Abstract
Background and Aims
Nursery pollination is a highly specialized interaction in which pollinators breed inside plant reproductive structures. Pollinator occupancy of host plants often depends on plant location, flowering synchrony and sex. The nursery pollination system between the dioecious dwarf palm Chamaerops humilis (Arecaceae) and the host-specific palm flower weevil Derelomus chamaeropsis was investigated. For the first time, sex, flowering synchrony and spatial distribution of plants was related to the occupancy probability and the abundance of D. chamaeropsis larvae, important traits influencing both pollinator and plant fitness.
Methods
During the flowering season, all inflorescences in anthesis were counted every 12 d and a flowering synchrony index was calculated taking into account all possible correlations with generalized linear mixed models. To analyse the spatial structure of plants, larva occupancy and abundance, different techniques of spatial point pattern analysis were used.
Key results
In total, 5986 larvae in 1063 C. humilis inflorescences were recorded over three consecutive seasons. Male inflorescences showed a higher presence and abundance of weevil larvae than females, but interestingly approx. 30 % of the females held larvae. Also, larvae occurred mainly in highly synchronous plants with a low number of inflorescences, perhaps because those plants did not lead to a resource dilution effect. There was no evidence of spatial patterns in larva occupancy or abundance at any spatial scale, suggesting high dispersal ability of adult weevil.
Conclusions
The results in a nursery-pollinated dioecious palm demonstrate that plant sex, flowering display and flowering synchrony act as additive forces influencing the presence and abundance of the specialized pollinator larvae. Contradicting previous results, clear evidence that female dwarf palms also provide rewarding oviposition sites was found, and thus the plant ‘pays’ for the pollination services. The findings highlight that plant local aggregation is not always the main determinant of pollinator attraction, whereas flower traits and phenology could be critical in specialized plant–pollinator interactions.
Keywords: Specialized pollination, pollinator weevil, flowering synchrony, spatial point pattern analysis, dioecious, presence–abundance pollinator, mutualism, Chamaerops humilis
INTRODUCTION
Pollination by animals is a key ecosystem service for the maintenance of both wild plant communities (Ashman et al., 2004; Aguilar et al., 2006) and agricultural productivity (Klein et al., 2007; Ricketts et al., 2008). Up to 87 % of all flowering plant species rely on this mutualistic interaction, involving several groups of insects and small vertebrates (Richards, 1986; Harder and Barrett, 2006; Buchmann and Nabhan, 2012). The plants offer a reward (pollen, nectar, refuge or oviposition sites) in exchange for pollination services. Resource availability (usually, open flowers) for pollinators varies markedly, both in time and in space, determining their patterns of activity and pollination effectiveness (Anker, 1990; Eckhart, 1991; Suzuki et al., 2003; Fedriani et al., 2015).
Pollinators are usually attracted to a given plant or group of plants only after a certain threshold density of flowers bloom (Rathcke, 1983; Marquis, 1988; Fagan et al., 2014). Thus, important traits governing successful pollination are the flowering synchrony of individuals (i.e. in relation to the population flowering peaks) and the number of flowers per plant (Augspurger, 1981; Melampy, 1987). For instance, pollination in mass flowering species experience high intraspecific pollinator competition that results in a dilution of pollinator visits among the multitude of flowering resources (Fritz and Nilsson, 1994; Larson and Barrett, 2000; Delmas et al., 2014). Additionally, the degree of plant aggregation across the landscape influences the successful discovery of host plants by their pollinators, with large and well-connected clumps of plants being generally more attractive to pollinators than isolated ones (Aizen and Vázquez, 2006; Fedriani et al., 2015). For instance, in species such as Cypripedium japonicum (Orchidaceae) or Pyrus bourgaeana (Rosaceae), flowering individuals growing in clumps generally showed higher flowering synchrony and were more attractive to pollinators (Sun et al., 2009; Fedriani et al., 2015).
The effect of the spatiotemporal flowering patterns on pollination success has been studied in several pollination systems (e.g. Eckhart, 1991; Traveset and Sáez, 1997; Dupont et al., 2009; Price et al., 2005). However, relatively few studies have focused on systems where the pollinators not only feed on the floral structures but also use them as oviposition sites (but see Aker, 1982). These ‘nursery pollination’ systems involve coevolution between two species, implying a variety of costs and benefits (see Dufaÿ and Anstett, 2003). In these systems, plants provide the pollinator with egg-laying sites (e.g. seeds, ovaries, inflorescences) and resources for larvae development in exchange for pollination services (Anstett et al., 1997; Cook and Rasplus, 2003; Dufaÿ and Anstett, 2003; Herre et al., 2008).
In dioecious plant species, the nursery pollination interaction consistently involves sex-specific components that influence egg-laying site selection and larva development (Norstog and Fawcett, 1989; Hossaert-McKey et al., 2016). The pollinators’ development takes place in sexual tissues that can occur in only one plant gender or differ between genders in their quality for pollinator reproduction. For instance, Dufaÿ and Anstett (2003) found that in all dioecious systems, except for that of Silene latifolia/Hadena bicruris (Jürgens et al., 1996; Bopp and Gottsberger, 2004; Labouche and Bernasconi, 2010), pollinators only reproduce on a single sexual type of the plants (usually male plants). This implies that they will develop on male plants and pollination of female plants, in general, will be by deceit of pollinators. The more extended strategies to ensure visitation and pollination are the visual and/or chemical mimicry between male and female flowers, which prevents pollinators from discriminating between them (Dufaÿ et al., 2004; Dötterl and Jürgens, 2005). For instance, odour mimicry has been extensively studied in fig/fig wasp systems because it occurs in more than 300 fig species (Janzen, 1979; Hossaert-McKey et al., 2016). To ensure pollination in this system, the hermaphrodite (but functionally male) trees mimic the female floral volatile organic compounds to attract and deceive female wasps to lay their eggs in the non-functional ovaries (Hossaert-McKey et al., 2016). The wasps’ offspring then exit their natal fig loaded with pollen, and search for receptive flowers of female figs that have long styles, where female wasps can pollinate them but cannot lay eggs (Kjellberg et al., 1987; Anstett et al., 1997). On the other hand, intersexual odour mimicry has also been proposed for the dioecious genus Siparuna (Feil, 1992) and Chamaerops humilis (Dufaÿ et al., 2004) where deceptive females emit the same scent as males. Furthermore, in dioecious species, the spatial arrangement and the availability of individuals of the opposite sex within a patch can strongly affect both plant reproductive success and pollinator spatial distribution (Kunin, 1993; Groom, 1998; Davis et al., 2004; Gascoigne et al., 2009). Understanding the variables that determine the pollinators’ occupancy patterns in these systems will provide insight into how mutualisms evolve and function.
