Abstract
Background
Drug memories become labile and reconsolidated after retrieval by presentation of environmental cues (conditioned stimulus) or drugs (unconditioned stimulus). Whether conditioned stimulus and unconditioned stimulus retrieval trigger different memory reconsolidation processes is not clear.
Methods
Protein synthesis inhibitor or β-adrenergic receptor (β-AR) antagonist was systemically administrated or intra-central amygdala infused immediately after cocaine reexposure in cocaine-conditioned place preference or self-administration mice models. β-ARs were selectively knocked out in the central amygdala to further confirm the role of β-adrenergic receptor in cocaine reexposure-induced memory reconsolidation of cocaine-conditioned place preference.
Results
Cocaine reexposure triggered de novo protein synthesis dependent memory reconsolidation of cocaine-conditioned place preference. Cocaine-priming-induced reinstatement was also impaired with post cocaine retrieval manipulation, in contrast to the relapse behavior with post context retrieval manipulation. Cocaine retrieval, but not context retrieval, induced central amygdala activation. Protein synthesis inhibitor or β1-adrenergic receptor antagonist infused in the central amygdala after cocaine retrieval, but not context retrieval, inhibited memory reconsolidation and reinstatement. β1-adrenergic receptor knockout in the central amygdala suppressed cocaine retrieval-triggered memory reconsolidation and reinstatement of cocaine conditioned place preference. β1-adrenergic receptor antagonism after cocaine retrieval also impaired reconsolidation and reinstatement of cocaine self-administration.
Conclusions
Cocaine reward memory triggered by unconditioned stimulus retrieval is distinct from conditioned stimulus retrieval. Unconditioned stimulus retrieval induced reconsolidation of cocaine reward memory depends on β1-adrenergic signaling in the central amygdala. Post unconditioned stimulus retrieval manipulation can prevent drug memory reconsolidation and relapse to cocaine, thus providing a potential strategy for the prevention of substance addiction.
Significance Statement
It is well known that drug memories become labile and reconsolidated upon retrieval by the presentation of conditioned stimulus (CS) or unconditioned stimulus (US). Whether CS and US retrieval trigger different memory reconsolidation processes is unknown. In this study, we found that US retrieval, but not CS retrieval, triggered memory reconsolidation of cocaine-conditioned place preference dependent on β1-AR and de novo protein synthesis in the central amygdala. Furthermore, cocaine priming-induced reinstatement was impaired with post US retrieval manipulation in contrast to the relapse behavior with post CS retrieval manipulation. In cocaine self-administration, β1-AR antagonism after US retrieval also impaired reconsolidation and reinstatement. Our study indicates that reconsolidation of cocaine reward memory triggered by US retrieval is distinct from CS retrieval. US retrieval induced reconsolidation of cocaine reward memory depends on β1-adrenergic signaling in the central amygdala.
Keywords: β-AR, unconditioned stimulus, conditioned stimulus, memory reconsolidation, cocaine
Significance Statement
It is well known that drug memories become labile and reconsolidated upon retrieval by the presentation of conditioned stimulus (CS) or unconditioned stimulus (US). Whether CS and US retrieval trigger different memory reconsolidation processes is unknown. In this study, we found that US retrieval, but not CS retrieval, triggered memory reconsolidation of cocaine-conditioned place preference dependent on β1-AR and de novo protein synthesis in the central amygdala. Furthermore, cocaine priming-induced reinstatement was impaired with post US retrieval manipulation in contrast to the relapse behavior with post CS retrieval manipulation. In cocaine self-administration, β1-AR antagonism after US retrieval also impaired reconsolidation and reinstatement. Our study indicates that reconsolidation of cocaine reward memory triggered by US retrieval is distinct from CS retrieval. US retrieval induced reconsolidation of cocaine reward memory depends on β1-adrenergic signaling in the central amygdala.
