only the contribution of C. medica and C. micrantha, probably resulted from an interspecific backcross with a diploid ovule of C. aurantiifolia (C. micrantha × C. medica) fertilized by C. medica. The actual phenotypic diversity around the ‘Tahiti’ and ‘Tanepao’ lime types should be the result of asexual variations (mutations or somaclonal variations). Today there is no evidence on the polyploidization mechanisms (interploid hybridization or 2n gametes) that produced triploid C. latifolia and C. aurantiifolia limes.
The genetic structure of diploid gamete populations and particularly the parental heterozygosity restitution (PHR) are driven by the diploid gamete origin. Therefore, molecular marker studies of diploid gametes may cast light on their origin. In the case of 2n gametes, PHR is a function of the genetic distance to the centromere (Zhao and Speed, 1998; Cuenca et al., 2015). In the centromeric area, PHR is null and total for SDR and FDR, respectively, increasing and decreasing with the genetic distance to the centromere. There are two extreme models for diploid gametes produced by tetraploid plants, i.e. disomic in allotetraploids and tetrasomic in autotetraploids (Stebbins, 1947; Stift et al., 2008; Sybenga, 2012). In allotetraploids resulting from the merging of two species’ genomes, there are two sets of homologous chromosomes. Each chromosome pairs only with its homologous form during meiosis (Sybenga, 2012), and only bivalents are formed (Stebbins, 1947). This results in disomic inheritance, with 100 % of the interspecific heterozygosity transmitted by each gamete (Stift et al., 2008). In autotetraploids, the presence of four homologous chromosomes instead of two results in equal opportunities to pair at meiosis, leading to multivalent formation and tetrasomic inheritance (Jackson and Jackson, 1996; Sybenga, 1996). For doubled diploids, it hypothetically leads to 66 % restitution of the heterozygosity of the diploid that led to the tetraploid (Sanford, 1983; Aleza et al., 2016). Allo- and autotetraploids (with disomic and tetrasomic inheritance, respectively) are the extremes of the range. In cases where parents are divergent but have retained enough homology to prevent exclusive preferential pairing, inheritance patterns intermediate between di- and tetrasomic can be expected (Stebbins, 1947; Sybenga, 1996; Stift et al., 2008; Jeridi et al., 2012). Many polyploid taxa display a combination of autopolyploid and allopolyploid pairing behaviour (Jackson and Jackson, 1996; Allendorf and Danzmann, 1997; Fjellstrom, et al., 2001), and several studies have revealed inheritance patterns intermediate between disomic and tetrasomic (Danzmann and Bogart, 1983; Hickok, 1978; Marsden et al., 1987; Stift et al., 2008). Stift et al. (2008) developed a likelihood-based approach to evaluate whether disomic, intermediate or tetrasomic inheritances best fitted the segregation of genetic markers and to estimate preferential pairing and double reduction (DR) rates. DR can occur for tetravalents and increases the homozygosity of diploid gametes (Sybenga, 1995; Ronfort et al., 1998; Stift et al., 2008; Aleza et al., 2016). This method was simplified for doubled diploids by Aleza et al. (2016).
In the present study, we analyzed the preferential chromosome pairing and inheritance of the interspecific (C. medica/C. micrantha) doubled-diploid ‘Mexican’ lime. This was performed by combining a meiotic cytogenetic study and the analysis of single nucleotide polymorphism (SNP) and simple sequence repeat (SSR) marker segregation. The interspecific recombination and interspecific structures of diploid ‘Mexican’ lime gametes were then analysed from the genetic marker data, and their compatibility with C. aurantiifolia and C. latifolia triploid lime phylogenomic structure was evaluated. The implications for lime breeding programmes are discussed.
MATERIALS AND METHODS
Plant materials
Sexual hybridization between doubled-diploid ‘Clemenules’ clementine (C. clementina Hort. Ex Tan.) as female parent and doubled-diploid ‘Mexican’ lime as male parent (Cl4x × ML4x hybridization) was performed at IVIA (Moncada, Spain) to obtain tetraploid hybrids (named ClemMex). The ClemMex hybrids were used to study the segregation model of doubled-diploid ‘Mexican’ lime. Doubled-diploid ‘Clemenules’ clementine was obtained by colchicine treatment of shoot-tip grafting in vitro (Aleza et al., 2009), whereas tetraploid ‘Mexican’ lime was identified by flow cytometry in seedlings of diploid ‘Mexican’ lime. Molecular marker analysis proved that it was a doubled diploid (Aleza et al., 2011). The ploidy level was verified by flow cytometry, as described in Aleza et al. (2010). Eighty-five tetraploid hybrids were obtained.
Pollen viability
Pollen viability was estimated using aceto-carmine colorimetric tests (Stanley and Linskens, 1974). Pollen viability was scored according to the staining level. Pollen with a bold red colour is viable and that which is colourless is unviable. The pollen viability rate was determined as the ratio of the number of viable grains to the total grain number.
Meiotic chromosome preparation
Fifty-one pollen mother cells (PMCs) of different anthers were observed and analysed for this work. Cytogenetic protocols that have been described for meiotic chromosome pairing for the Musa genus (Shepherd, 1999) were used. Flower buds, with diameters <5 mm, were collected from the IVIA-490 tetraploid ‘Mexican’ lime accession of the Citrus Germplasm Bank of pathogen-free plants (Navarro et al., 2002). For each bud, two opposite anthers were desiccated, coloured by aceto-carmine and observed. The buds with the two anthers in the first metaphase stage were conserved for further analysis. They were fixed in a mixture of absolute ethanol:chloroform:acetic acid (6:3:1) for 24 h. Later they were transferred to 70 % alcohol for storage. The next steps of the protocol were done directly on the slides. The anthers were dissected and stained in a drop of 1 % carmine in 45 % acetic acid. A cover glass was placed on top, and the slides were warmed to well short of boiling point. The anthers were lightly pressed under the cover glass. Finally the cover glass edges were sealed with nail varnish to avoid drying out of the smear.
Genotyping of progeny using SSR and SNP markers
Genomic DNA of the hybrids and their parents was isolated using the Plant DNAeasy kit from Qiagen Inc. (Valencia, CA, USA), according to the manufacturer’s protocol. For SSRs, PCR amplifications were performed using a Thermocycle rep gradient S (Eppendorf®) in 10 µL final volume containing 0.8 U of Taq DNA polymerase (Fermentas®), 2 ng µL–1 citrus DNA, 0.2 mm wellRED (Sigma®) dye-labelled forward primer, 0.2 mm non dye-labelled reverse primer, 0.2 mm of each dNTP, 10× PCR buffer and 1.5 mm MgCl2. The PCR protocol was as follows: denaturation at 94 °C for 5 min followed by 40 repeats of 30 s at 94 °C, 1 min at 50 or 55 °C, 45 s at 72 °C and a final 4 min elongation step at 72 °C. Capillary electrophoresis was carried out using a CEQ™ 8000 Genetic Analysis System (Beckman Coulter Inc.). PCR products were initially denatured at 90 °C for 2 min, injected at 2 kV for 30 s and subsequently separated at 6 kV for 35 min. Alleles were sized, based on a DNA size standard (400 bp). GenomeLab™ GeXP v.10.0 genetic analysis software was used for data collection. Allele dosage was calculated using the MAC-PR (microsatellite DNA allele counting-peak ratio) method (Esselink et al., 2004), validated in citrus by Cuenca et al. (2011).
Progeny were also genotyped with SNP markers using KASPar technology. The KASPar™ Genotyping System is a competitive, allele-specific dual Förster resonance energy transfer (FRET)-based assay for SNP genotyping. Primers were directly designed by LGC Genomics based on the SNP locus flanking sequence. Detailed explanation of the specific conditions and reagents used the in KASPar technique can be found in Cuppen (2007). Identification of allele dosages in heterozygous tetraploid hybrids was carried out on the basis of relative allele signals, as described by Cuenca et al. (2013).
Control of the hybrid origin of tetraploid plants and inference of the diploid gamete genotype
The hybrid origin was confirmed using two SSRs (mCrCIR07F11 and MEST001) with total differentiation between the parents (A1A2 × A3A4).
To study the genetic structure of the diploid gametes produced from the doubled-diploid ‘Mexican’ lime, the male and female parents and 85 hybrids were genotyped using a total of 35 molecular markers (27 SSRs and eight SNPs) that were heterozygous for ‘Mexican’ lime and displayed polymorphism between ‘Mexican’ lime and ‘Clemenules’ clementine (Table 1). These markers were described by Kijas et al. (1997), Ahmad et al. (2003), Luro et al. (2008), Froelicher et al. (2008), Ollitrault et al. (2010), Cuenca et al. (2011), Garcia-Lor et al. (2013b), Ollitrault et al. (2012a, b) and Curk et al. (2015). They are distributed across all linkage groups (LGs) of the clementine genetic map (Ollitrault et al., 2012b), with a minimum of three molecular markers in LG07 and a maximum of five in LG06 and LG09. Distances of the markers to the centromere were estimated from the clementine genetic map (Ollitrault et al., 2012b) and the estimated centromere genetic location (Aleza et al., 2015). When the markers were not on the clementine genetic map, their genetic positions were inferred from their physical position and local correlations between the physical and genetic maps. Marker 6P7496245 was the closest to a centromere (0.10 cM from the LG06 centromere), while marker MEST131 was the furthest (88.74 cM from the LG03 centromere). Overall, 18 markers were considered as centromeric markers (15 SSRs and three SNPs), and the rest were either telomeric or intermediate markers. For ‘Mexican’ lime, the alleles inherited from C. medica and C. micrantha ancestors are respectively in the first and second positions in Table 1.
Table 1.