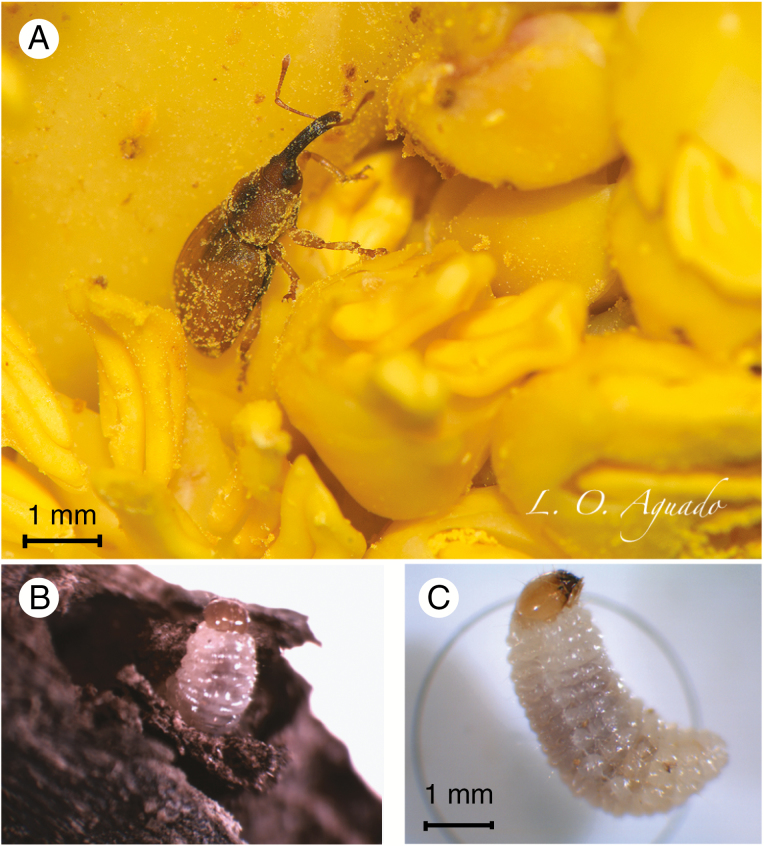
Here, we studied the nursery pollination system of the dioecious dwarf palm C. humilis with the host-specific palm flower weevil Derelomus chamaeropsis (Anstett, 1999; Fig. 1A). In this specialized mutualism, once pollinating weevils have found a dwarf palm (either female or male), they typically stay until the end of anthesis, finding shelter, egg-laying sites and food (i.e. nectar drops or pollen) within the inflorescences (Dufaÿ et al., 2004). Oviposition occurs mainly inside inflorescence rachises during summer and autumn; larvae develop then during autumn and winter in the same old inflorescences attached to the plant (Anstett, 1999; Dufaÿ and Anstett, 2003; Dufaÿ et al., 2004; Fig. 1B, C). Weevil larva development should occur almost exclusively in male inflorescences, due to mechanisms that arrest larval development in female inflorescences (Dufaÿ and Anstett, 2004). However, it seems that weevil larvae can also develop in female inflorescences that were not bearing fruits (Anstett, 1999). Thus, C. humilis has developed two strategies to avoid any kind of selection from weevils between sexes and to ensure effective partner encounter and pollen transportation. The first strategy is odour mimicry. Dufaÿ et al. (2003) found that leaves of both sexes produce almost the same amount of volatile compounds during flowering anthesis and that both attracted weevils in the same proportion. The second strategy is flowering synchrony of male and female plants. Dufaÿ (2010) found that increased synchrony between sexes reduces the capacity of weevils to distinguish between male and deceptive female plants.
Fig. 1.
Images of D. chamaeropsis: (A) adult on male inflorescence (image by Luis Oscar Aguado); (B) larva obtained from an old male inflorescence; and (C) larva placed for identification.
Our main objective in this study was to analyse how plant sex, flowering synchrony, plant density and spatial distribution are related to the occupancy probability of inflorescences by nursery pollinator larva. In this context, we tested the following hypotheses. (1) We expected higher larva occupancy in male inflorescences, and also that the female plants with higher larva occupancy would be those with non-pollinated inflorescences. (2) Weevils are attracted to plants during flowering anthesis, so we expected that palms with higher flowering synchrony would attract more pollinators than palms with lower flowering synchrony (plants that flower earlier or later). (3) Once weevils are in a plant, we expected that a high number of inflorescences would decrease larva presence and abundance per inflorescence due to a resource dilution effect. (4) Aggregated plants tend to be more attractive to pollinators because they have to move relatively shorter distances to travel among individual plants (Aizen and Vázquez, 2006; Fedriani et al., 2015). We therefore expect that larva presence and abundance in inflorescences will be higher in palm aggregations and that larva presence will be spatially structured. (5) High-density plant neighbourhoods can attract more pollinators (Aguilar et al., 2006; Fedriani et al., 2015). (6) We expect that inflorescences of palms in high-density areas will be occupied, on average, by more weevil larvae than palms located in low-density areas.
MATERIAL AND METHODS
Study area
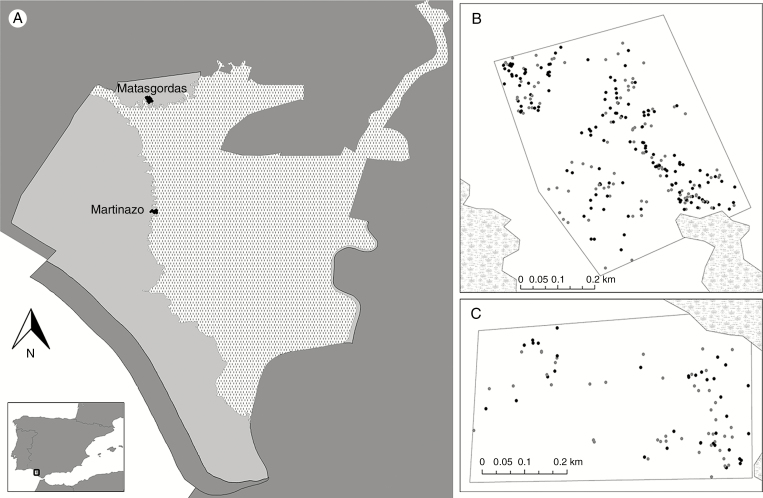
We selected two focal populations of C. humilis (hereafter, Matasgordas and Martinazo; Fig. 2) located in Doñana National Park (510 km2; 37°9′N, 6°26′W) on the right bank of the Guadalquivir estuary in south-west Spain. The climate is Mediterranean characterized by average annual temperatures between 15.4 and 18. 7 °C (mean = 16.91 ± 1.06 °C) and annual rainfall between 170 and 1028 mm (mean = 542.6 ± 120.02 mm). Between November and December 2011 we identified and georeferenced (with a submetric GPS) all breeding individuals of C. humilis (a total of 399). At Matasgordas, our study site occupied 22.1 ha and contained 308 marked individuals (177 females, 131 males) and at Martinazo, our study site occupied 20.93 ha and contained 91 adult individuals (42 females, 49 males).
Fig. 2.
Location of Doñana National Park (black square). (A) The two study sites limited by the marshland (grass pattern) within the Doñana National Park area. The study sites (B) Matasgordas and (C) Martinazo with the georeferenced plants: points in black represent female plants, while grey points represent males.
Data collection and statistical analyses
Presence and abundance of larvae.
Larvae development occurs during autumn and winter in the same old inflorescences attached to the plant from the previous flowering season. Thus, in December 2011 and 2012 we identified the sex of each reproductive dwarf palm and randomly collected two old inflorescences from the past flowering season (n = 584 inflorescences). In 2013, we randomly selected two or four inflorescences of each reproductive plant during the flowering season and collected them in December (n = 479 inflorescences). In the lab, we carefully dissected each rachis by making a longitudinal cut along the base through all the inflorescence ramifications to avoid the accidental destruction of the larva. Finally, we extracted, identified and counted the number of D. chamaeropsis larvae (overall, n = 5986 larvae).
For statistical analysis of the presence and abundance of larvae we performed generalized linear mixed models (GLMMs) through the GLIMMIX procedure (SAS; Littell et al., 2006). Specifically, the presence of larvae was analysed using a binomial GLMM with a logit link function and larva abundance using a Poisson GLMM with a log link function. We quantified the effect of plant sex and study site over larva presence and abundance in inflorescences over the period 2011–2013. Study site (SS) and plant sex (S), as well as their second-order interactions, were included as fixed factors.
Correlation between the number of inflorescences, flowering synchrony and weevil larvae.
During the 2013 flowering season (February–May) we counted all inflorescences produced by each reproductive individual during the flowering season (n = 290 dwarf palms). Every 12 d we also counted the number of inflorescences in anthesis and calculated a flowering synchrony index Xi for each individual i in relation to all the other individuals j, using the method of Augspurger (1983):
| (1) |
where ej≠i is the number of days that individuals i and j overlap in their flowering, fi is the total number of days individual i is flowering, and n is the number of individuals in the sample. Xi = 1 when the flowering time of an individual i overlaps completely with all other individuals, and Xi = 0 when there is no overlap in an individual’s flowering time with that of any other individuals. The overall synchrony of the population is obtained by averaging individual synchronies. We analysed the differences in the synchrony index and the number of inflorescences between sexes with a binomial and Poisson GLMMs, respectively, where individual plants were included as a random factor.