Introduction
Drug addiction is commonly considered a learning and memory disorder (Boening, 2001; Hyman, 2005). The reinforcing effects of a drug (known as unconditioned stimulus [US]), such as drug-elicited euphoria, are strongly associated with environmental cues (known as conditioned stimulus [CS]) (Hyman and Malenka, 2001; Shalev et al., 2002; Koob and Volkow, 2016). Consequently, exposure to drug-associated environmental cues can evoke various degrees of psychological dependence or compulsive drug taking (Cadet et al., 2014; Wise and Koob, 2014). A major challenge in the treatment of drug addiction is drug relapse, which can occur even after prolonged abstinence (Meil and See, 1996; Li et al., 2016). Therefore, treatments that weaken the strength of drug-associated memories represent a potential clinical prevention method for addicted individuals.
Disruption of the association between cues and a drug might attenuate cravings induced by cue reexposure. One way to achieve this goal is through interference with the reconsolidation process (Lee et al., 2005). Consolidated memory enters an unstable state after memory retrieval, thus rendering a memory process temporarily susceptible to disruption by an intervention. Memory then undergoes an additional consolidation-like process and is restabilized as long-term memory (Misanin et al., 1968; Nader et al., 2000a; Tronson and Taylor, 2007). This dynamic process is known as memory reconsolidation (Rodriguez-Ortiz and Bermudez-Rattoni, 2007; Jones et al., 2012). Manipulation of memory reconsolidation can update previously learned memories with new information and strengthen or weaken preexisting memories (Tronson et al., 2006). In fact, intervention in the reconsolidation process provides a potential way to prevent memory storage in various learning paradigms in animals, including spatial learning (Przybyslawski and Sara, 1997; Flint et al., 2007), fear conditioning (Debiec and Ledoux, 2004), and cocaine-induced reward memory (Milton and Everitt, 2010).
Studies have shown that cue reexposure, as CS retrieval, renders memory temporarily susceptible to suppression by post retrieval interventions (Nadel and Land, 2000; Nader and Hardt, 2009). In addition to cue reexposure, footshock reexposure can also trigger a reconsolidation process as cue reexposure (Debiec et al., 2010; Diaz-Mataix et al., 2011; Liu et al., 2014). Studies have reported that propranolol, the nonselective β-adrenergic receptor (β-AR) antagonist, treated after nicotine reexposure inhibits nicotine cravings in rat and human models (Xue et al., 2017a), and noncontingent cocaine injection 1 hour before an extinction session decreases cocaine relapse in the tests of reinstatement, spontaneous recovery, and renewal of cocaine seeking in rats (Luo et al., 2015). Garcinol, a histone acetyltransferase inhibitor, impairs cocaine reexposure-induced memory reconsolidation of cocaine self-administration (SA) (Dunbar and Taylor, 2017). These studies suggest that drug reexposure, as US retrieval, can also trigger drug memory reconsolidation. A key issue is whether US retrieval and CS retrieval are different in the induction of the memory reconsolidation process.
Many studies have demonstrated that β-AR is involved in reconsolidation, because systemic or intra-basolateral amygdala (BLA) injection of a β-AR antagonist immediately after CS retrieval prevents memory reconsolidation of cocaine-SA or conditioned place preference (CPP) (Milton et al., 2008; Otis et al., 2013). In the signaling cascades downstream of β-AR, amygdala protein kinase A and ERK are both involved in the reconsolidation of cocaine-related contextual memories (Wells et al., 2013; Arguello et al., 2014). However, the underlying molecular mechanisms involved in US retrieval-induced drug memory reconsolidation remain unknown.
In this study, we investigated possible differences between US retrieval and CS retrieval-triggered reconsolidation of cocaine reward memory and the underlying molecular mechanism of US retrieval-induced memory reconsolidation. Our data suggest that US is more efficient than CS at triggering a reconsolidation process for cocaine reward memory, thus suggesting that a post US retrieval intervention may be a potential approach to prevent substance addiction.