Molecular markers used to study the genetic structure of diploid gametes produced from tetraploid ‘Mexican’ lime, with their GenBank accession number, genetic position, noted alleles and bibliographical reference
| Locus | GenBank accesion | LG | Geneticposition (cM) | Distance to centromere | Alleles* | Bibliographic reference | |
|---|---|---|---|---|---|---|---|
| Clementine | ‘Mexican’ lime† | ||||||
| 1P199494 | Ciclev10010680m.g | 1 | 1.00 | 59.66 | C–C | T–C | Curk et al. (2015) |
| CIBE5720 | ET082224 | 1 | 58.45 | 2.21 | 325–337 | 320–308 | Ollitrault et al. (2010) |
| MEST001 | DY262452 | 1 | 70.61 | 9.95 | 170–174 | 186–190 | Luro et al. (2008) |
| JK-taa15 | none | 1 | 119.73 | 59.07 | 188–192 | 164–168 | Kijas et al. (1997) |
| mCrCIR02D09 | FR677569 | 2 | 11.37 | 45.50 | 230–238 | 233–248 | Cuenca et al. (2011) |
| 2P25198777 | Ciclev10015267m.g | 2 | 67.6 | 10.73 | A–A | G–A | Curk et al. (2015) |
| JK-TAA41 | None | 2 | 131.86 | 74.99 | 147–154 | 132–170 | Kijas et al. (1997) |
| mCrCIR04F12 | FR692369 | 3 | 29.66 | 60.93 | 261–263 | 263–259 | Ollitrault et al. (2012) |
| CIBE1644 | ET097780 | 3 | 70.23 | 20.36 | 346–364 | 350–368 | Ollitrault et al. (2010) |
| JI-TC01 | CK934237 | 3 | 109.68 | 19.09 | 333–347 | 349–335 | (Ollitrault et al., 2012b) |
| MEST131 | DY276912 | 3 | 179.4 | 88.81 | 141–147 | 141–124 | Garcia-Lor et al. (2012a) |
| MEST070 | DY268779 | 4 | 4.25 | 11.89 | 218–220 | 224–193 | Unpul. res. |
| CID6458 | ET086604 | 4 | 15.88 | 0.259 | 385–397 | 397–388 | Ollitrault et al. (2012a) |
| mCrCIR07D06 | FR677581 | 4 | 16.33 | 0.19 | 165–188 | 172–167 | Cuenca et al. (2011) |
| mCrCIR03G05 | FR677578 | 4 | 75.06 | 58.92 | 226–228 | 219–215 | Cuenca et al. (2011) |
| mCrCIR07G11 | AM489751 | 5 | 20.2 | 2.92 | 202–210 | 208–145 | Froelicher et al. (2008) |
| cms30 | None | 5 | 36.84 | 13.72 | 152–156 | 150–154 | Ahmad et al. (2003) |
| mCrCIR01F08a | AM489737 | 5 | 54 | 30.88 | 118–118 | 131–128 | Froelicher et al. (2008) |
| mCrCIR04H12 | FR692371 | 6 | 0 | 6.4 | 160–160 | 166–178 | Ollitrault et al. (2012) |
| 6P7496245 | Ciclev10013603m.g | 6 | 6.3 | 0.1 | C–C | G–C | Curk et al. (2015) |
| MEST488 | DY297637 | 6 | 68.48 | 62.08 | 126–130 | 120–128 | Garcia-Lor et al. (2012a) |
| PSY-C461 | AB037975 | 6 | 69.72 | 63.32 | A–A | T–A | Ollitrault et al. (2012b) |
| AOC-C593 | DY293375 | 6 | 89.88 | 83.48 | T–T | A–T | Ollitrault et al. (2012b) |
| MEST107 | DY274062 | 7 | 8.899 | 87.531 | 175–183 | 175–181 | Garcia-Lor et al. (2012a) |
| DXS-C545 | Ciclev10024949m.g | 7 | 40 | 56.43 | G–G | C–G | Garcia-Lor et al. (2013b) |
| mCrCIR03B07 | FR677573 | 7 | 83.39 | 13.04 | 263–265- | 273–277 | Cuenca et al. (2011) |
| mCrCIR01F04a | AM489736 | 8 | 5.92 | 48.29 | 186–202 | 202–171 | Froelicher et al. (2008) |
| CiBE0214 | ET088913 | 8 | 40.41 | 13.8 | 313–324 | 316–307 | Ollitrault et al. (2010) |
| 8P18684429 | Ciclev10028449m.g | 8 | 55 | 0.79 | C–C | T–C | Curk et al. (2015) |
| mCrCIR02A09 | FR677568 | 8 | 98.63 | 44.42 | 160–163 | 163–178 | Cuenca et al. (2011) |
| Ci02B07 | AJ567403 | 9 | 0 | 52.16 | 163–165 | 165–154 | Froelicher et al. (2008) |
| mCrCIR07F11 | FR677567 | 9 | 49.57 | 2.59 | 152–160 | 168–158 | Kamiri et al. (2011) |
| JI-TCT01 | CV704385 | 9 | 52.8 | 0.64 | 148–154 | 145–148 | Unpubl. res |
| Ci08C05 | AJ567415 | 9 | 55.14 | 2.98 | 154–175 | 135–152 | Froelicher et al. (2008) |
| 9P31143176 | Ciclev10006644m.g | 9 | 88 | 35.84 | A–A | A–G | Curk et al. (2015) |
*The numbers indicate the size of alleles in nucleotides for SSR markers, and letters correspond to SNP marker alleles.
†The first allele is the one inherited from C. medica and the second one from C. micrantha.
For markers with total allelic differentiation between parents (A1A1A2A2 × A3A3A4A4 and A1A1A1A1 × A2A2A3A3), the genotypes of the diploid gametes from ‘Mexican’ lime were inferred directly from the presence/absence of the specific alleles of the Mexican lime in the hybrid. When the male and female genitor shared one allele (A1A1A1A1 × A1A1A2A2 and A1A1A2A2 × A2A2A3A3), the diploid male gamete structure was inferred from the estimated allele dosage in the tetraploid hybrid. For markers with an A1A1A1A1 × A1A1A2A2 configuration, A1A1, A1A2 and A2A2 male gametes were inferred from A1A1A1A1, A1A1A1A2 and A1A1A2A2 hybrid genotypes, respectively. For markers with an A1A1A2A2 × A2A2A3A3 allelic configuration, the nine potential combinations of the two parental diploid gametes produced nine tetraploid hybrid genotypes totally differentiated by allele dosages: A1A1A2A2, A1A1A2A3, A1A1A3A3, A1A2A2A2, A1A2A2A3, A1A2A3A3, A2A2A2A2, A2A2A2A3 and A2A2A3A3. The male diploid gametes inferred from these tetraploid hybrid genotypes were, respectively, A2A2, A2A3, A3A3, A2A2, A2A3, A3A3 A2A2, A2A3 and A3A3.
Statistical analysis of preferential pairing
Stift et al. (2008) proposed a segregation model to interpret the inheritance model in allotetraploid citrus. Aleza et al. (2016) simplified it for the doubled diploid, considering that the expected gamete frequencies depend only on the ‘tetrasomic’ parameter (τ) corresponding to the proportion of gametes formed by random meiotic chromosome associations (random bivalent or tetravalent pairing), while taking values from zero (full disomic) to one (full tetrasomic). τ was estimated by a maximum likelihood approach, as proposed by Aleza et al. (2016), from the analysis of the closest marker to the centromere for each chromosome. Having a τ value estimated for each chromosome, the preferential pairing (PP) was calculated as 1 – τ. Parental heterozygosity restitution (PHR) was calculated for each marker as the percentage of inferred heterozygous diploid gametes.
Genetic mapping
With the aim of studying the interspecific recombination (C. medica/C. micrantha) at the tetraploid level, we anchored LGs inferred from ‘Mexican’ lime diploid gametes on the reference clementine genome sequence (https://phytozome.jgi.doe.gov/pz/portal.html) and compared it with genetic maps of clementine at the diploid and tetraploid levels, also anchored in the reference sequence. The diploid clementine genetic map (Ollitrault et al., 2012b) was used as the reference map for the Citrus genome reference sequence assembly (Wu et al., 2014). For the tetraploid clementine map, we used the 57 molecular marker segregation analysis published by Aleza et al. (2016). For tetraploid ‘Mexican’ lime, 34 out of the 35 markers analysed for this study were used. Indeed, SNP marker 8P18684429 showed no segregation, with 100 % heterozygosity. Each tetraploid progeny was analysed with Tetraploid Map Software (Hackett et al., 2007) using the default parameters to establish the different map distances in centiMorgans. Then, the genetic and physical maps were drawn using the MapChart program (Voorrips, 2002).
RESULTS
Pollen viability and cytogenetic analysis
A total of 1179 pollen grains were monitored and 64 % pollen viability was recorded (Fig. 1). For the cytogenetic observations, two-thirds of the chromosomes were implicated in bivalent pairing (Fig. 2A, B; Table 2). The majority of the other chromosomes were involved in tetravalent pairing. Both closed and chain tetravalents were distinguishable. We found that 25.05 and 6.32 %, respectively, of the chromosomes were in chain tetravalent and closed tetravalent configurations (Fig. 2C, D). The average number of bivalent and tetravalent configurations per PMC was 11.71 and 2.82, respectively, while that of monovalent and trivalent configurations per PMC was very low (mean 0.41 and 0.29, respectively, per PMC).
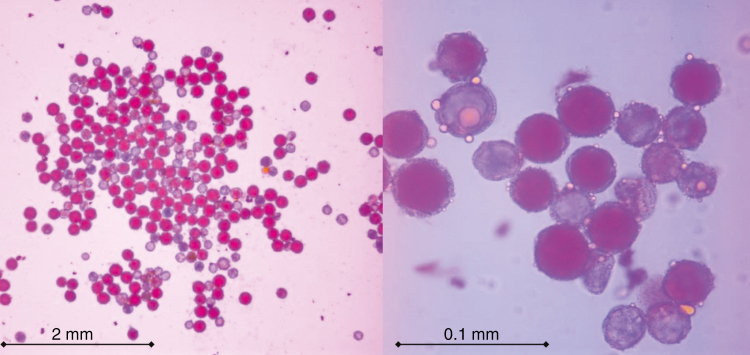
Fig. 1.
Pollen grains of tetraploid ‘Mexican’ lime stained with aceto-carmine. Bold red colour indicates viable and colourless (blue) non-viable.