We analysed in two GLMMs the correlation between flowering synchrony and number of inflorescences with the larva presence and abundance data of 2013. As described above, we used the GLIMMIX procedure (SAS; Littell et al., 2006), using a binomial GLMM with a logit link function for larva presence. For larva abundance, we used a Poisson GLMM with a log link function. Study site, plant sex, synchrony (Sn) and number of inflorescences (Ni), as well as their second-order interactions, were included as fixed factors. For this analysis we established several competing models (see Table 1) with different variable combinations and selected the best model based on Akaike’s information criterion (AIC) as proposed by Burnham and Anderson (2002).
Table 1.
Type III effects: relevance of an individual’s inflorescences traits for larva abundance and presence in 2013
| Model | AICc | ΔAICc | wAICc | Rank |
|---|---|---|---|---|
| (A) Presence models | ||||
| 1. Sex | 1117.56 | 18.04 | 0.0001 | 10 |
| 2. S + Ni + Sn | 1109.47 | 9.95 | 0.0036 | 9 |
| 3. S + Ni + Sn + SS | 1105.52 | 6 | 0.0257 | 6 |
| 4. S + Ni + Sn + SS + S × Ni | 1104.28 | 4.76 | 0.0478 | 3 |
| 5. S + Ni + Sn + SS + S × Sn | 1107.27 | 7.75 | 0.0107 | 8 |
| 6. S + Ni + Sn + SS + Ni × Sn | 1104.42 | 4.9 | 0.0446 | 4 |
| 7. S + Ni + Sn + SS + S × Ni + S × Sn | 1105.09 | 5.57 | 0.0319 | 5 |
| 8. S + Ni + Sn + SS + S × Ni + Ni × Sn | 1099.52 | 0 | 0.5166 | 1 |
| 9. S + Ni + Sn + SS + S × Sn + Ni × Sn | 1106.07 | 6.55 | 0.0195 | 7 |
| 10. S + Ni + Sn + SS + S × Ni + S × Sn + Ni × Sn | 1100.07 | 1 | 0.2995 | 2 |
| (B) Abundance models | ||||
| 1. Sex | 2019.82 | 11.32 | 0.0022 | 9 |
| 2. S + Ni + Sn | 2012.89 | 4.42 | 0.0700 | 6 |
| 3. S + Ni + Sn + SS | 2011.12 | 2.59 | 0.1748 | 5 |
| 4. S + Ni + Sn + SS + S × Ni | 2010.79 | 2.31 | 0.5520 | 4 |
| 5. S + Ni + Sn + SS + S × Sn | 2008.48 | 0 | 0.253 | 1 |
| 6. S + Ni + Sn + SS + Ni × Sn | 2012.98 | 4.5 | 0.033 | 7 |
| 7. S + Ni + Sn + SS + S × Ni + S × Sn | 2008.77 | 0.29 | 0.267 | 2 |
| 8. S + Ni + Sn + SS + S × Ni + Ni × Sn | 2014.35 | 5.87 | 0.016 | 8 |
| 9. S + Ni + Sn + SS + S × Sn + Ni × Sn | 2010.42 | 1.94 | 0.117 | 3 |
| 10. S + Ni + Sn + SS + S × Ni + S × Sn + Ni × Sn | 2010.79 | 2.31 | 0.2010 | 4 |
(A) Models for the presence/absence of larvae. (B) Models for the abundance of larvae. The best models are indicated in bold. Sex (S), number of inflorescences/individual (Ni), synchrony index (Sn) and study site (SS). ΔAICc is the relative difference of a given AICc value compared with the smallest AICc. AIC weights indicate the relative support for every model (the weights of all the models in the candidate set have the sum of 1.
For all GLMMs, plant individual (nested within study site) was included as a random a factor to control for their potential effects. When appropriate, year was also specified in the models as a random factor (Bennington and Thayne, 1994). We calculated the adjusted means and standard errors using LSMEANS and back-transformed them using the appropriate Taylor’s series approach (Littell et al., 2006). In the case of significant interactions, we tested for the effect of a given factor at the different levels of the other factor (i.e. tests of slices). The test of slices looks for separate effects for dwarf palms from fixed factors, it calculates them by extracting the appropriate rows from the coefficient matrix for the interaction using LSMEANS and by using them to form an F test (SAS, 2013). We performed the GLMMs using the SLICE option in the LSMEANS statement of the MIXED procedure (Littell et al., 2006).
Spatial structure in larva occupancy and abundance
Jácome-Flores et al. (2016) demonstrated that the dwarf palms in our study sites show aggregated spatial patterns. To determine if the spatial pattern of dwarf palms and/or a dependency on the neighbourhood density of dwarf palms had effects on larvae occupancy, we used techniques of marked point patterns (Jacquemyn et al., 2010; Wiegand and Moloney, 2014; Velázquez et al., 2016). A marked point pattern is given by the coordinates of ecological objects (here dwarf palms) with a given observation window and attached marks that further characterize the objects. We used here the qualitative mark ‘presence vs. absence’ of weevil larva and the quantitative mark ‘weevil larva abundance’. The goal of the marked point pattern analysis is to determine if the marks show spatial correlations (e.g. occupancy is spatially aggregated over the plants). To this end, we estimated several summary functions that captured different aspects of the spatial structure of larva presence/absence on the dwarf palms (e.g. mark connection and mark correlation functions; Wiegand and Moloney, 2014) and compared them to that of 999 realizations of a null model that represents the null hypothesis of absence of spatial correlations in the marks. For this we used the random labelling (or random marking) null model that randomly shuffles the marks over the palms (Wiegand and Moloney, 2014).
To detect spatial correlations in the occupancy pattern of weevil larvae (qualitatively marked pattern) we used three summary functions to detect the following possibilities of larva occupancy patterns: (1) the conditional probability that, from two dwarf palms that are separated by distance r, both are occupied by larvae [testing for aggregation of occupancy; the mark connection function p11(r)]; (2) similar to (1) the conditional probability that the first palm is occupied but the second palm not [testing if dwarf palms with and without larvae are spatially segregated or aggregated; the mark connection function p12(r)]; and (3) whether dwarf palms containing larvae are preferably located in areas of overall high density of dwarf palms [e.g. high-density clusters) (g1,1 + 2(r) – g2,1 + 2(r)] (details in the Supplementary Data, Appendix).
To detect spatial correlation patterns in larva abundance (quantitatively marked pattern) we used non-normalized uni- and bivariate r-mark correlation functions cm(r) (Illian et al., 2008; Law et al., 2009; Wiegand and Moloney, 2014: section 3.1.7) as summary functions. The univariate function cm(r) is the mean number of larva in palms that have another palm at an approximate distance r. This allows us to determine if palms with a nearby neighbour tend to host a higher number of larvae than the ‘average’ palm. We analysed the univariate pattern of males and females separately (univariate analysis). We use also a bivariate function cm2(r) given by the mean number of larva in female palms that have a male palm at approximate distance r to determine if a female palm hosts fewer larvae if a male palm is nearby (compared to the ‘average’ female palm). Finally, we used the density correlation function Cm,K(r) proposed by Fedriani et al. (2015) to test whether the number of larvae in a focal palm is correlated with the number of palms in its neighbourhood. The density correlation function Cm,K(r) is the classical Pearson correlation coefficient between the larva abundance mi of palm i and the number of neighbours Ki(r) of plant i within distance r [=λKi(r)]. Details can be found in the Appendix.
In all analyses we used 999 Monte Carlo simulations of null models for construction of pointwise simulation envelopes, being the 25th highest and 25th lowest values of the summary function of the simulated patterns (Velázquez et al., 2016). If the observed summary function wanders outside the pointwise simulation envelopes we may have a spatial structure in the marks. To determine if the departure is significant we accounted for effects of multiple testing (Wiegand et al., 2016). For this we used the goodness-of-fit (GoF) promoted by Loosmore and Ford (2006). A departure from the null hypothesis occurred if the P-value of the GoF test was <0.05. For all point pattern analyses, we used the software Programita (Wiegand and Moloney, 2014), which can be accessed at www.programita.org.