Materials and Methods
Animals
Six-week-old male C57BL/6J mice or male Sprague-Dawley rats, weighing about 22 or 280 g, respectively, were purchased from Slaccas Lab Animal. Adrb1flox/flox and Adrb2flox/flox mice with C57BL/6J background were developed by our laboratory. According to the gene structure and the size of exons, exon of Adrb1 (ENSMUSE00000294435) or Adrb2 (ENSMUSE00000399288) can be conditionally removed and will result in no β1-AR or β2-AR expression. 5’-loxP site is inserted about 1.4 or 1.2 kb upstream of start codon, where the promoter of Adrb1 or Adrb2 is located. 3’-loxP site is inserted downstream of 3’UTR. Removal of the flanked exon will result in no protein translation. Mice or rats used for experiments were housed with a reversed 12-h-light/-dark cycle and access to food and water available ad libitum. All animal treatments were strictly in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Animal Care and Use Committee of Shanghai Medical College of Fudan University. The male mice or rats 8 to 10 weeks old were used for all behavioral tests. Adrb1flox/flox mice and subsequent offspring were genotyped using the following primer sets: 5’-CTGTTCGCATCGGAATGAAGC-3’; 5’-TGACGTCATGAACTGGGATTTCAG-3’. Adrb2flox/flox mice and subsequent offspring were genotyped using the following primer sets: 5’-GGTTGCACAGCAGCCCTAGAT-3’; 5’-CCGTTATGTG CACCAGACTTTAGG -3’.
Reagents
Cocaine hydrochloride (Qinghai Pharmaceutical Firm) was dissolved in saline at 4 mg/mL for rat cocaine-SA model and 3 mg/mL for mouse cocaine-CPP model. Propranolol, betaxolol, ICI118,551, and cyclohemixide (Tocris Bioscience) were dissolved in saline and administered at a dose of 10 mg/kg (i.p.), 5 mg/kg (i.p.), 2 mg/kg (i.p.), and 60 mg/kg (s.c.), respectively. Anisomycin (Sigma-Aldrich) was dissolved in saline of equal molar of HCl, diluted with ACSF, and adjusted to pH = 7.4 with NaOH and administered at 150 mg/kg (i.p.) in mice. Propranolol (6.0 μg/μL), betaxolol (10 μg/μL), or ICI 118, 551 (10 μg/μL), cycloheximide (7.0 μg/μL) was injected into each side of the central amygdala (CeA) at the velocity of 0.1 μL/min for 5 minutes. Control animals received an equivalent volume of vehicle.
Cannula Implantation and Drug Delivery
Mice were anesthetized with 10% chloral hydrate and placed in a stereotaxic apparatus. Pedestal guide cannulas (27 gauge, RWD Life Science Co., Ltd) were implanted bilaterally 1 mm above the CeA (AP: -1.80 mm; ML: ±2.70 mm; DV: -3.30 mm) (Paxinos and Franklin, 2004). With a 2-week recovery, the behavioral tests were performed in the animals. Immediately after memory retrieval of cocaine CPP, the cannula dummy caps were gently removed. A 34-gauge infusion cannula was inserted into the guide cannula and infusion began. The mice were restrained in homecage throughout the infusion for 5 minutes. The infusion cannula was left for an additional 5 minutes to avoid the diffusion of the drug back into guide cannula.
Viral Constructs and Microinjection
Titre of AAV9 was exceeding 5 × 1012 v.g. mL-1 (Neuron Biotech Co., Ltd). To conditional knockout β1-AR in the CeA, the packaged virus with an EF1α promoter-driven AAV vector to express Cre recombinase and eGFP reporter (AAV-EF1α:eGFP-T2A-Cre) was injected into the CeA of Adrb1flox/flox mice. For the microinjection of the virus (Lowery and Majewska, 2010), anesthetized mice were positioned in a stereotaxic apparatus with the injection syringe of 36 gauge tips (World Precision Instruments, Inc.) aimed at the CeA. The intended stereotaxic coordinates were: AP: -1.80 mm; ML: ±2.70 mm; DV: -4.30 mm. Then 0.15 μL of the virus was infused into the CeA at 0.05 μL/min. The needle was left in place for an additional 5 minutes. The viral infection area determined by eGFP expression in the CeA was evaluated after behavioral experiments. In pilot experiments, expression of eGFP by AAV injection into the CeA was detectable 7 days after the surgery and lasted for at least 2 months.