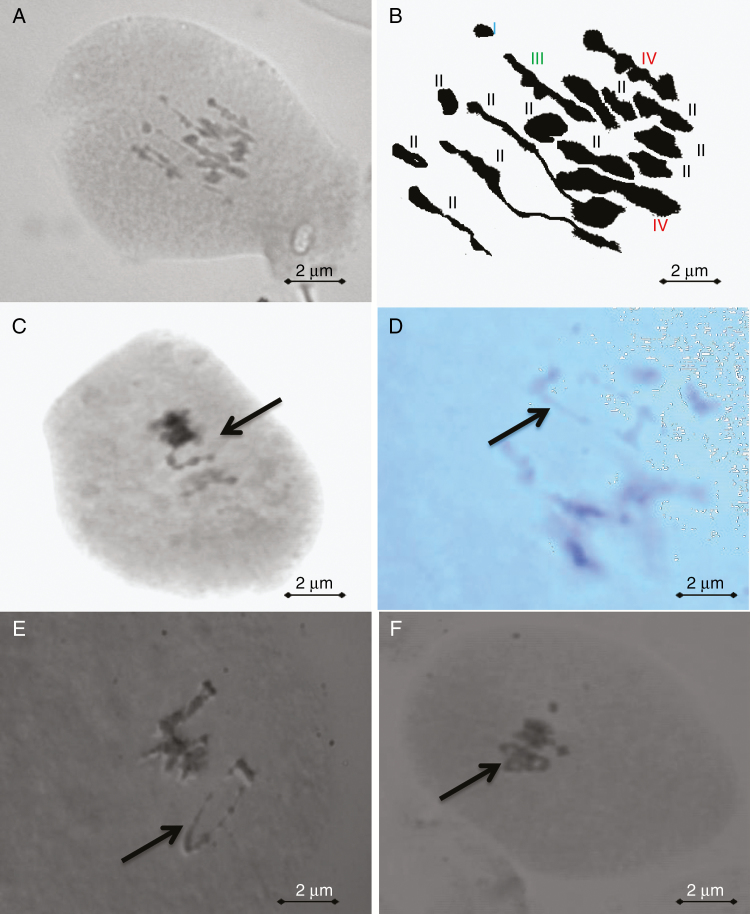
Fig. 2.
Chromosome pairing configuration. (A) Pollen mother cells (PMCs) of tetraploid ‘Mexican’ lime. (B) Schematic interpretation of (A) one univalent (blue) + 12 bivalents (black) + one trivalent (green) + two tetravalents (red). (C) Open tetravalent (arrow). (D–F) Closed (ring) tetravalents (arrows).
Table 2.
Chromosome configuration at meiosis in pollen mother cells (PMCs) of tetraploid ‘Mexican’ lime
| Univalents | Bivalents | Trivalents | Tetravalents | ||
|---|---|---|---|---|---|
| Ring | Chain | ||||
| n | 21 | 597 | 15 | 29 | 115 |
| % IC | 1.14 | 65.03 | 2.45 | 6.32 | 25.05 |
| C/PMC | 0.41 | 11.71 | 0.29 | 0.57 | 2.25 |
n, number of association structures; % IC, percentage of involved chromosomes; C/PMC, average number of configurations per PMC.
The analysis of configurations at the individual PMC level (Supplementary Data Fig. S1) revealed at least eight bivalents and two tetravalents per PMC. Twelve bivalents per PMC was the most frequent situation (19 PMCs), with a maximum of 14 bivalents observed in 13 PMCs. Up to four tetravalents per PMC were found in 14 PMCs. Fifteen PMCs had at least one monovalent and one trivalent.
Configurations with one trivalent and one monovalent may be interpreted as a broken tetravalent or incomplete tetravalent in which one chromosome is disjoined as monovalent by the absence of chiasmata on its two arms (Sybanga, 1975; Jeredi et al., 2012). Under this hypothesis, three configurations were predominant: 12 bivalents/three tetravalents (18 PMCs; 35 %), 14 bivalents/two tetravalents (13 PMCs; 25 %) and ten bivalents/four tetravalents (13 PMCs; 25 %).
Molecular marker analysis
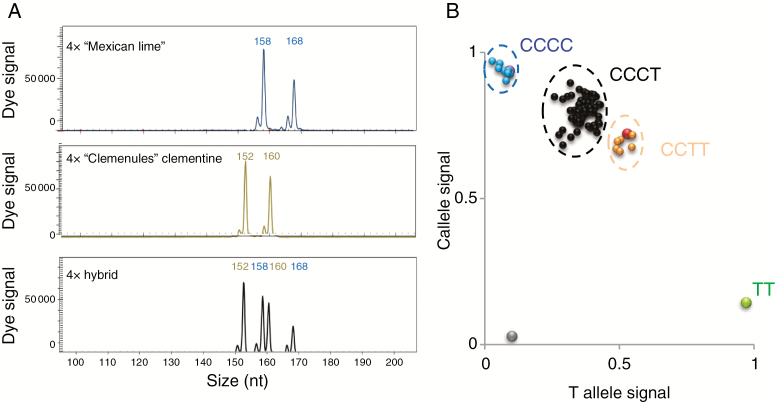
Eighty-five plants were obtained from the tetraploid clementine × tetraploid ‘Mexican’ lime hybridization. Flow cytometry analysis demonstrated that all were tetraploids. They were analysed with two SSR markers without a common allele between the two parents, i.e. mCrCIR07F11 and MEST001, to study their genetic origin. For each marker, at least one specific allele of ‘Mexican’ lime was observed in all plants. Moreover, in many plants, the two specific alleles of ‘Mexican’ lime were observed in combination with clementine alleles (Fig. 3). The Cl4x × ML4x hybrid origin of all analysed plants was thus confirmed.
Fig. 3.
Illustration of SSR and SNP genotyping for ‘Clemenules’ clementine × ‘Mexican’ lime tetraploid hybrids and their parents. (A) Electropherogram of a tetraploid hybrid recovered from hybridization between tetraploid ‘Clemenules ‘clementine and tetraploid ‘Mexican’ lime, and its parents with mCrCIR07F11 SSR marker. nt, nucleotides. Top, tetraploid ‘Mexican’ lime; middle, tetraploid clementine; bottom, tetraploid hybrid. (B) Plot of C and T allele signals of the 1P199494 SNP marker representing tetraploid hybrids from the same hybridization. Letters indicate the allelic configuration for each genotype. Blue and purple dots, CCCC tetraploid hybrids and clementine, respectively; black dots, CCCT tetraploid hybrids; orange and red dots, CCTT tetraploid hybrids and tetraploid ‘Mexican’ lime, respectively; green dot, TT diploid control; grey dot, water control.
These 85 hybrids were analysed with 35 co-dominant markers. The genotypes of the ‘Mexican’ lime diploid gamete were inferred along with their phylogenomic structure [C. micrantha or C. medica homozygosity or interspecific heterozygosity (Supplementary Data Table S1)]. On average, for all loci, 90.2 % PHR was observed (Table 3; Table S1) and it ranged from 82.7 % for LG05 to 95.6 % for LG08 (Table 3). At the individual marker level (Fig. 4A; Supplementary Data Table S1), PHR ranged from 74.1 to 100 % for mCrCIR04F12 (LG03) and 8P18684429 (LG08) markers, respectively.
Table 3.
Interspecific structures of ‘Mexican’ lime diploid gametes for the nine LGs
| PHR | FH | Fmed | Fmic | Mixed | |
|---|---|---|---|---|---|
| LG1 | 90.3 | 76.5 | 2.4 | 1.2 | 20.0 |
| LG2 | 95.3 | 90.6 | 0.0 | 0.0 | 9.4 |
| LG3 | 85.0 | 57.6 | 1.2 | 0.0 | 41.2 |
| LG4 | 89.7 | 77.6 | 1.2 | 1.2 | 20.0 |
| LG5 | 82.7 | 72.9 | 2.4 | 4.7 | 20.0 |
| LG6 | 88.0 | 80.0 | 0.0 | 3.5 | 16.5 |
| LG7 | 92.9 | 82.4 | 0.0 | 0.0 | 17.6 |
| LG8 | 95.6 | 82.4 | 0.0 | 0.0 | 17.6 |
| LG9 | 92.0 | 80.0 | 0.0 | 0.0 | 20.0 |
| Total | 90.2 | 77.8 | 0.8 | 1.2 | 20.3 |
PHR, parental heterozygosity restitution; FH, percentage of fully heterozygous gametes for the LG; Fmed, percentage of fully C. medica homozygous gametes for the LG; Fmic, percentage of fully C. micrantha homozygous gametes for the LG; Mixed, percentage of gametes with mixed heterozygosity and homozygosity for the LG.
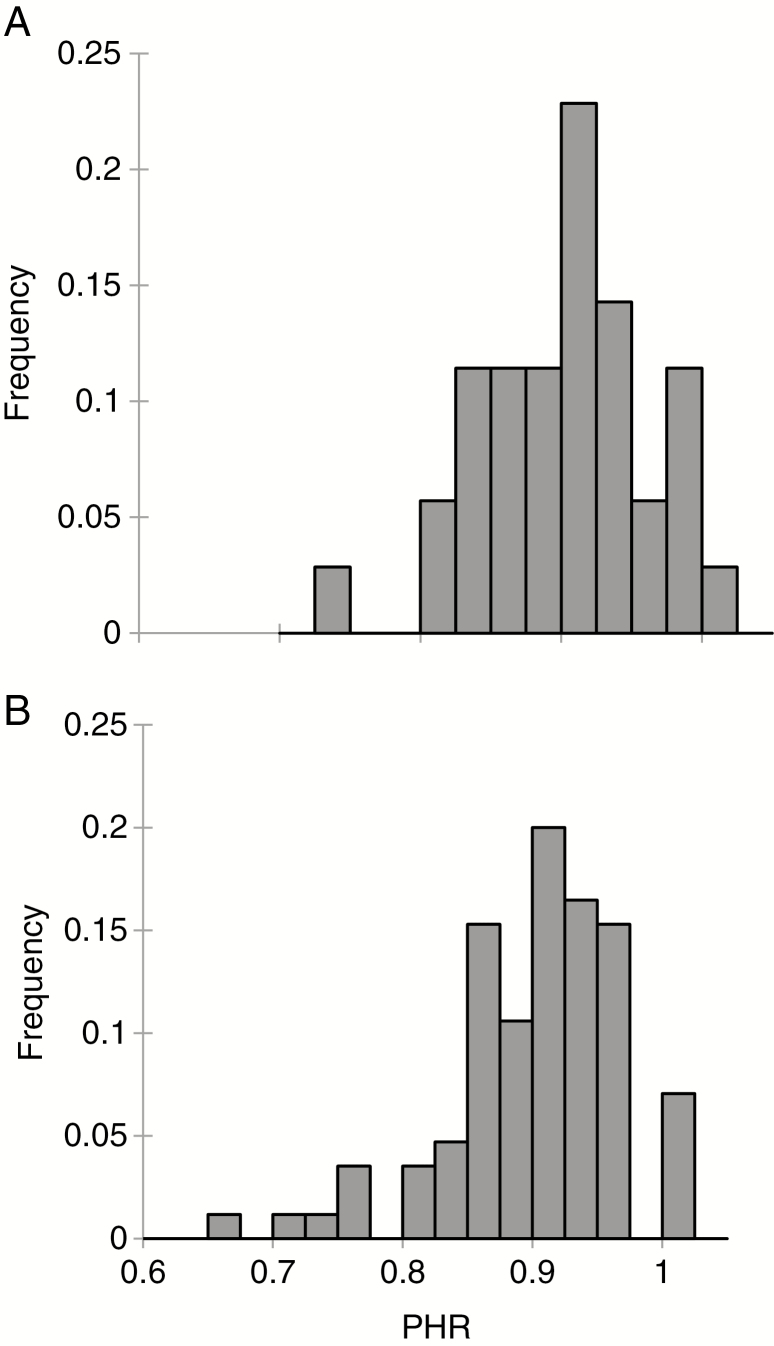
Fig. 4.