All the data are available from the Figshare repository: https://figshare.com/s/084be486a974b0d85033 (Jácome-Flores et al., 2016).
RESULTS
Do D. chamaeropsis larvae develop only in male inflorescences?
The effect of sex and study site on the probability of presence and the average number of larvae was evaluated for the 2011, 2012 and 2013 seasons in Martinazo and for the 2011 and 2013 seasons in Matasgordas. In 2012, the Matasgordas area suffered a fire, burning 97 % of dwarf palm individuals, so we had no data from that year.
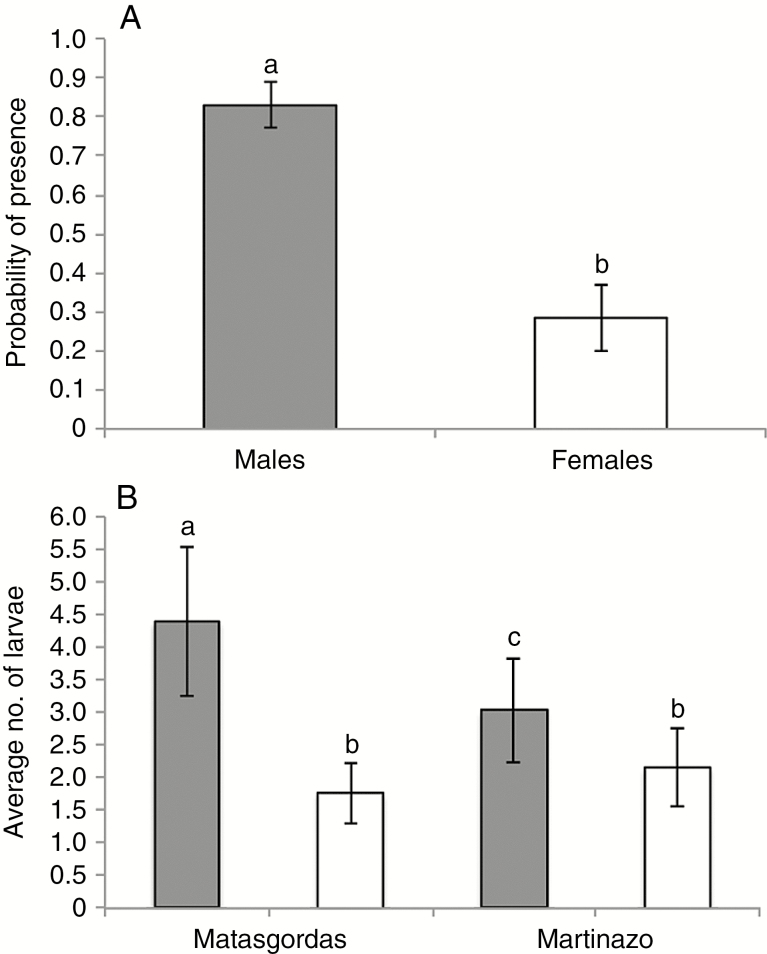
The overall composition of the two populations was 54.9 % female and 45.1 % male individuals. The sex ratio was different for the two populations: Matasgordas was female-biased (females/males 1.35: 1), whereas Martinazo was male-biased (1: 1.16). For the presence model, we found significant differences between the sexes (F1,2074 = 123.23, P < 0.0001). Inflorescences of male plants had a 3.6 times higher probability of having larvae than those of female plants (Fig. 3A). We additionally found significant differences (F1,2074 = 13.45, P = 0.0003) between study sites, with inflorescences in Matasgordas having a lower probability of hosting larva (0.48) than Martinazo (0.68), both before and after the fire. Although the second-order interaction between sex and study site was not significant (F1,2074 = 0.06, P = 0.805), we found that males in Martinazo had a higher probability of hosting larva (0.88) than males in Matasgordas (0.77).
Fig. 3.
(A) Probability of larva presence by plant sex (both sites pooled) and (B) average number of larvae by plant sex and study site. Grey bars represent male plants and white bars represent female plants. Error bars represent the standard error. Different lower-case letters indicate significant differences between bars.
In the abundance model, we excluded plants without larva. In this model, the interaction between sex and study site was significant (F1,293 = 15.09, P = 0.0001). Although in both populations inflorescences of male plants had more larvae than those of female plants, the difference was greater in Matasgordas (Fig. 3B). Tests of slices indicated that in both locations, the differences between males and females were significant (Matasgordas, F1,293 = 120.07, P < 0.0001; Martinazo, F1,293 = 8.04, P < 0.0049). Moreover, inflorescences of male plants in Matasgordas had significantly more larvae than those of males in Martinazo (test of slices, F1,293 = 2.51, P < 0.0001). There were no significant differences between study sites (test of slices, F1,293 = 22.03, P < 0.1139) in abundance of larvae on female plants.
Correlation between the number of inflorescences, flowering synchrony and weevil larvae
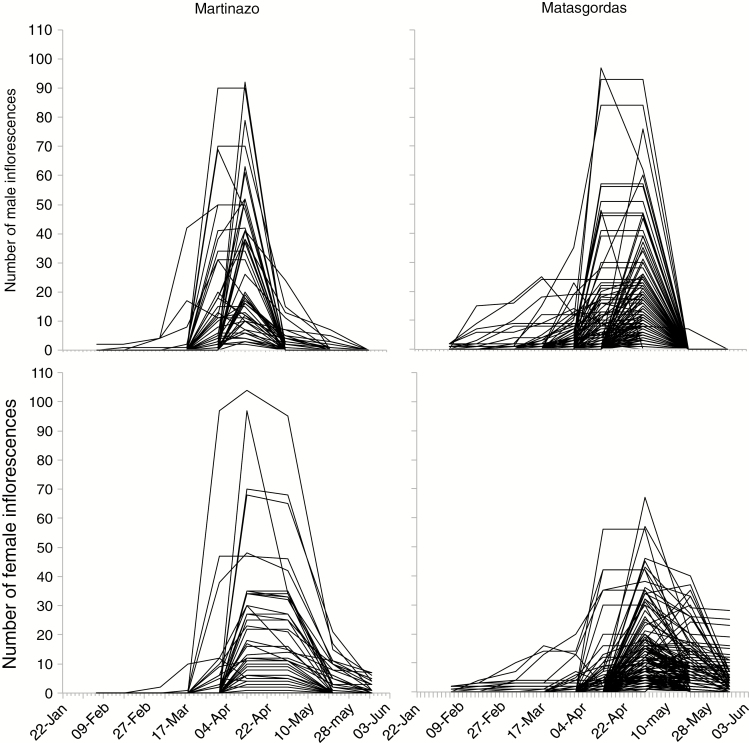
We assessed correlation between the number of inflorescences, flowering synchrony and weevil larvae for the 2013 data. In general, plants followed a unimodal distribution in flower production, beginning in February with an abrupt decrease in May (Fig. 4). The production of female and male inflorescences peaked around 14–16 April in both populations. By the end of April, 97 % of female plants and 94 % of male plants had reached anthesis. Total flowering time was 121 d in Martinazo and 109 d in Matasgordas. Overall, population synchrony ranged from 0.18 to 0.92, with a mean of 0.63 and a standard error of 0.001.
Fig. 4.
Flowering phenology patterns for all plants in Martinazo (left column; 50 males and 41 females) and Matasgordas (right column; 145 males and 169 females). Each line represents an individual plant’s flowering pattern.
The GLMM for the synchrony index (which was corrected for the effects of random factors) indicated significant differences between sexes (F1,10 = 21.69, P = 0.0009). Male inflorescences were on average more synchronous (0.75 ± 0.01, mean ± s.e.) than females (0.67 ± 0.02). We found no significant differences between the two study sites (F1,10 = 3.93, P = 0.08). Our model did not reveal any significant differences in the mean number of inflorescences produced by males (40.84 ± 2.37) and females (38.99 ± 2.29; F1,1 = 0.32 P = 0.673), again with no significant differences between the study sites (F1,1 = 37.69, P = 0.1028).