Cocaine-CPP
A 2-chamber, unbiased CPP paradigm was applied as described previously (Liu et al., 2015). The CPP apparatus was consisted of 2 compartments with distinct floorings and walls. Before each session, mice were habituated to the experimental room for at least 30 minutes for 3 days.
Pre-Test Session
Mice were allowed free access to the entire apparatus for 15 minutes. Mice with an initial preference (>65% of total time) for either chamber were excluded from the experiment.
CPP Training Session
Mice were confined in one of the conditioning compartments for 30 minutes after injection of cocaine (15 mg/kg i.p.) or the other compartment after injection of saline (4 mL/kg i.p.). The cocaine- or saline-paired training was performed alternatively in the morning or afternoon and repeated for 3 days.
CPP Retrieval Session
Mice were injected with a low dose of cocaine (1.5 mg/kg i.p.) and kept in their homecage as cocaine reexposure or allowed to explore the entire apparatus for 5 minutes after injection of saline as context reexposure. Immediately after cocaine or context reexposure, mice were infused with β-AR antagonist or protein synthesis inhibitor systemically or into the CeA and returned to homecage.
CPP Memory Retention Test
At 24 hours after memory retrieval, mice were allowed free access to the entire apparatus (15 minutes), and the duration in the cocaine paired side was recorded.
CPP Extinction Session
At 24 hours after memory retention tests, mice were injected with saline and immediately confined to the compartment that was previously paired with cocaine or saline for 30 minutes alternatively for 3 days.
Reinstatement Test Session
After the extinction session, mice received a priming injection of saline (4 mL/kg i.p.) and cocaine (15 mg/kg i.p.) on the following day and were allowed free access to both compartments for 15minutes. The amount of time the mice spent in each compartment was recorded. The sessions were taped by a digital video camera, and the time spent in each chamber was recorded by a trained observer blind to the genotype and treatment. The CPP preference was determined as score with time spent in cocaine paired side minus the time in saline side in each minute (s/min).
Cocaine-SA
Cocaine-SA training was in accordance with the method our laboratory previously employed (Wang et al., 2010; Le et al., 2017). Rats weighing 280 to 320 g were used for surgery. They were housed in pairs before surgery and singly after. Before cocaine-SA training session, rats were initially maintained at 85% of original body weight and trained to lever press under a fixed-ratio 1 schedule of food pellets in the operant chambers (Med-Associates, Inc). After stable lever press for food pellets was achieved, rats were anaesthetized with 10% chloral hydrate and implanted with a single silastic catheter in the right jugular. Catheters were flushed every day with 0.1 mL saline solution containing gentamycin (0.5 mg/mL) and heparin (30 U/mL). They were allowed recovery for 7 days before the start of the behavioral experiment.
Cocaine SA Training Session
Rats were trained to self-administer i.v. injections of cocaine (0.75 mg/kg/infusion delivered in 4 seconds) during a 4-h session daily for 10 days under the FR1 reinforcement schedule. Each injection was accompanied with the CS, illumination of the stimulus light, and an audible tone for 20 seconds simultaneously. Inactive lever presses were also recorded but had no programmed consequences.
SA Retrieval Session
Rats received 4 i.v. cocaine infusions (0.75 mg/kg/infusion) in the operant chambers with the withdrawal levers and with no light or tone. Immediately after cocaine reexposure, rats were injected with β-AR antagonist and returned to the home cage. In the control group, rats were only returned to the operant chambers without cocaine infusion.
SA Memory Retention Test
At 24 hours after memory retrieval, rats were returned to the operant chamber for 30 minutes, during which the active lever press was accompanied with conditioned cues but no cocaine was delivered. The number of active lever presses was counted to show the craving for cocaine and the effect of drug on memory reconsolidation of cocaine SA.