Distribution of PHR values among markers (A) and diploid gametes (B) obtained from tetraploid ‘Mexican’ lime.
A slight decrease in PHR was observed in most LGs from centromeric to telomeric markers. For instance, for LG01, the PHR values were 92.9 and 94.1 % for the two centromeric SSR markers CIBE5720 and MEST001, respectively, and they decreased to 85.9 and 88.2 % for the telomeric markers 1P199494 and JK-TAA15, respectively. This reduction could be associated with DR in the case of tetravalent associations.
At the individual gamete level, PHR displayed a unimodal distribution and ranged from 0.66 to 1; six gametes were fully heterozygous and 58.8 % of the diploid gametes displayed a PHR >90 % (Fig. 4B; Supplementary Data Table S1).
On average, for all loci and gametes, the percentages of C. micrantha and C. medica homozygosity were 4.7 and 5.1 %, respectively. A more accurate analysis of the data at the gamete level (Table 3; Supplementary Data Table S1) revealed that the majority (77.8 %) of individual LGs of the different hybrids were fully heterozygous. Conversely, only nine (1.2 %) and six (0.8 %) fully homozygous LGs for C. micrantha and C. medica, respectively, were observed. A total of 20.26% of the individual LGs displayed a mixed structure with homozygosity and heterozygosity, and all nine citrus LGs were affected. Homozygous and mixed LGs testified for pairing of C. micrantha and C. medica chromosomes, and mixed LGs indicated interspecific recombination. The number of LGs with homozygosity for both markers flanking the centromere was low (5.23 %) and varied between chromosomes, ranging from 12.9 % in LG05 to <0.01 % for LG03, LG07 and LG08.
Estimation of preferential association frequency
The values of τ and PP were estimated from the likelihood models (Table 4) for each LG. Disomic inheritance with high preferential pairing values was observed for LG07 and LG08 (PP = 0.965) and for LG02 (PP = 0.86). A PP trend was found for LG01, LG03, LG04, LG06 and LG09 (0.68 < PP < 0.79). For LG05, the intermediate model fitted better than the disomic or tetrasomic models (PP = 0.50).
Table 4.
Estimation of τ and PP from centromeric loci of the nine LGs of tetraploid ‘Mexican’ lime
| LG | Locus | DC | Mic/Mic | Med/Mic | Med/Med | τ | PP |
|---|---|---|---|---|---|---|---|
| 1 | Cibe5720 | 2.21 | 1 | 79 | 5 | 0.210 | 0.790 |
| 2 | 2P25198777 | 10.73 | 1 | 81 | 3 | 0.140 | 0.860 |
| 3 | JI-TC01 | 19.09 | 1 | 78 | 6 | 0.245 | 0.755 |
| 4 | mCrCIR07D06 | 0.19 | 3 | 79 | 3 | 0.210 | 0.790 |
| 5 | mCrCIR07G11 | 2.93 | 7 | 71 | 7 | 0.495 | 0.505 |
| 6 | 6P7496245 | 0.10 | 4 | 76 | 5 | 0.320 | 0.680 |
| 7 | mCrCIR03B07 | 13.04 | 0 | 84 | 1 | 0.035 | 0.965 |
| 8 | CiBE0214 | 13.80 | 0 | 84 | 1 | 0.035 | 0.965 |
| 9 | JI-TCT01 | 0.64 | 0 | 78 | 7 | 0.245 | 0.755 |
LG, linkage group; DC, distance to the centromere [from reference genetic map data (Ollitrault et al. 2012b) and location of centromere (Aleza et al. 2015)]; Med/Med, Med/Mic and Mic/Mic, number of individuals with such allelic configuration; τ, tetrasomic rate; PP, preferential pairing.
Genetic mapping and recombination rate analysis
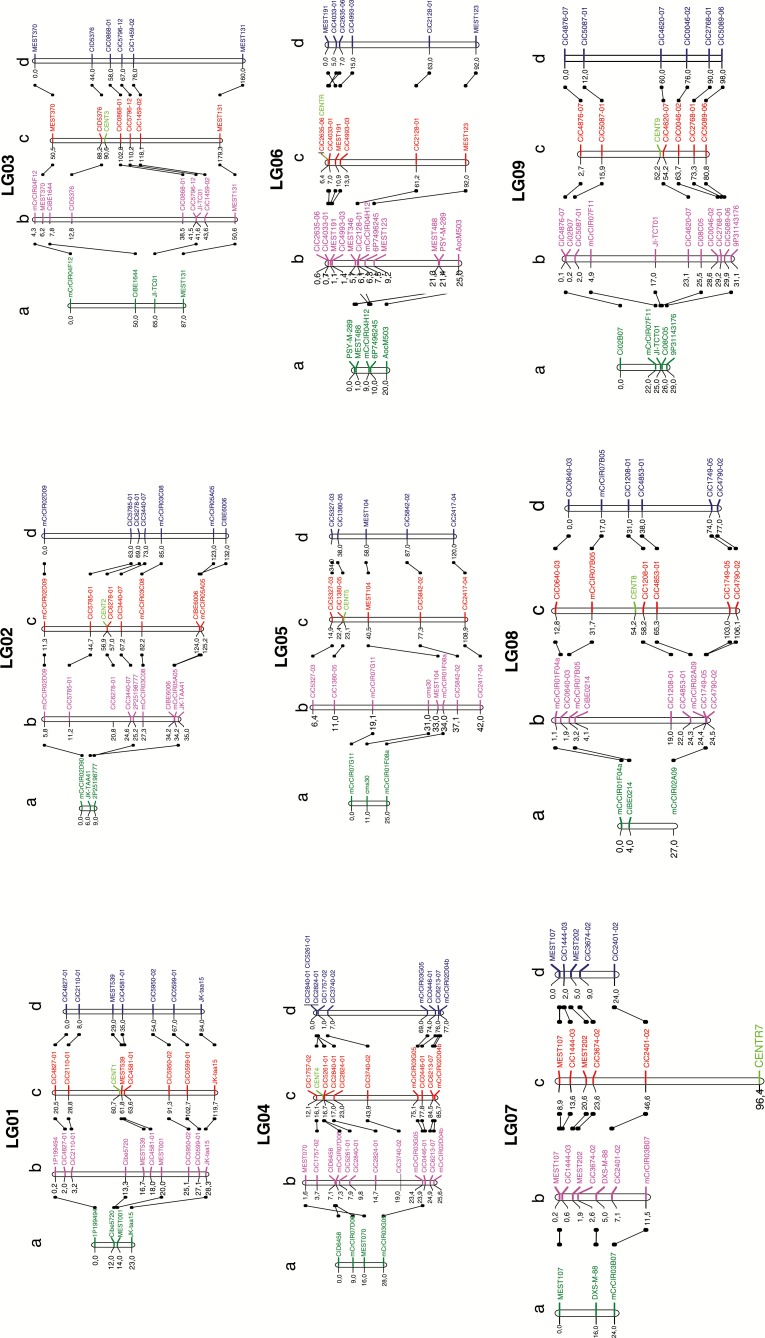
The genetic maps were established on the basis of the SSR and SNP marker segregations and then compared using the physical positions as common reference (Fig. 5). The average recombination rates per megabase were estimated for each LG and each population considering the extreme marker positions on the genetic and physical maps (Table 5).
Fig. 5.
Comparative mapping between tetraploid ‘Mexican’ lime and diploid and tetraploid ‘Clemenules’ clementine. (A) Tetraploid ‘Mexican’ lime (cM); (B) diploid clementine (physical; Mb); (C) diploid clementine (genetics; cM); (D) tetraploid clementine (cM). The centromere of each linkage group is indicated in green on the diploid clementine map.
Table 5.
Average recombination rates per LG (cM Mb–1) for diploid and tetraploid clementine and tetraploid ‘Mexican’ lime
| 2x Clementine | 4x Clementine | 4x ‘Mexican’ lime | |
|---|---|---|---|
| LG1 | 3.53 | 2.99 | 0.82 |
| LG2 | 3.97 | 4.65 | 0.31 |
| LG3 | 2.79 | 3.46 | 1.88 |
| LG4 | 3.37 | 3.53 | 1.28 |
| LG5 | 2.64 | 2.42 | 1.68 |
| LG6 | 3.51 | 3.78 | 1.07 |
| LG7 | 5.49 | 3.49 | 2.12 |
| LG8 | 4.12 | 3.40 | 1.16 |
| LG9 | 2.63 | 3.30 | 0.94 |
| Total | 3.29 | 3.41 | 1.21 |
LG, linkage group.
For the diploid clementine genetic and physical maps, the considered positions were those published by Ollitrault et al. (2012b) and Wu et al. (2014), respectively. These maps were compared with the two tetraploid genetic maps. For the genetic maps, only markers common to the tetraploid clementine map were selected. For the physical map, we retained the previous markers plus those of the tetraploid ‘Mexican’ lime map.
For the tetraploid clementine map, the positions of 57 molecular markers of tetraploid clementine published previously by Aleza et al. (2016) were inferred. The map size was 864 cM. Compared with the clementine genetic map, synteny was complete and the marker order preserved, except for very close telomeric markers in LG02 and LG06. The genetic distances of the two clementine maps were very similar, with average rates of 3.29 and 3.41 cM Mb–1 for the diploid and tetraploid clementines and limited variation among LGs.
For the tetraploid ‘Mexican’ lime map, the 34 segregating molecular markers of the present study (the 8P18684429 marker was heterozygous for the 85 analysed hybrids) were mapped. The map spanned only 272 cM. The majority of the markers maintained the same order as on the clementine physical map, although three inversions were observed on LG02, LG04 and LG06. The distances between markers were considerably lower than on the diploid and tetraploid clementine maps. Indeed, the average rate of recombination of the tetraploid ‘Mexican’ lime was 1.21 cM Mb–1, close to 3-fold lower than that observed for the tetraploid clementine.
DISCUSSION
Meiotic behavior of the doubled-diploid ‘Mexican’ lime revealed by cytogenetics
Asynapsis, dependent on low temperature, has been described in diploid ‘Mexican’ lime (Iwamasa and Iwasaki, 1963). No evidence of asynapsis was observed in the tetraploid ‘Mexican’ lime. Indeed, the very low rate of monovalents observed during microsporogenesis indicated that such an abnormality in meiosis was not induced in the tetraploid lime cultivated in Spain. The asynaptic behaviour was recorded at temperatures <10 °C (Iwamasa and Iwasaki, 1963), while our sampling was done at >16 °C.