Incorporating the measures of flowering synchrony and number of inflorescences into the presence GLMMs and using the ΔAICc < 2 model selection criterion, we found two best-supported models (Table 1A; models 8 and 10). The most parsimonious model was model 8 without an interaction between sex and flowering synchrony, where the differences in sex and flowering synchrony did not have an effect on the presence of larvae. Instead, for the abundance GLMMs we found three best-supported models (Table 1B, models 5, 7 and 9) where the interaction between sex and flowering synchrony seems to be constant. We selected the most parsimonious model, model 5, with only one interaction. The results of the selected models were used to evaluate the effect of each variable (Table 2).
Table 2.
Type III effects: relevance of an individual’s inflorescence traits on larva abundance and presence in 2013; the explanatory variables for each response variable are those included in the best supported models
| Explanatory variables | d.f. | F | P |
|---|---|---|---|
| Presence | |||
| Sex | 1, 529 | 27.3 | <0.0001 |
| Ninflor | 1, 529 | 4.23 | 0.0401 |
| Synchrony | 1, 529 | 0.63 | 0.4268 |
| Study site | 1, 529 | 4.51 | 0.0341 |
| Sex × Ninflor | 1, 529 | 6.37 | 0.0119 |
| Ninflor × Synchrony | 1, 529 | 6.17 | 0.0133 |
| Abundance | |||
| Sex | 1, 135 | 14.86 | 0.0002 |
| Ninflor | 1, 135 | 1.19 | 0.2782 |
| Synchrony | 1, 135 | 17.35 | <0.0001 |
| Study site | 1, 135 | 4.23 | 0.0416 |
| Synchrony × Sex | 1, 135 | 4.65 | 0.0328 |
For the presence model, we found significant effects of all main factors except flower synchrony (Table 2). As we know, males were more likely to hold larvae (0.80 ± 0.03) than females (0.33 ± 0.04). Study site had an effect on larva presence: plants in the high-density study site had a lower probability of holding larvae (Matasgordas = 0.52) than those of the low-density study site (Martinazo = 0.65). In the first significant interaction (sex and number of inflorescences) we found that the inflorescences with a greater probability of having larvae were those male inflorescences on plants with a low number of inflorescences (estimate = −0.97); the same negative correlation was found in female inflorescences within a plant with a low number of inflorescences but the intensity of this interaction was higher (estimate = −0.114). The second interaction (number of inflorescences and flowering synchrony) was related to an increased probability of having larvae not only in the plants that are more synchronous, but also in those with a low number of inflorescences.
For the abundance model (where we exclude palms with zero larvae), we found that sex was again a variable with an important effect, along with the number of inflorescences and study site (Table 2). We found that male inflorescences had more larvae (8.45 ± 0.53) than females (3.96 ± 0.36). This means that male inflorescences were not only more prone to having larvae but also tended to have larvae in greater numbers. The flowering synchrony of dwarf palms had a significant positive effect (estimate = 0.72) on abundance. Furthermore, we found that dwarf palms in the Matasgordas study site had on average more larvae (6.4) than those in Martinazo (5.2). Finally, the interaction between synchrony and sex had an important effect: the most synchronous female and male plants had on average more larvae, but the effect was stronger in males (estimate males = 1.75, females = 1.45).
Spatial structure in larval occupancy
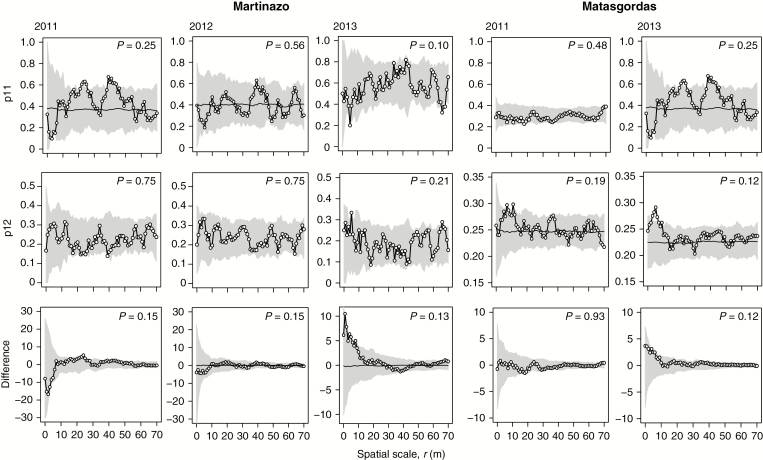
Random labelling analysis showed that the presence of larvae in dwarf palms followed largely random patterns. We did not find evidence for spatial correlations in larva presence; the observed mark connection functions for the two study sites in the two years were inside the simulation envelopes (Fig. 5). The results of the mark connection function p11(r) indicated that the occupancy of palms was not enhanced if another occupied palm was nearby (i.e. no aggregation of dwarf palms with larvae within the pattern of all dwarf palms). The bivariate function p12(r) showed that dwarf palms with and without larvae were not segregated or aggregated within all palms. Finally, the test of g1,1 + 2(r) – g2,1 + 2(r) showed that the density of dwarf palms did not influence their occupancy; larvae were not more likely to be present in palms with more palm neighbours.
Fig. 5.
Random labelling for larva presence and absence in all the plants. The summary functions used were: p11(r): tests the conditional probability that, from two dwarf palms that are separated by distance r, both are type 1; p12(r): gives the conditional probability that, from two dwarf palms that are separated by distance r, the first is type 1 (i.e. with larvae) and the second is type 2 (i.e. without larvae); Difference: compares the density of dwarf palms (i.e. 1 + 2) around dwarf palms with larvae with the density of dwarf palms around dwarf palms without larvae (i.e. type 2). The white circles represent the distribution for the used statistic. Filled circles: empirical mark connection function; grey shading: expectation under the null model; and black lines: simulation envelopes constructed from 25th lowest and highest values taken from 999 simulations of the null model where plants with larvae and without larvae were independently shuffled.
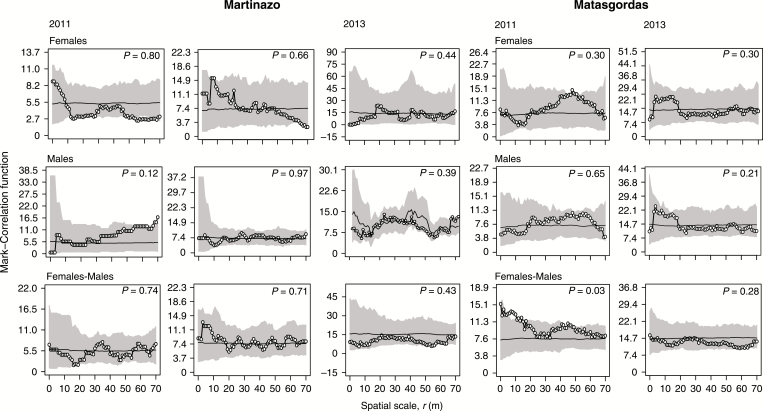
Spatial structure in larva abundance of male and female palms
There was no significant aggregation of larva abundance at any spatial scale. The number of larvae in dwarf palms was randomly distributed in both study sites (Fig. 6). The only spatial structure that we found was for the year 2011 in Matasgordas, where female palms hosted more larvae if they were close to males (P = 0.03, rank = 196) at small spatial scales (5–10 m).
Fig. 6.
Univariate r-mark correlation function for each sex (males and females) and bivariate r-mark correlation function (females vs. males), both used to quantify potential spatial associations of the larva abundance in both populations. The expected non-normalized mark correlation function (white circles) corresponds to the number of larva. Other conventions are as described in Fig. 5.