SA Extinction Session
Rats were reintroduced into an operant chamber similar to the training procedure, except that cocaine was not infused with the active lever press. The rats experienced 4 hours daily extinction sessions for 7 days, during which the active lever press resulted in an infusion of the same volume of saline with the light and tone cues simultaneously. Extinction training continued until the active lever presses were <10 times in 4 hours for 2 consecutive days.
Reinstatement Test Session
Rats received a priming injection of 0.9% saline (4 mL/kg i.p.) and were returned to the operant chamber. At 24 hours later, rats received 5 mg/kg cocaine injection (i.p.) and were returned to the operant chamber. The procedure of the reinstatement test was the same as the test for memory retention, during which active lever press was accompanied with CS but no cocaine was delivered. Lever presses during the 60 minutes were counted.
Immunohistochemistry and Immunofluorescence
The mice were perfused intracardiacally with saline first, then with 4% paraformaldehyde in 0.1 M Na2HPO4/NaH2PO4 buffer (pH 7.5) and the brains were removed. After post-fixation in 4% paraformaldehyde for 4 hours, the samples were stored in 30% sucrose/PBS for 3 days. Brain slices 30 μm thick were incubated in primary antibody (anti-c-Fos 1: 1000, Santa Cruz; anti-β1-AR 1:100, Santa Cruz) at 4°C overnight. After being washed with PBS 3 times, the slices were incubated with fluorescence conjugated secondary antibody at room temperature (1:50000, Jackson ImmunoResearch) for 1 hour. Then the brain sections were rinsed in PBS and mounted with antiquenching mounting medium (Thermo Fisher Scientific). The sections were visualized under a LSM 510 laser confocal fluorescence microscope (Carl Zeiss) and analyzed by Image-Pro Plus. Labeled cells above the same threshold determined from control animals were counted (Trifilieff et al., 2006).
High-Resolution Fluorescent in Situ Hybridization (FISH) by RNAscope
FISH was performed on the fixed frozen brain tissue following the RNAscope procedures (Advanced Cell Diagnostics, Inc). Hybridization of a probe against the Bacillus subtilis dihydrodipicolinate reductase gene was used as negative control. In brief, frozen sections (10 µm thick) were cut coronally through the amygdala formation. Sections were thaw-mounted onto Superfrost Plus microscope slides (Fisher Scientific) and pretreated for heat retrieval and protease digestion. Sections were then incubated with probes of mouse β1-AR and β2-AR (Adrb1, accession no: NM_001039652.1, target region 1135-2162; Adrb2, accession no: NM_007420.3, target region 55-962) for 2 hours at 40°C with labeled probe mixture per slide. The nonspecifically hybridized probe was removed by washing the sections, 3 times for 2 minutes each in 1× wash buffer at room temperature, followed by Amplifier 1 for 30 minutes, Amplifier 2 for 30 minutes at 40°C and Amplifier 3 for 15 minutes at 40°C. Each amplifier was removed by washing with 1× wash buffer for 2 minutes at room temperature. The slides were incubated with HRP-C1 or HRP-C2, followed by TSA-fluorophore each. Then the slides were viewed, analyzed, and photographed with an LSM 510 microscope (Zeiss). At least 3 independent experiments have been performed and imaged from 3 male C57BL/6J mice.
Statistical Analysis
Experimental data were presented as the mean ± SEM and analyzed by Sigma plot 12.5. Data of immunofluorescence were non-normally distributed and analyzed by Kruskal-Wallis 1-way ANOVA on ranks. Data from behavioral tests were analyzed by 1-way or 2-way ANOVA with repeated measures followed by the Bonferroni’s posthoc test with sessions or levers as a within-subjects factor and drug treatment or genotype as a between-subjects factor.