A common approach to distinguish autotetraploids from allotetraploids is to assess the tetravalent formation frequency. In genuine autotetraploids, about two-thirds of the chromosomes are usually involved in tetravalent configurations (Morrison and Rajhathy, 1960). However, this should be considered with caution since genetic systems involving diploidization or preferential pairing could exist.
The predominance of bivalents (65 %) in the meiosis of tetraploid ‘Mexican’ lime was consistent with observations in several allotetraploid somatic hybrids, including C. deliciosa + C. limon (Kamiri et al., 2011), C. sinensis + C. limon (Del Bosco et al., 1999; Chen et al., 2004) and Tangelo (C. reticulata × C. paradisi) + C. grandis (Xie et al., 2015). These studies, as in our work, also revealed tetravalent formation and a low percentage of monovalents and trivalents. Multivalent frequency in tetraploids is usually related to the pairing affinity (Jeredi et al., 2012). The chromosome length and centromere position may also influence the multivalent frequency (McCollum, 1958). Homeologous pairing can also be under genetic control in some species (Jenczewski et al., 2003; Griffiths et al., 2006; Qi et al., 2007; Cifuentes et al., 2010).
Structural variations strongly affect chromosome pairing. A large heterozygous inversion resulting in partial gametophyte sterility was described in diploid ‘Mexican’ lime (Iwamasa, 1966; Iwamasa and Nito, 1988). In Valencia (Spain), the pollen viability of diploid ‘Mexican’ lime was estimated to be <10 % (Pons et al., 2011). Interestingly, we observed 64 % pollen viability for the doubled-diploid ‘Mexican’ lime, i.e. higher than the rates (31–41 %) reported by Aleza et al. (2012) and Del Bosco et al. (1999) for different doubled-diploid and somatic hybrids. When interspecific diploid hybrid sterility is due to improper chromosome pairing, the generation of allotetraploids by chromosome doubling results in a homologue for each chromosome to pair with during meiosis and can allow for the development of fertile gametes (Zadoo et al., 1975; Lu and Bridgen, 1997; Van Tuyl and De Jeu, 1997; Contreras et al., 2007).
We observed that 6.3 % of the chromosomes were involved in closed tetravalents. Moreover, more than one closed tetravalent was observed in some PMCs. In diploid species, the observation of closed tetravalents is considered as evidence of the presence of heterozygous reciprocal translocation (Sybenga, 1975). Reciprocal translocation is defined as the interchange of part of a chromosome with part of another (Sybenga, 1995), resulting in meiotic configuration alterations. The affected chromosomes may form a ring or a chain tetravalent structure (Sybenga, 1975, 2012). In citrus, reciprocal translocation was described in diploid ‘Valencia’ and ‘Lue Gin Gong’ sweet oranges (C. sinensis), in which tetravalents were frequently observed (Iwamasa and Iwasaki, 1963). Del Bosco et al. (1999) studied meiosis of an allotetraploid somatic hybrid between ‘Valencia’ sweet orange and ‘Femminello’ lemon, and revealed that reciprocal translocation still existed in the somatic hybrid. Closed tetravalents were not observed in the previous cytogenetic study of diploid ‘Mexican’ lime, while inversion was described (Iwamasa and Nito, 1988; Iwamasa, 1966). The presence of a double inversion affecting the two arms of the same chromosome should be in line with our observations for the doubled diploid and the previous data for the diploid ‘Mexican’ lime (Fig. 6). This double inversion pattern may result from chromosome structural variation between C. medica and C. micrantha, i.e. the two parental species of ‘Mexican’ lime (Nicolosi et al, 2000; Carvalho et al., 2005; Curk et al., 2016).
Fig. 6.
Interpretation for reconciliation of microsporogenesis observations in diploid and doubled-diploid ‘Mexican’ lime: double inversion can produce a closed tetravalent during doubled-diploid meiosis.
Doubled-diploid ‘Mexican’ lime has intermediary inheritance with a preferential disomic trend
Froelicher et al. (2000) were the first to analyse the inheritance of molecular markers in a tetraploid Aurantioideae, i.e. Clausena excavata. They concluded that strict disomic inheritance occurred.
Our study reveals preferential disomic inheritance for the doubled-diploid ‘Mexican’ lime, with an average PHR value of 90 %. This value is higher than those observed by Kamiri et al. (2011), who reported PHR values ranging from 54 to 79 % for a C. deliciosa + C. limon tetraploid somatic hybrid. Xie et al. (2015) reported 76.2 % PHR for a somatic hybrid between Tangelo and a pummelo, while it was 65 % for a doubled-diploid clementine (Aleza et al., 2016). In direct relation to PHR, the preferential pairing rate was high for most LGs for the tetraploid ‘Mexican’ lime. Disomic inheritance with high preferential pairing values was observed for LG02, LG07 and LG08. Preferential pairing trends were found for five LGs (LG01, LG03, LG04, LG06 and LG09). For LG05, the intermediate models fitted better than the disomic or tetrasomic model (PP = 0.50). Lower values were estimated by Kamiri et al. (2011) and Aleza et al. (2016). For instance, Aleza et al. (2016) concluded that non-preferential pairing (PP = 0) was observed for five LGs in DD clementine.
Most interspecific diploid hybrids within the Citrus genus, except ‘Mexican’ lime, display high fertility (Ollitrault and Navarro, 2012). It indicates good pairing affinity and therefore limited chromosome variations between species. Tetraploid genotypes offer a choice of chromosome partners, which is not available at the diploid level. Therefore, doubled-diploid meiosis may reveal chromosomal variations between ancestral species not identified at the diploid level. The different meiotic behaviours observed by Kamiri et al. (2011), Aleza et al. (2016) and in our study could be due to the phylogenomic structure of the different genotypes. Regarding the C. reticulata + C. limon hybrid studied by Kamiri et al. (2011), the phylogenomic structure is highly complex as C. limon results from (C. maxima × C. reticulata) × C. medica natural hybridization (Curk et al., 2016). Therefore this somatic hybrid harbours two-ancestor heterozygosity (C. reticulata/C. reticulata/C. reticulata/C. medica) or three-ancestor heterozygosity (C. reticulata/C. reticulata/C. maxima/C. medica) at each locus. The doubled-diploid clementine studied by Aleza et al. (2016) had a simpler structure with a predominant C. reticulata genomic constitution, with some genomic segments showing C. reticulata/C. reticulata/C. maxima/C. maxima heterozygosity. Citrus aurantiifolia is a hybrid of two distant species (C. micrantha × C. medica), so each locus displays C. micrantha/C. micrantha/C. medica/C. medica heterozygosity. Molecular studies (García et al., 2013a; Curk et al., 2014, 2015; Carbonell-Caballero et al., 2015) have shown that C. medica is the cultivated citrus ancestor most distant from the three other ancestral taxa. Both sequence divergence and structural variations between C. medica and C. micrantha probably drive the preferential pairing and intermediary preferential disomic inheritance observed for the doubled-diploid ‘Mexican’ lime. As stated by Stebbins (1950), the extent of differentiation may vary between the different sets of chromosomes and could explain the difference in PP rates between the chromosomes. Interestingly, in the tetraploid ‘Mexican’ lime, none of the nine chromosomes displayed tetrasomic inheritance, suggesting that pairing is more affected by overall differentiation rather than discrete and local large structural variations, such as the inversion described in diploid ‘Mexican’ lime.
Interspecific recombination occurs in each LG but is markedly lower compared with the recombination rates in doubled-diploid clementine
Despite the disomic trend, mixed heterozygous/homozygous structures were observed for the nine citrus LGs, revealing interspecific recombination between C. medica and C. micrantha, the parents of the ‘Mexican’ lime (Nicolosi et al., 2000; Curk et al., 2016) for the nine citrus chromosomes. For LG05, 12.9 % of the gametes displayed homozygosity on both sides of the centromere, suggesting higher homology between C. micrantha and C. medica for the corresponding chromosome than for the others (5.3 % in average). This was confirmed by the intermediate preferential pairing findings for chromosome 5, while the other chromosomes displayed intermediate inheritances with a disomic trend or disomic inheritance. Interspecific recombination was observed in distal areas for most chromosomes. It appeared, however, to be very limited for LG08, with only 2.4 % of identified interspecific recombined gametes in one of the two chromosome arms.
Genetic mapping revealed effective recombination rates (1.2 cM Mb–1) that were markedly lower compared with diploid (3.3 cM Mb–1) and tetraploid clementine (3.4 cM Mb–1). This was, at least partly, a direct consequence of the medium to high preferential pairing that prevented interspecific chiasmata and thus interspecific recombination. Sequence divergence between C. medica and C. micrantha might also decrease the recombination frequency when interspecific pairing is effective. Interspecificity is well known to decrease recombination rates (Manrique-Carpintero et al., 2016). The impact of structural heterozygosity on the recombination frequency is variable, as discussed by Parker et al. (1982). It is, however, well established that sequence divergence at the interspecific level has an inhibitory effect on sexual recombination (Chambers et al., 1996; Liharska et al., 1996; Chetelat et al., 2000; Opperman et al., 2004; Li et al., 2006). In citrus, variations in recombination rates were observed between clementine and sweet orange (Ollitrault et al., 2012b). The authors proposed that this may be related to the higher C. reticulata/C. maxima heterozygosity in sweet orange than in clementine. Genetic mapping of diploid ‘Mexican’ lime would cast light on a potential limitation of sexual recombination due to interspecific genome divergence.
Synteny was observed in diploid clementine, doubled-diploid clementine and ‘Mexican’ lime. Collinearity was high between diploid and tetraploid clementine maps, while the alignment of the genetic maps of tetraploid ‘Mexican’ lime with diploid clementine revealed three inversions in LG02, LG04 and LG06. However, the low number of observed recombinations and markers analysed questions the reliability of these observations (a few genotyping errors can lead to erroneous ordering). Saturated mapping of larger populations would be necessary to be able to associate the inversion concluded from the cytogenetic studies (Iwamasa, 1970) and the inverted LGs.