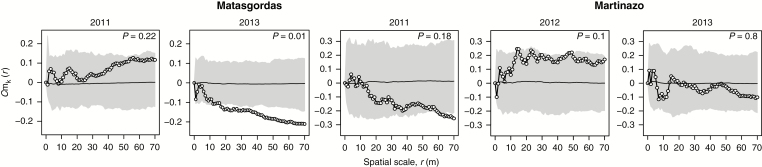
The density correlation function Cm,K(r) did not detect any effect of palm density on larval abundance in any year at Martinazo (P > 0.05; Fig. 7). However, we found a highly significant and negative density dependence at distances larger than 16 m for Matasgordas in 2013.This means that palms with more neighbours at distances >16 m tended to host fewer larvae (Fig. 7).
Fig. 7.
Larva abundance. The density correlation function Cm,K is the correlation between the mark mi (number of larvae) and the number of neighbours within distance r. Other conventions are as described in Fig. 5.
DISCUSSION
To our knowledge, this is the first study addressing the effects of flowering synchrony and plant spatial patterns on the presence and abundance of larvae of its nursery pollinator. At the plant level, we found a strong correlation between larva presence/abundance and plant sex. Also, flowering synchrony and number of inflorescences influenced weevil larva abundance and presence. At the population level, we found that study site did not affect larva presence and abundance, except for the differences in larval abundance in the male inflorescences in each location. Incorporating flowering phenology traits such as synchrony and number of inflorescences (only data for 2013), we found an unexpected negative correlation between larva occupancy and the density of plants in each study site. Finally, we were unable to detect any spatial effect of density and distance dependence on larva occupancy and abundance.
One of the most interesting findings of our study relates to plant sex. We found that male plants showed a higher probability of hosting larvae as well as hosting higher numbers of larvae. According to Dufaÿ and Anstett (2004), weevil larvae only develop within male inflorescences, with benefits to the reproductive success of the dwarf palm, because fruit development would not be affected by weevil occupancy. Furthermore, these authors suggested that weevil larvae could not develop on female inflorescences due to a defence mechanism associated with the process of fruit development. In our 3-year field study, we found that in each year almost 30 % of the female dwarf palms held weevil larvae. However, we observed that female inflorescences with larvae were those with a low mean proportion of fruit-set (0.07) and in those without fruits, in the same vein as the findings of Anstett (1999) and Dufaÿ and Anstett (2004). The occurrence of these poorly or non-pollinated inflorescences could be related to a low abundance of pollinators, leaving some inflorescences unvisited. This is a common pattern in plants with a high number of flowers, where intraspecific pollinator competition results in pollinator visits being diluted among the multitude of flowering resources (Fritz and Nilsson, 1994; Larson and Barrett, 2000; Delmas et al., 2014). We found, contrary to our predictions, that the number of inflorescences was negatively correlated with the presence of larvae on both sexes. Plants with higher numbers of inflorescences might have a lower probability of holding weevil larvae due to a resource dilution effect because an increase in available inflorescences in a plant outstrips the increase in arriving weevils. This has been described in similar systems such as for the globeflower Trollius europaeus and its specialist pollinator and seed predator, the fly Chiastocheta spp. (Klank et al., 2010). From the weevils’ perspective, the non-pollinated inflorescences represent an available resource for oviposition and larval development. We therefore concluded that for low pollinator availability, there would be fewer pollinated female inflorescences, which would increase the number of oviposition sites for weevils. Although weevils cannot avoid deceptive flowers because the arrest of larval development occurs only after fruits have started to develop (Dufaÿ and Anstett, 2004), our findings suggest that females, in general, do not cheat pollinators by killing their larvae as proposed by Dufaÿ and Anstett (2004), but instead provide a reduced reward relative to males.
Many studies find that highly synchronous flowering plants are more attractive to pollinators (Eckhart, 1991; Ashman, 2005; Castilla et al., 2011). However, we know very little about how floral synchrony relates to the pollinators’ survival and reproduction in nursery pollination systems. In our system, this pattern could be related to synchronicity between two events: weevil emergence and emission of volatile compounds during flower anthesis (Dufaÿ et al., 2004). If a plant flowers before weevil emergence, there will be a lower probability that a weevil oviposits on it. On the other hand, if a plant flowers too late, there will be few available weevils because the majority would have already oviposited in another plant. Dufaÿ (2010) found that the number of freshly emerged weevils decreased strongly with time, suggesting lower availability of pollinators that could oviposit in late-flowering plants. Thus, synchronous flowering may be a good strategy to attract pollinators (Elzinga et al., 2007). Of course, the effect of this variable was stronger when we analysed its interaction with plant sex, as explained above. Furthermore, similar to Dufaÿ (2010) we found that male dwarf palms tended to flower more synchronously in our study sites, and females ended their anthesis later, with some overlap with male flowering. This could have a positive effect on the oviposition and pollination of female dwarf palms. Highly synchronous males reach the end of anthesis at the same time, leaving no resources for weevils, so they have to abandon these plants and travel to less rewarding females with belated available inflorescences in anthesis. Dufaÿ (2010) found that weevils visit female plants entering anthesis by the time many males were finished flowering, and produced the highest proportions of fully pollinated fruits, suggesting better pollination. Finally, the interaction between synchrony and number of inflorescences had a significant effect only for larva presence. We found that the dwarf palms with the highest probability of holding larvae were those with high flowering synchrony values (where plant anthesis coincides with weevil emergence) and with few inflorescences, which increased the probability of oviposition. Individual plants in other nursery pollination systems present a similar pattern (Klank et al., 2010). It seems that flowering synchronously with a high number of inflorescences is a strategy developed in these systems to mitigate the antagonistic effect of pollinators over seed predation, through a form of predator satiation.
In terms of plant population, we found a slight effect of study site, possibly related to plant density. Matasgordas had a density three times higher than Martinazo, which could impinge upon larva presence and abundance in each plant. Although high plant density could be more attractive to weevils due to a higher presence of volatiles, plants in dense patches could experience high competition for pollinators. Thus, the resource dilution effect is consistent at the population level, where dwarf palms in high-density neighbourhoods ‘diminish the capacity’ of weevils to oviposit in all available dwarf palms. Furthermore, in another study in the same areas (M. E. Jácome-Flores et al., unpubl. data), we found that pollination success (measured as fruit initiation) was 1.42-fold higher at Martinazo. This result provides a good proxy for adult weevil abundance and suggests that Matasgordas had pollinator limitation.
In terms of spatial patterns, we expected a higher probability of weevil presence and higher weevil abundance in palms surrounded by more neighbours, where resources are more abundant (Aizen and Vázquez, 2006; Fedriani et al., 2015). Plant selection by an insect (e.g. suitable oviposition sites) has been described as a major cause of non-random patterns in insect–host associations (Kunin, 1993; Groom, 1998; Singer et al., 2002; Davis et al., 2004; Gascoigne et al., 2009), and the dwarf palm distribution shows a strong aggregated pattern (Jácome-Flores et al., 2016). We believe that, in our study sites, D. chamaeropsis weevils use only dwarf palms as hosts, so plant distribution should have a very strong effect on the distribution of larva presence and abundance. However, we did not find a distance-dependent effect on the number of larvae.
One explanation for the absence of spatial patterns would be low statistical power due to low sample sizes. In general, the width of the simulation envelopes is inversely related to the number of points of the pattern (Wiegand et al., 2016). Thus, if the sample size is small (<100; Wiegand et al., 2016) we may not detect spatial patterns because the simulation envelopes become too wide. Thus, our analysis at Martinazo may be prone to low statistical power because the sample size was low (42 female and 49 male palms), but this should not be the case at Matasgordas with sufficiently high sample sizes (167 female and 127 male palms). An alternative biological explanation for the absence of spatial effects is that the pollinator is highly mobile. This was demonstrated with a ‘natural experiment’ caused by the 2012 fire in Matasgordas, where most inflorescences with their weevil larvae were eliminated. Fortunately, 95 % of the burned palms survived, due to their capacity to resist fires (Granados et al., 1988; Herrera, 1989). Flowering the following year occurred normally, and weevils coming from unburned palms were capable of ovipositing even in the most distant individuals (235 m away from any conspecific), with no changes in the distance dependence patterns. Applying a density dependence function, we found that the number of larvae decreased with an increase in the number of plants, which is a transient dilution effect created by disturbance (Otway et al., 2005). We expect that once the weevil’s populations recover, the pattern will again be random, as found in the Martinazo study site and the first year at Matasgordas. It seems that a lack of density and distance dependence of host occupancy are not rare in nursery pollination systems. For instance, studies of the Lophocereus–Upiga (Holland and Fleming, 1999) and Trollius–Chiastocheta (Klank et al., 2010) systems revealed no direct effects of plant population size or population-level plant density on the abundance of nursery pollinators, with occupancy rates being spatially uniform.