Results
Cocaine Reexposure Triggered Protein Synthesis Dependent Memory Reconsolidation of Cocaine CPP
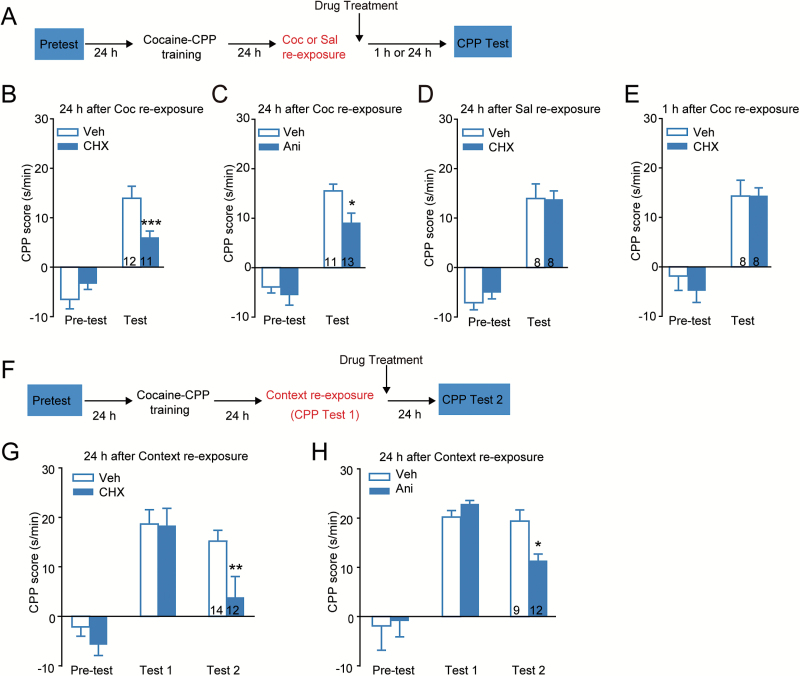
We first investigated whether cocaine reexposure might trigger reward memory reconsolidation in a similar manner to contextual cue reexposure. In this study, cycloheximide (CHX), a protein synthesis inhibitor, was administrated immediately (<5 minutes) after an injection of cocaine (1.5 mg/kg) 1 day after cocaine-CPP training. Twenty-four hours or 1 hour after cocaine reexposure, a memory retention test was performed (Figure 1A). A significant inhibitory effect of CHX on the preference for the cocaine paired side in the memory retention test and a treatment-by-session interaction were observed 24 hours later. Bonferroni’s posthoc comparison confirmed that CHX administered immediately after cocaine reexposure significantly decreased memory expression (Figure 1B, Ftreatment × session (1, 21) = 17.116, P < .001; Bonferroni posthoc test: t = 4.009, P < .001, vs Veh in test, 2-way RM ANOVA). Treatment with anisomycin, another protein synthesis inhibitor, also significantly decreased the preference for the cocaine paired side in the memory retention test (Figure 1C, Ftreatment × session (1, 22) =5.620, P=.027; Bonferroni posthoc test: t = 2.606, P=.014, vs Veh in test, 2-way RM ANOVA). When administered after saline reexposure, CHX had no effects on memory expression of cocaine-CPP (Figure 1D, Ftreatment × session (1, 14) = 0.024, P=.879, 2-way RM ANOVA). When a retention test was carried out 1 hour post cocaine reexposure, no significant inhibitory effect of CHX on memory expression was detected (Figure 1E, Ftreatment × session (1, 14) = 0.330, P=.575, 2-way RM ANOVA). Our data suggested that protein synthesis inhibition after cocaine reexposure impaired long-term memory of cocaine-CPP. A number of studies have demonstrated that protein synthesis inhibitors are classic amnestic agents that can impair memory reconsolidation when administered after CS reexposure-induced memory retrieval. Thus, the effects of protein synthesis inhibition on context retrieval-induced reward memory reconsolidation were also examined. CHX or anisomycin injected immediately after context reexposure inhibited the preference for cocaine paired side in the memory retention test 24 hours later (Figure 1F–H, CHX: Ftreatment × session (2, 48) = 3.335, P=.044; Bonferroni posthoc test: t = 2.767, P=.008, vs Veh in test2; Ani: Ftreatment × session (2, 38) = 3.744, p = 0.033; Bonferroni posthoc test: t = 2.568, P=.014, vs Veh in test2, 2-way RM ANOVA). Together, our data indicate that cocaine reexposure (1.5 mg/kg), as US retrieval, triggered memory reconsolidation of cocaine-CPP in a manner dependent on de novo protein synthesis.