Diploid gamete structures of doubled-diploid ‘Mexican’ lime are compatible with the origin of triploid C. aurantiifolia and C. latifolia limes
‘Persian’, ‘Tahiti’ and ‘Bears’ are different lime varieties classified as C. latifolia, and representing the same ideotype, producing large seedless lime fruits. It is believed that they are a group of clones derived from the same ancestral hybrid. ‘Tahiti’ lime was introduced into the Mediterranean region through Iran (where it is called ‘Persian’ lime), while it reached California from Tahiti between 1850 and 1880 and was introduced in Florida by 1883. The genetic origin of ‘Tahiti’ lime was unclear until a recent publication of Curk et al. (2016). Previous cytoplasmic studies showed that ‘Tahiti’ lime shared the same cytoplasm as C. limon and C. aurantium (Bayer et al., 2009; Froelicher et al., 2011). Reece and Childs (1962) proposed, on the basis of morphological trait segregation studies in ‘Tahiti’ lime seedlings, that this variety may result from lime by citron or lemon hybridization, but they did not recognize the triploid status of ‘Tahiti’ lime. Another ideotype of triploid lime producing large seedy lime fruits, represented by several cultivars (such as ‘Tanepao’, ‘Coppenrhad’, ‘Ambilobe’ and ‘Mothasseb’ limes and ‘Madagascar’ lemon) is cultivated to a lesser extent. Curk et al. (2016) demonstrated that these varieties, classified as C. aurantiifolia, are genetically very close and probably derive from the same ancestral hybrid by mutation or epigenetic variations. They share the same cytoplasm as ‘Mexican’ lime (Curk et al., 2016). On the basis of cytoplasmic and nuclear molecular data, Curk et al. (2016) proposed that the two main types of triploid limes, i.e. ‘Tahiti’ lime and ‘Tanepao’ lime, were interspecific hybrids involving a diploid gamete of C. aurantiifolia combined with a haploid ovule of C. limon and a haploid pollen of C. medica, respectively. Moreover, their data suggest that the PHRs of the concerned diploid gamete were 88 and 95 %, respectively. Curk et al. (2016) hypothesized that these diploid gametes could have originated from a natural doubled diploid of ‘Mexican’ lime similar to the ‘Giant Key’ lime selected in a seedling of diploid ‘Key’ lime (a ‘Mexican’ lime clone), or could be unreduced gametes from a diploid ‘Mexican’ lime-like variety. The average PHR value (90.2 %) and range (between 65.7 and 100 %) observed in the present study for doubled-diploid ‘Mexican’ lime are compatible with those estimated by Curk et al. (2016) for ‘Tahiti’ and ‘Tanepao’ types. Conversely, SDR, which is described as the main mechanism of unreduced mega-gametophyte production in citrus (Luro et al., 2004; Cuenca et al., 2011, 2015; Aleza et al., 2016), results in lower PHR values (40 % on average) (Peloquin, 1983; Hutten et al., 1994; Carputo et al., 2003). Therefore, SDR 2n gametes are not compatible with the interspecific genetic structure of ‘Tahiti’ and ‘Tanepao’ limes. FDR, identified as the predominant mechanism for diploid pollen formation in a clementine × sweet orange hybrid (Rouiss et al., 2017a), and secondary mechanisms in lemon 2n gamete ovule production (Rouiss et al., 2017b), should produce diploid gametes with high PHR (80 % on average; Peloquin, 1983; Hutten et al., 1994; Carputo et al., 2003), particularly if combined with asynapsis. Indeed, FDR associated with strict asynapsis for all chromosomes would result in 100 % PHR. Studies on the mechanisms and structure of unreduced gametes of diploid ‘Mexican’ lime would be necessary to conclude definitively on the origin of C. latifolia and C. aurantiifolia triploid limes. However, the interploid hybridization hypothesis is consistent with the actual molecular data on these two types of triploid limes, the natural occurrence of tetraploid ‘Mexican’ limes and our present results on the phylogenetic diploid gamete structure produced by doubled-diploid ‘Mexican’ lime.
Implications for ‘Tahiti’ and ‘Tanepao’ lime-like breeding
As discussed earlier, doubled-diploid ‘Mexican’ lime can produce diploid gametes with a genetic structure similar to those that led to the ‘Tahiti’ and ‘Tanepao’ lime types. This offers the possibility of developing a reconstruction breeding strategy for these limes using a doubled-diploid ‘Mexican’ lime-like parent, selected for interesting variations (disease resistance, improved phenology, primary and secondary metabolite contents, etc.). These parents should be mutational variants of ‘Mexican’ lime but also genotypes of similar interspecific origin such as C. macrophylla, C. excelsa or C. aurata (Curk et al., 2016) or recent hybrids. Similarly, for the lemon-like parent, they should be natural mutants of the ‘Mediterranean lemon’ but also genotypes derived from a similar C. aurantium × C. medica hybridization such as C. limeta (Curk et al., 2016) or recent hybrids.
Chromosome doubling in diploid ‘Mexican’ lime restored good pollen viability, so this could be used in extensive breeding programmes to produce ‘Tahiti’ and ‘Tanepao’ lime-like hybrids. This could be much more efficient than searching for triploid hybrids resulting from unreduced gametes of ‘Mexican’ limes. Indeed, the partial apomixis of parents, which markedly limits hybrid recovery, combined with the relatively low frequency of 2n gametes described in citrus (Esen and Soost, 1971; Geraci et al., 1977; Cuenca et al., 2015), could be a real impediment for efficient triploid lime breeding from unreduced gametes. The successful use of unreduced gametes for triploid mandarin breeding was based on the selection of non-apomictic female parents (Ollitrault et al., 2008; Aleza et al., 2010).
The predominant intermediate segregation with a disomic inheritance pattern observed for the different LGs of doubled-diploid ‘Mexican’ lime results in highly heterozygous gametes. Indeed, about 90 % of ‘Mexican’ lime heterozygosity would be transmitted to triploid progenies via diploid gametes. This should avoid inbreeding depression in triploid hybrids that can occur when using doubled-diploid parents with tetrasomic inheritance (Gallais, 2003). The low diversity between diploid gametes produced by such meiotic mechanisms is also favourable for reconstructing phylogenomic structures similar to the two triploid lime ideotypes, thus optimizing the probability of selecting new varieties phenotypically close to the ideotypes. The development and application of diagnostic molecular markers of the four ancestral taxa of cultivated citrus (Curk et al., 2015) will help improve the efficiency of such reconstruction breeding strategies.
On the other hand, the limited effective interspecific recombination associated with predominant disomic inheritance, as illustrated by the decrease in the genetic length of the different genetic LGs for tetraploid ‘Mexican’ lime as compared with diploid and tetraploid clementine maps, should impact the breeding efficiency due to an increased linkage drag. This would require developing large progeny if needed to separate a given locus of genetic importance from another linked undesired locus. However, although limited, interspecific recombination has been observed for each chromosome, thus enhancing the possibilities for lime breeding considering the high pollen viability of doubled-diploid ‘Mexican’ lime and thus the capacity for generating large triploid progenies.
Conclusion
Disomic segregation was found to predominate in doubled-diploid ‘Mexican’ lime. Preferential pairing varied between chromosomes. Disomic inheritance with high PP values was observed for three LGs (LG02, LG07 and LG08), while intermediate segregation with a PP trend was found for five LGs (LG01, LG03, LG04, LG06 and LG09), and intermediate segregation for LG05. However, interspecific (C. medica/C. micrantha) chromosome pairing and recombination was revealed for each LG in the molecular marker study. The disomic pattern limits effective interspecific recombination and the diversity of the diploid gamete population. The interspecific phylogenetic structures of the produced diploid gametes with high C. medica/C. micrantha heterozygosity were compatible with those that generate triploid C. latifolia and C. aurantiifolia varieties that may therefore result from interploid hybridization. The restored pollen fertility of the doubled-diploid ‘Mexican’ lime compared with the diploid and the genetic structures of the diploid gametes, consistent with the origin of C. aurantiifolia and C. latifolia triploid limes, paves the way for efficient reconstruction breeding programmes based on interploid hybridization for triploid lime diversification.
SUPPLEMENTARY DATA
Supplementary data are available at https://academic.oup.com/aob and consist of the following. Figure S1: individual meiotic configuration of the 51 observed PMCs for the tetraploid ‘Mexican’ lime. Table S1: phylogenomic structure of ‘Mexican’ lime diploid gametes for the nine LGs.
ACKNOWLEGEMENTS
This work was supported by grant RTA2015-00069-00-00, from the Ministry of ‘Economía y Competividad’, ‘Instituto Nacional de Investigación y Tecnología Agraria y Agroalimentaria’ and ‘Fondo Europeo de Desarrollo Regional’ (FEDER); the European Regional Development Fund and the Guadeloupe Region through the ‘Cavalbio’ project.