In summary, our results demonstrate that in a nursery-pollinated dioecious palm, plant sex, flowering display and flowering synchrony act as additive forces influencing the presence and abundance of the specialized pollinator larvae. Furthermore, despite the fact that D. chamaeropsis use mainly male inflorescences, we found clear evidence that female dwarf palms also provide rewarding oviposition sites, and thus female plants ‘pay’ for the pollination services. Our findings highlight that plant local aggregation is not always the primary determinant of pollinator attraction, whereas flower traits and phenology could be critical in specialized plant–pollinator interactions. This study raises questions about nursery pollination in dioecious species. In particular, future research should focus on whether and how the number of visits by adult weevils impinges upon C. humilis fruit initiation and development. On the other hand, the spatial arrangement of floral resources in our highly synchronous populations did not influence the larva occupancy pattern, suggesting a high dispersal ability of adult weevils. Our study exhaustively documents the spatial and temporal patterning of pollinator occurrence and abundance in a poorly studied system; it reveals new costs/benefits associated with such interactions and thus furthers our understanding of the ecology and evolution of nursery pollination.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Appendix: Spatial structure in larva occupancy and abundance with respect to host plant sex.
ACKNOWLEDGEMENTS
We thank Gemma Calvo, Encarnación Rico and Irene Castañeda for their field assistance. We sincerely thank the staff of the National Park Service and Doñana Biological Station, in particular Sofía Conradi, for their invaluable support throughout different stages of our study. Xavier Picó and Juan Arroyo provided helpful comments on early versions of the manuscript. We thank Bill Shipley and two anonymous referees for constructive criticism on early versions of the manuscript. MEJF was supported by a postdoctoral fellowship from the Consejo Nacional de Ciencia y Tecnología (265369). JMF was funded by a Marie Curie Intraeuropean fellowship (FP7-PEOPLE-2011-IEF-298137) and a Portuguese Fundaçao para a Ciência e a Tecnologia grant (IF/00728/2013). TW was supported by European Research Council advanced grant 233066. This research was carried out under project CGL2010-21926 of the Spanish Ministry of Science and Innovation.
LITERATUTE CITED
- Aguilar R, Ashworth L, Galetto L, Aizen MA. 2006. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters 9: 968–980. [DOI] [PubMed] [Google Scholar]
- Aizen MA, Vázquez DP. 2006. Flower performance in human-altered habitats. In: Harder LD, Barrett SCH, eds. Ecology and evolution of flowers. Oxford University Press on Demand, 159–182. [Google Scholar]
- Aker CL. 1982. Spatial and temporal dispersion patterns of pollinators and their relationship to the flowering strategy of Yucca whipplei (Agavaceae). Oecologia 54: 243–252. [DOI] [PubMed] [Google Scholar]
- Anker R. 1990. The ecology and evolution of reproductive synchrony. Trends in Ecology & Evolution 5: 135–140. [DOI] [PubMed] [Google Scholar]
- Anstett M-C. 1999. An experimental study of the interaction between the dwarf palm (Chamaerops humilis) and its floral visitor Derelomus chamaeropsis throughout the life cycle of the weevil. Acta Oecologica 20: 551–558. [Google Scholar]
- Anstett MC, Hossaert-McKey M, Kjellberg F. 1997. Figs and fig pollinators: evolutionary conflicts in a coevolved mutualism. Trends in Ecology and Evolution 12: 94–99. [DOI] [PubMed] [Google Scholar]
- Ashman T-L. 2005. The limits on sexual dimorphism in vegetative traits in a gynodioecious plant. The American Naturalist 166 (Suppl): S5–S16. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Knight TM, Steets JA et al. 2004. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 85: 2408–2421. [Google Scholar]
- Augspurger CK. 1981. Reproductive synchrony of a tropical shrub: experimental studies on effects of pollinators and seed predators in Hybanthus prunifolius (Violaceae). Ecology 62: 775–788. [Google Scholar]
- Augspurger CK. 1983. Phenology, flowering synchrony, and fruit set of six neotropical shrubs. Biotropica 15: 257–267. [Google Scholar]
- Bennington C, Thayne W. 1994. Use and misuse of mixed model analysis of variance in ecological studies. Ecology 75: 717–722. [Google Scholar]
- Bopp S, Gottsberger G. 2004. Importance of Silene latifolia ssp. alba and S. dioica (Caryophyllaceae) as host plants of the parasitic pollinator Hadena bicruris (Lepidoptera, Noctuidae). Oikos 105: 221–228. [Google Scholar]
- Buchmann SL, Nabhan GP. 2012. The forgotten pollinators. Washington, DC: Island Press. [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer. [Google Scholar]
- Castilla AR, Alonso C, Herrera CM. 2011. Exploring local borders of distribution in the shrub Daphne laureola: Individual and populations traits. Acta Oecologica 37: 269–276. [Google Scholar]
- Cook JM, Rasplus JY. 2003. Mutualists with attitude: coevolving fig wasps and figs. Trends in Ecology and Evolution 18: 241–248. [Google Scholar]
- Davis HG, Taylor CM, Civille JC, Strong DR. 2004. An allee effect at the front of a plant invasion: Spartina in a Pacific estuary. Journal of Ecology 92: 321–327. [Google Scholar]
- Delmas CEL, Escaravage N, Cheptou PO et al. 2014. Relative impact of mate versus pollinator availability on pollen limitation and outcrossing rates in a mass-flowering species. Plant Biology 17: 209–218. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jürgens A. 2005. Spatial fragrance patterns in flowers of Silene latifolia: Lilac compounds as olfactory nectar guides?Plant Systematics and Evolution 255: 99–109. [Google Scholar]
- Dufaÿ M. 2010. Impact of plant flowering phenology on the cost/benefit balance in a nursery pollination mutualism, with honest males and cheating females. Journal of Evolutionary Biology 23: 977–986. [DOI] [PubMed] [Google Scholar]
- Dufaÿ M, Anstett M-C. 2003. Conflicts between plants and pollinators that reproduce within inflorescences : evolutionary variations on a theme. Oikos 1: 3–14. [Google Scholar]
- Dufaÿ M, Anstett M-C. 2004. Cheating is not always punished : killer female plants and pollination by deceit in the dwarf palm Chamaerops humilis. Journal of Evolutionary Biology 17: 862–868. [DOI] [PubMed] [Google Scholar]
- Dufaÿ M, Hossaert-McKey M, Anstett M-C. 2003. When leaves act like flowers: how dwarf palms attract their pollinators. Ecology Letters 6: 28–34. [Google Scholar]
- Dufaÿ M, Hossaert-McKey M, Anstett M-C. 2004. Temporal and sexual variation of leaf-produced pollinator-attracting odours in the dwarf palm. Oecologia 139: 392–398. [DOI] [PubMed] [Google Scholar]
- Dupont YL, Padrón B, Olesen JM, Petanidou T. 2009. Spatio-temporal variation in the structure of pollination networks. Oikos 118: 1261–1269. [Google Scholar]
- Eckhart VM. 1991. The effects of floral display on pollinator visitation vary among populations of Phacelia linearis (Hydrophyllaceae). Evolutionary Ecology 5: 370–384. [Google Scholar]
- Elzinga JA, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G. 2007. Time after time : flowering phenology and biotic interactions. Trends in Ecology & Evolution 22: 432–439. [DOI] [PubMed] [Google Scholar]
- Fagan WF, Bewick S, Cantrell S, Cosner C, Varassin IG, Inouye DW. 2014. Phenologically explicit models for studying plant–pollinator interactions under climate change. Theoretical Ecology 7: 289–297. [Google Scholar]
- Fedriani JM, Wiegand T, Calvo G et al. 2015. Unraveling conflicting density and distance dependent effects on plant reproduction using a spatially explicit approach. Journal of Ecology 103: 1344–1353. [Google Scholar]
- Feil JP. 1992. Reproductive ecology of dioecious Siparuna (Monimiaceae) in Ecuador, a case of gall midge pollination. Botanical Journal of the Linnean Society 110: 171–203. [Google Scholar]
- Fritz A-L, Nilsson LA. 1994. How pollinator-mediated mating varies with population size in plants. Oecologia 100: 451–462. [DOI] [PubMed] [Google Scholar]
- Gascoigne J, Berec L, Gregory S, Courchamp F. 2009. Dangerously few liaisons: a review of mate-finding allee effects. Population Ecology 51: 355–372. [Google Scholar]
- Granados M, Martin A, García Novo F. 1988. Long-term vegetation changes on the stabilized dunes of Doñana National. Vegetatio 75: 73–80. [Google Scholar]
- Groom MJ. 1998. Allee effects limit population viability of an annual plant. The American Naturalist 151: 487–96. [DOI] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. (eds) 2006. Ecology and evolution of flowers. Oxford: Oxford University Press. [Google Scholar]
- Herre EA, Jandér K, Machado CA. 2008. Evolutionary ecology of figs and their associates: recent progress and outstanding puzzles. Annual Review of Ecology, Evolution, and Systematics 39: 439–458. [Google Scholar]
- Herrera J. 1989. On the reproductive biology of the dwarf palm, Chamaerops humilis in southern Spain. Principes 33: 27–33. [Google Scholar]
- Holland JN, Fleming TH. 1999. Geographic and population variation in pollinating seed-consuming interactions between senita cacti (Lophocereus schottii) and senita moths (Upiga virescens). Oecologia 121: 405–410. [DOI] [PubMed] [Google Scholar]
- Hossaert-McKey M, Proffit M, Soler CCL et al. 2016. How to be a dioecious fig: chemical mimicry between sexes matters only when both sexes flower synchronously. Scientific Reports 6: 21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illian DJ, Penttinen PA, Stoyan DH, Stoyan D. 2008. Statistical analysis and modelling of spatial point patterns. Chichester: John Wiley & Sons. [Google Scholar]
- Jácome-Flores ME, Delibes M, Wiegand T, Fedriani JM. 2016. Spatial pattern of an endemic Mediterranean palm at its colonization front. Ecology and Evolution 6: 8556–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H, Endels P, Honnay O, Wiegand T. 2010. Evaluating management interventions in small populations of a perennial herb Primula vulgaris using spatio-temporal analyses of point patterns. Journal of Applied Ecology 47: 431–440. [Google Scholar]
- Janzen DH. 1979. How to be a fig. Annual Review of Ecology and Systematics 10: 13–51. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 1996. Reproduction and pollination in central European populations of Silene and Saponaria species. Botanica Acta 109: 316–324. [Google Scholar]
- Kjellberg F, Gouyon P, Ibrahim M, Raymond M, Valdeyron G. 1987. The stability of the symbiosis between dioecious figs and their pollinators: a study of Ficus. Evolution 41: 693–704. [DOI] [PubMed] [Google Scholar]
- Klank C, Pluess AR, Ghazoul J. 2010. Effects of population size on plant reproduction and pollinator abundance in a specialized pollination system. Journal of Ecology 98: 1389–1397. [Google Scholar]
- Klein A-M, Vaissière BE, Cane JH et al. 2007. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society, Biological Sciences 274: 303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin WE. 1993. Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology 74: 2145–2160. [Google Scholar]
- Labouche A-M, Bernasconi G. 2010. Male moths provide pollination benefits in the Silene latifolia - Hadena bicruris nursery pollination system. Functional Ecology 24: 534–544. [Google Scholar]
- Larson BMH, Barrett SCH. 2000. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society 69: 503–520. [Google Scholar]
- Law R, Illian J, Burslem DFRP, Gratzer G, Gunatilleke CVS, Gunatilleke IAUN. 2009. Ecological information from spatial patterns of plants: insights from point process theory. Journal of Ecology 97: 616–628. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. 2006. SAS for mixed models. Cary, NC: SAS Institute Inc. [Google Scholar]
- Loosmore NB, Ford ED. 2006. Statiscal inference using the G or K point pattern spatial statistics. Ecology 87: 1925–1931. [DOI] [PubMed] [Google Scholar]
- Marquis RJ. 1988. Phenological variation in the neotropical understory shrub Piper arielanum. Ecology 69: 1552–1565. [Google Scholar]
- Melampy MN. 1987. Flowering phenology, pollen flow and fruit production in the andean shrub Befaria resinosa. Oecologia 73: 293–300. [DOI] [PubMed] [Google Scholar]
- Norstog KJ, Fawcett PKS. 1989. Insect-cycad symbiosis and its relation to the pollination of Zamia furfuracea (Zamiaceae) by Rhopalotria mollis (Curculionidae). American Journal of Botany 76: 1380–1394. [Google Scholar]
- Otway SJ, Hector A, Lawton JH. 2005. Resource dilution effects on specialist insect herbivores in a grassland biodiversity experiment. Journal of Animal Ecology 74: 234–240. [Google Scholar]
- Price MV, Waser NM, Irwin RE, Campbell DR, Brody AK. 2005. Temporal and spatial variation in pollination of a montane herb: a seven-year study. Ecology 86: 2106–2116. [Google Scholar]
- Rathcke B. 1983. Competition and facilitation among plants for pollination In: Pollination biology, 305–329. [Google Scholar]
- Richards AJ. 1986. Plant breeding systems. London: George Allen & Unwin. [Google Scholar]
- Ricketts TH, Regetz J, Steffan-Dewenter I et al. 2008. Landscape effects on crop pollination services: are there general patterns?Ecology Letters 11: 499–515. [DOI] [PubMed] [Google Scholar]
- SAS 2013. Base SAS 9.4 ® Procedures Guide: Statistical Procedures. Cary, NC: SAS Institute Inc. [Google Scholar]
- Singer MC, Stefanescu C, Pen I. 2002. When random sampling does not work: standard design falsely indicates maladaptive host preferences in a butterfly. Ecology Letters 5: 1–6. [Google Scholar]
- Sun H, Cheng J, Zhang F, Luo Y, Ge S. 2009. Reproductive success of non-rewarding Cypripedium japonicum benefits from low spatial dispersion pattern and asynchronous flowering. Annals of Botany 103: 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki RO, Kudoh H, Kachi N. 2003. Spatial and temporal variations in mortality of the biennial plant, Lysimachia rubida: effects of intraspecific competition and environmental heterogeneity. Journal of Ecology 91: 114–125. [Google Scholar]
- Traveset A, Sáez E. 1997. Pollination of Euphorbia dendroides by lizards and insects: spatio-temporal variation in patterns of flower visitation. Oecologia 111: 241–248. [DOI] [PubMed] [Google Scholar]
- Velázquez E, Martínez I, Getzin S, Moloney KA, Wiegand T. 2016. An evaluation of the state of spatial point pattern analysis in ecology. Ecography 39: 1–14. [Google Scholar]
- Wiegand T, Moloney KA. 2014. Summary Statistics for Quantitatively Marked Point Patterns In: Smith R, ed. Handbook of spatial point-pattern analysis in ecology. Boca Raton, FL: CRC Press, 212–234. [Google Scholar]
- Wiegand T, Grabarnik P, Stoyan D. 2016. Envelope tests for spatial point patterns with and without simulation. Ecosphere 7: 1–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.