LITERATURE CITED
- Ahmad R, Struss D, Southwick SM. 2003. Development and characterization of microsatellite markers in Citrus. Journal of the American Society for Horticultural Science 128: 584–590. [Google Scholar]
- Aleza P, Juárez J, Ollitrault P, Navarro L. 2009. Production of tetraploid plants of non-apomictic citrus genotypes. Plant Cell Reports 28: 1837–1846. [DOI] [PubMed] [Google Scholar]
- Aleza P, Juárez J, Cuenca J, Ollitrault P, Navarro L. 2010. Recovery of citrus triploid hybrids by embryo rescue and flow cytometry from 2x × 2x sexual hybridisation and its application to extensive breeding programs. Plant Cell Reports 29: 1023–1034. [DOI] [PubMed] [Google Scholar]
- Aleza P, Froelicher Y, Schwarz S et al. . 2011. Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment. Annals of Botany 108: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleza P, Juárez J, Hernández M, Ollitrault P, Navarro L. 2012. Implementation of extensive citrus triploid breeding programs based on 4x × 2x sexual hybridizations. Tree Genetics and Genomes 8: 1293–1306. [Google Scholar]
- Aleza P, Cuenca J, Hernández M, Juárez J, Navarro L, Ollitrault P. 2015. Genetic mapping of centromeres in the nine Citrus clementina chromosomes using half-tetrad analysis and recombination patterns in unreduced and haploid gametes. BMC Plant Biology 8: 15–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleza P, Cuenca J, Juárez J, Navarro L, Ollitrault P. 2016. Inheritance in doubled-diploid clementine and comparative study with SDR unreduced gametes of diploid clementine. Plant Cell Reports 35: 1573–1586. [DOI] [PubMed] [Google Scholar]
- Allendorf FW, Danzmann RG. 1997. Secondary tetrasomic segregation of MDH-B and preferential pairing of homeologs in rainbow trout. Genetics 145: 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley NA, Roose ML, Krueger RR, Federici CT. 2006. Assessing genetic diversity and population structure in a citrus germplasm collection utilizing simple sequence repeat markers (SSRs). Theoretical and Applied Genetics 112: 1519–1531. [DOI] [PubMed] [Google Scholar]
- Bayer RJ, Mabberley DJ, Morton C et al. . 2009. A molecular phylogeny of the orange subfamily Rutaceae, Aurantioideae using nine cpDNA sequences. American Journal of Botany 96: 668–685. [DOI] [PubMed] [Google Scholar]
- Bretagnolle F, Thompson JD. 1995. Gametes with the somatic chromosome number – mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytologist 129: 1–22. [DOI] [PubMed] [Google Scholar]
- Cameroon J, Frost H. 1968. Genetic, breeding and nucellar embryony. In: Reuther W, Batchelor LD, Webber HJ, eds. The citrus industry, Vol. 2 Berkeley, CA: University of California, 199–205. [Google Scholar]
- Carbonell-Caballero J, Alonso R, Ibañez V, Terol J, Talon M, Dopazo JA. 2015. Phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus citrus. Molecular Biology and Evolution 32: 2015–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carputo D, Frusciante L, Peloquin SJ. 2003. The role of 2n gametes and endosperm balance number in the origin and evolution of polyploids in the tuber-bearing Solanums. Genetics 163: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R, Soares Filho WS, Brasileiro-Vidal AC, Guerra M. 2005. The relationships among lemons, limes and citron: a chromosomal comparison. Cytogenetic and Genome Research 109: 276–282. [DOI] [PubMed] [Google Scholar]
- Chambers SR, Hunter N, Louis EJ, Borts RH. 1996. The mismatch repair system reduces meiotic homeologous recombination and stimulates recombination-dependent chromosome loss. Molecular and Cellular Biology 1611: 6110–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Guo W, Yi H, Deng X. 2004. Cytogenetic analysis of two interspecific Citrus allotetraploid somatic hybrids and their diploid fusion parents. Plant Breeding 123: 332–337. [Google Scholar]
- Chetelat RT, Meglic V, Cisneros PA. 2000. Genetic map of tomato based on BC1 Lycopersicon esculentum×Solanum lycopersicoides reveals overall synteny but suppressed recombination between these homeologous genomes. Genetics 1542: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes M, Grandont L, Moore G, Chèvre AM, Jenczewski E. 2010. Genetic regulation of meiosis in polyploid species: new insights into an old question. New Phytologist 1861: 29–36. [DOI] [PubMed] [Google Scholar]
- Contreras RN, Ranney T, Tallury SP. 2007. Reproductive behavior of diploid and allotetraploid Rhododendron L., ‘Fragrant Affinity’. HortScience 42: 31–34. [Google Scholar]
- Cuenca J, Froelicher Y, Aleza P, Juárez J, Navarro L, Ollitrault P. 2011. Multilocus half-tetrad analysis and centromere mapping in citrus: evidence of SDR mechanism for 2n megagametophyte production and partial chiasma interference in mandarin cv ‘Fortune’. Heredity 107: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca J, Aleza P, Navarro L, Ollitrault P. 2013. Assignment of SNP allelic configuration in polyploids using competitive allele-specific PCR: application to citrus triploid progeny. Annals of Botany 111: 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca J, Aleza P, Juárez J et al. . 2015. Maximum-likelihood method identifies meiotic restitution mechanism from heterozygosity transmission of centromeric loci: application in citrus. Scientific Reports 5: 9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppen E. 2007. Genotyping by allele-specific amplification (KASPar). Cold Spring Harbor Protocols 2007: npdb.prot4841. doi: 10.1101/pdb.prot4841. [DOI] [PubMed] [Google Scholar]
- Curk F, Ancillo G, Garcia-Lor A et al. . 2014. Next generation haplotyping to decipher nuclear genomic interspecific admixture in Citrus species, analysis of chromosome 2. BMC Genetics 15: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curk F, Ancillo G, Ollitrault F et al. . 2015. Nuclear species-diagnostic SNP markers mined from 454 amplicon sequencing reveal admixture genomic structure of modern citrus varieties. PLoS One 10: e0125628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curk F, Ollitrault F, Garcia-Lor A, Luro F, Navarro L, Ollitrault P. 2016. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Annals of Botany 117: 565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzmann RG, Bogart JP. 1983. Further evidence for a polymorphism in gametic segregation in the tetraploid treefrog Hyla versicolor using a glutamate oxaloacetic transaminase locus. Genetics 103: 753–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bosco SF, Tusa N, Conicella C. 1999. Microsporogenesis in a Citrus interspecific tetraploid somatic hybrid and its fusion parents. Heredity 83: 373–377. [DOI] [PubMed] [Google Scholar]
- Duportal M, Jorda E, Sanchez C, Imbert É, Loeillet D, Vannière H. 2013. FruiTrop Focus Citron. FruiTrop Cirad 2013: 140. [Google Scholar]
- Esen A, Soost RK. 1971. Unexpected triploids in Citrus: their origin, identification, and possible use. Journal of Heredity 62: 329–333. [Google Scholar]
- Esen A, Soost RK, Geraci G. 1979. Genetic evidence for the origin of diploid megagametophytes in Citrus. Journal of Heredity 70: 5–8. [Google Scholar]
- Esselink G, Nybom H, Vosman B. 2004. Assignment of allelic configuration in polyploids using the MAC-PR (microsatellite DNA allele counting-peak ratios) method. Theoretical and Applied Genetics 109: 402–408. [DOI] [PubMed] [Google Scholar]
- Fjellstrom RG, Beuselinck PR, Steiner JJ. 2001. RFLP marker analysis supports tetrasomic inheritance in Lotus corniculatus L. Theoretical and Applied Genetics 102: 718–725. [Google Scholar]
- Folimonova SY, Robertson CJ, Garnsey SM, Gowda S, Dawson WO. 2009. Examination of the responses of different genotypes of citrus to Huanglongbing (citrus greening) under different conditions. Phytopathology 99: 1346–1354. [DOI] [PubMed] [Google Scholar]
- Froelicher Y, Luro F, Ollitrault P. 2000. Analysis of meiotic behaviour of tetraploid Clausena excavata species by molecular marker segregation studies. In: 9th International Society of Citriculture Congress, South Africa, 116. [Google Scholar]
- Froelicher Y, Dambier D, Bassene JB et al. . 2008. Characterization of microsatellite markers in mandarin orange (Citrus reticulata Blanco). Molecular Ecology Resources 8: 119–122. [DOI] [PubMed] [Google Scholar]
- Froelicher Y, Mouhaya W, Bassene JB et al. . 2011. New universal mitochondrial PCR markers reveal new information on maternal citrus phylogeny. Tree Genetics and Genomes 7: 49–61. [Google Scholar]
- Gallais A. 2003. Quantitative genetics and breeding methods in autopolyploids plants, 2nd edn Paris: INRA. [Google Scholar]
- Garcia-Lor A, Luro F, Navarro L, Ollitrault P. 2012. Comparative use of InDel and SSR markers in deciphering the interspecific structure of cultivated citrus genetic diversity: a perspective for genetic association studies. Molecular Genetics and Genomics 287: 77–94. [DOI] [PubMed] [Google Scholar]
- Garcia-Lor A, Curk F, Snoussi-Trifa H et al. . 2013a. A nuclear phylogenetic analysis: SNPs, indels and SSRs deliver new insights into the relationships in the ‘true citrus fruit trees’ group (Citrinae, Rutaceae) and the origin of cultivated species. Annals of Botany 111: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lor A, Ancillo G, Navarro L, Ollitrault P. 2013b. Citrus (Rutaceae) SNP markers based on competitive allele-specific PCR; transferability across the Aurantioideae subfamily. Applications in Plant Sciences 1: apps.1200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci G, De Pasquale F, Tusa N. 1977. Percentages of spontaneous triploids in progenies of diploid lemons and mandarins. In: Proceedings of the Second International Citrus Congress. International Society of Citriculture, Vol. 2 Orlando, Florida, 596–597. [Google Scholar]
- Griffiths S, Sharp R, Foote TN et al. . 2006. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439: 749–752. [DOI] [PubMed] [Google Scholar]
- Hackett CA, Milne I, Bradshaw JE, Luo Z. 2007. TetraploidMap for Windows: linkage map construction and QTL mapping in autotetraploid species. Journal of Heredity 98: 727–729. [DOI] [PubMed] [Google Scholar]
- Hickok LG. 1978. Homoeologous chromosome pairing: frequency differences in inbred and intraspecific hybrid polyploid ferns. Science 202: 982–984. [DOI] [PubMed] [Google Scholar]
- Hutten R, Schippers M, Hermsen JT, Ramanna M. 1994. Comparative performance of FDR and SDR progenies from reciprocal 4x–2x crosses in potato. Theoretical and Applied Genetics 89: 545–550. [DOI] [PubMed] [Google Scholar]
- Iwamasa M. 1966. Study on the steriliy in genus Citrus with special reference to the seedlessness. Bulletin of the Horticultural Research Station of Japan, Series B 6: 2–77. [Google Scholar]
- Iwamasa M. 1970. Chromosome aberrations in citrus in relation to sterility and seedlessness. In: 1st International Citrus Symposium, Riverside, Vol. 1 175–181. [Google Scholar]
- Iwamasa M, Iwasaki T. 1963. On the sterility phenomenon caused by low temperatures in the Mexican lime (Citrus aurantifolia Swing.). Bulletin of the Horticultural Research Station of Japan, Series B 2: 25–45. [Google Scholar]
- Iwamasa M., Nito N. 1988. Cytogenetics and the evolution of modern cultivated citrus. In: Proceedings of the Sixth International Citrus Congress, Vol. 1 Tel Aviv: Margraf, 265–275. [Google Scholar]
- Jackson R, Jackson JW. 1996. Gene segregation in autotetraploids: prediction from meiotic configurations. American Journal of Botany 83: 673–678. [Google Scholar]
- Jenczewski E, Eber F, Grimaud A et al. . 2003. PrBn, a major gene controlling homeologous pairing in oilseed rape Brassica napus haploids. Genetics 164: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeridi M, Perrier X, Rodier-Goud M, Ferchichi A, D’Hont A, Bakry F. 2012. Cytogenetic evidence of mixed disomic and polysomic inheritance in an allotetraploid AABB musa genotype. Annals of Botany 110: 1593–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiri M, Stift M, Srairi I et al. . 2011. Evidence for non-disomic inheritance in a Citrus interspecific tetraploid somatic hybrid between C. reticulata and C. limon using SSR markers and cytogenetic analysis. Plant Cell Reports 30: 1415–1425. [DOI] [PubMed] [Google Scholar]
- Kijas JMH, Thomas MR, Fowler JCS, Roose ML. 1997. Integration of trinucleotide microsatellites into a linkage map of Citrus. Theoretical and Applied Genetics 94: 701–706. [Google Scholar]
- Krug C. 1943. Chromosome number in the subfamily Aurantioideae with special reference to the genus Citrus. Botanical Gazette 104: 602–611. [Google Scholar]
- Lapin WK. 1937. Investigation on polyploidy in Citrus. USSR. All-Union Science Research Institute for Humid Subtropic Workshop 1(4): 1–68. [Google Scholar]
- Lee LS. 1988. Citrus polyploidy: origins and potential for cultivar improvement. Australian Journal of Agricultural Research 39: 735–747. [Google Scholar]
- Legge J. 1865. The shoo king and the tribute of Yu. In: The Chinese classics. London: Trubner and Co, Vol. 3, Pt. 1 and Pt. 3, Bk. 1, 111–112. [Google Scholar]
- Li L, Jean M, Belzile F. 2006. The impact of sequence divergence and DNA mismatch repair on homeologous recombination in Arabidopsis. The Plant Journal 456: 908–916. [DOI] [PubMed] [Google Scholar]
- Liharska T, Wordragen M, Kammen A, Zabel P, Koornneef M. 1996. Tomato chromosome 6: effect of alien chromosomal segments on recombinant frequencies. Genome 393: 485–491. [DOI] [PubMed] [Google Scholar]
- Longley AE. 1925. Polycarpy, polyspory and polyploidy in Citrus and Citrus relatives. Journal of the Washington Academy of Sciences 15: 347–357. [Google Scholar]
- Lu C, Bridgen MP. 1997. Chromosome doubling and fertility study of Alstroemeria aurea × A. caryophyllaea. Euphytica 94: 75–81. [Google Scholar]
- Luro F, Maddy F, Jacquemond C et al. . 2004. Identification and evaluation of diplogyny in clementine (Citrus clementina) for use in breeding. Acta Horticulturae 663: 841–848. [Google Scholar]
- Luro F, Costantino G, Terol J et al. . 2008. Transferability of the EST-SSRs developed on Nules clementine (Citrus clementina Hort ex Tan) to other Citrus species and their effectiveness for genetic mapping. BMC Genomics 9: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique-Carpintero NC, Coombs JJ, Veilleux RE, Buell CR, Douches DS. 2016. Comparative analysis of regions with distorted segregation in three diploid populations of potato. G3, Genes, Genomes, Genetics 68: 2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden JE, Schwager SJ, May B. 1987. Single-locus inheritance in the tetraploid treefrog Hyla versicolor with an analysis of expected progeny ratios in tetraploid organisms. Genetics 116: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum CD. 1958. Comparative studies of chromosome pairing in natural and induced tetraploid Dactylis. Chromosoma 9: 571–605. [DOI] [PubMed] [Google Scholar]
- Morrison JW, Rajhathy T. 1960. Frequency of quadrivalents in autotetraploid plants. Nature 187: 528–530. [DOI] [PubMed] [Google Scholar]
- Navarro L, Pina J, Juárez J et al. . 2002. The citrus variety improvement program in Spain in the period 1975–2001. In: Proceedings of the 15th Conference of the International Organization of Citrus Virologists, IOCV, Riverside: 306–316. [Google Scholar]
- Nicolosi E, Deng ZN, Gentile A, Malfa S, Continella G, Tribulato E. 2000. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theoretical and Applied Genetics 100: 1155–1166. [Google Scholar]
- Ollitrault P, Navarro L. 2012. Citrus. In: Badenes M, Byrne D, eds. Fruit breeding. Heidelberg: Springer, 623–662. [Google Scholar]
- Ollitrault P, Dambier D, Luro F, Froelicher Y. 2008. Ploidy manipulation for breeding seedless triploid citrus. Plant Breeding Reviews 30: 323–352. [Google Scholar]
- Ollitrault F, Terol J, Pina JA, Navarro L, Talon M, Ollitrault P. 2010. Development of SSR markers from Citrus clementina (Rutaceae) BAC end sequences and interspecific transferability in Citrus. American Journal of Botany 97: e124–9. [DOI] [PubMed] [Google Scholar]
- Ollitrault P, Terol J, Garcia-Lor A et al. . 2012a. SNP mining in C. clementina BAC end sequences: transferability in the Citrus genus (Rutaceae), phylogenetic inferences and perspectives for genetic mapping. BMC Genomics 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollitrault P, Terol J, Chen C et al. . 2012b. A reference genetic map of C. clementina hort. ex Tan.; citrus evolution inferences from comparative mapping. BMC Genomics 13: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman R, Emmanuel E, Levy AA. 2004. The effect of sequence divergence on recombination between direct repeats in Arabidopsis. Genetics 1684: 2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JS, Palmer RW, Whitehorn MAF, Edgar LA. 1982. Chiasma frequency effects of structural chromosome change. Chromosoma 85: 673–686. [Google Scholar]
- Peloquin S. 1983. Genetic engineering with meiotic mutants. In: Mulcahy DL, Mulcahy Bergamini G, Ottaviano E, eds. Pollen: biology and implications for plant breeding. New York: Elsevier, 311–316. [Google Scholar]
- Pons E, Navarro A, Ollitrault P, Peña L. 2011. Pollen competition as a reproductive isolation barrier represses transgene flow between compatible and co-flowering citrus genotypes. PLoS One 610: e25810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Friebe B, Zhang P, Gill BS. 2007. Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Research 15: 3–19. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics 29: 467–501. [Google Scholar]
- Ramsey J, Schemske DW. 2002. Neopolyploidy in flowering plants. Annual Review of Ecology and Systematics 33: 589–639. [Google Scholar]
- Reece PC, Childs JFL. 1962. Character differences among seedlings of the Persian lime. Florida State Horticultural Society 75: 110–116. [Google Scholar]
- Ronfort J, Jenczewski E, Bataillon T, Rousset F. 1998. Analysis of population structure in autotetraploid species. Genetics 1502: 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiss H, Cuenca J, Navarro L, Ollitrault P, Aleza P. 2017a. Tetraploid citrus progenies arising from FDR and SDR unreduced pollen in 4x × 2x hybridizations. Tree Genetics and Genomes 13: 10. [Google Scholar]
- Rouiss H, Cuenca J, Navarro L, Ollitrault P, Aleza P. 2017b. Unreduced megagametophyte production in lemon occurs via three meiotic mechanisms, predominantly second-division restitution. Frontiers in Plant Science 8: 1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F, Torrisi M. 1951. Il poliploidismo nei Citrus autopoliploidi e allopoliploidI. Annali della Sperimentazione Agraria 5: 1041–1062. [Google Scholar]
- Sanford JC, Moore JN, Janick J. 1983. Ploidy manipulations. In: Moore JN, Janick J, eds. Methods in fruit breeding. West Lafayette, IN: Purdue University Press, 100–123. [Google Scholar]
- Shepherd K. 1999. Cytogenetics of the genus Musa. Montpellier: International Network for the Improvement of Banana and Plantain. [Google Scholar]
- Soltis DE, Soltis PS. 1993. Molecular data and the dynamic nature of polyploidy. Critical Reviews in Plant Sciences 12: 243–273. [Google Scholar]
- Stanley RG, Linskens HF. 1974. Pollen, biology biochemistry management. New York: Springer. [Google Scholar]
- Stebbins G. 1947. Types of polyploids: their classification and significance. Advances in Genetics 1: 1939. [DOI] [PubMed] [Google Scholar]
- Stebbins C., Jr 1950. Variation and evolution in plants. New York: Columbia University Press. [Google Scholar]
- Stift M, Berenos C, Kuperus P, van Tienderen PH. 2008. Segregation models for disomic, tetrasomic and intermediate inheritance in tetraploids: a general procedure applied to Rorippa (yellow cress) microsatellite data. Genetics 179: 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybenga J. 1975. Meiotic configurations. Monograph on theoretical and applied genetics. Berlin: Springer-Verlag. [Google Scholar]
- Sybenga J. 1995. Meiotic pairing in autohexaploid lathyrus, a mathematical model. Heredity 754: 343–350. [Google Scholar]
- Sybenga J. 1996. Chromosome pairing affinity and quadrivalent formation in polyploids: do segmental allopolyploids exist?Genome 39: 1176–1184. [DOI] [PubMed] [Google Scholar]
- Sybenga J. 2012. Cytogenetics in plant breeding. Monograph on theoretical and applied genetics. Berlin: Springer-Verlag. [Google Scholar]
- Tanaka T. 1961. Citologia: semi-centennial commemoration papers on Citrus studies. Osaka: Citologia Supporting Foundation. [Google Scholar]
- Terol J, Naranjo MA, Ollitrault P, Talon M. 2008. Development of genomic resources for Citrus clementina, characterization of three deep-coverage BAC libraries and analysis of 46,000 BAC end sequences. BMC Genomics 9: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuyl JM, De Jeu MJ. 1997. Methods for overcoming interspecific crossing barriers. In: Sawhney VK. Shivanna KR, eds. Pollen biotechnology for crop production and improvement. New York: Cambridge University Press, 273–292. [Google Scholar]
- Voorrips RE. 2002. MapChart: software for the graphical presentation of linkage maps and QTLs. Journal of Heredity 93: 77–78. [DOI] [PubMed] [Google Scholar]
- Webber HJ, Reuther W, Lawton H. 1967. History and development of the citrus industry. In: Reuther W, Webber HJ, Batchelor LD, eds. The citrus industry. The botany of Citrus and its wild relatives, Vol. 1 Berkeley, CA: University of California, 1–39. [Google Scholar]
- Wendel J, Doyle J. 2005. Polyploidy and evolution in plants. In: Henry RJ, ed. Plant diversity and evolution. Genotypic and phenotypic variation in higher plants. Wallingford, UK: CAB International, 97–117. [Google Scholar]
- Wu GA, Prochnik S, Jenkins J et al. . 2014. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nature biotechnology 32: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Xia Q, Wang X et al. . 2015. Cytogenetic and SSR-marker evidence of mixed disomic, tetrasomic, and intermediate inheritance in a citrus allotetraploid somatic hybrid between ‘Nova’ tangelo and ‘HB’ pummelo. Tree Genetics and Genomes 11: 112. [Google Scholar]
- Zadoo SN, Roy RP, Khoshoo TN. 1975. Cytogenetics of cultivated bougainvilleas. V. Induced tetraploidy and restoration of fertility in sterile cultivars. Euphytica 24: 517–524. [Google Scholar]
- Zhao H, Speed TP. 1998. Statistical analysis of half-tetrads. Genetics 150: 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.