Figure 1.
Cocaine reexposure induced protein synthesis-dependent memory reconsolidation of cocaine-conditioned place preference (CPP). Mice were trained for 3 days to acquire cocaine-CPP. For cocaine retrieval, cocaine (1.5 mg/kg, i.p.) was injected 24 hours after the last conditioning. The protein synthesis inhibitor was injected after cocaine reexposure. CPP memory retention tests were carried out at the time indicated (A–E). (A) Experimental design. (B–E) Memory retention tests 24 hours (B, C, D) or 1 hour (E) after cocaine or saline reexposure followed by the treatment of cycloheximide (CHX) (60 mg/kg, s.c.) or anisomycin (Ani, 150 mg/kg, i.p.). *P < .05, *** P < .001 vs vehicle-treated group. For context retrieval, a 5-minute reexposure to the conditioned chamber (CPP test1) was performed followed by treatment of protein synthesis inhibitor (F–H). (F) Experimental design. (G–H) Memory retention tests 24 hours after context reexposure followed by the treatment of CHX (60 mg/kg, s.c.) or Ani (150 mg/kg, i.p.). *P < .05, **P < .01 vs vehicle-treated group. Values in the bar indicate number of mice per group.
Protein Synthesis Inhibition Post Cocaine Retrieval, Not Context Retrieval, Led to Impaired Reinstatement
To test whether the amnesia effect of protein synthesis inhibitor treated after US retrieval was long-lasting or irreversible, we tested cocaine priming-induced reinstatement of cocaine-CPP. With a confined extinction training after memory retrieval and treatments in Figure 1, the same cohort of mice was subjected to saline priming as a extinction test and cocaine priming as a reinstatement test (Figure 2A). Our data showed that post cocaine retrieval treatment of CHX or anisomycin greatly inhibited cocaine priming-induced reinstatement (Figure 2B–C, CHX: Ftreatment × session (1, 21) = 9.240, P=.006; Bonferroni posthoc test: t = 3.453, P=.001, vs Veh in cocaine priming; Ani: Ftreatment × session (1, 22) = 5.152, P=.033; Bonferroni posthoc test: t = 3.483, P=.001, vs Veh in cocaine-priming, 2-way RM ANOVA). Without retrieval, CHX had no effects on reinstatement of cocaine-CPP (Figure 2D, Ftreatment×session (1, 14)=0.527, P=.480, 2-way RM ANOVA). However, when CHX or anisomycin was injected after context retrieval, the preference for the cocaine paired side was restored after cocaine priming (Figure 2E–F, CHX: Ftreatment × session (1, 24)=0.009, P=.927; Ani: Ftreatment × session (1, 19) = 0.363, P=.554, 2-way RM ANOVA). These data suggest that treatments post US retrieval, not CS retrieval, led to failure of reinstatement of cocaine CPP and the amnesia effects induced by the manipulations post US retrieval were persistent. Thus, US retrieval might be more efficient than CS retrieval at triggering memory reconsolidation of cocaine-CPP.
Figure 2.
Protein synthesis inhibition after cocaine retrieval prevented reinstatement for cocaine- conditioned place preference (CPP). After memory retention test, the confined extinction was carried out for 3 days followed by saline and cocaine priming-induced reinstatement tests. (A) Experimental design. Cycloheximide (CHX) or anisomycin was injected systemically after cocaine or context retrieval. (B–C) Reinstatement tests of cocaine-CPP with post cocaine retrieval treatment of CHX (60 mg/kg, s.c.) or anisomycin (Ani, 150 mg/kg, i.p.). **P < .01 vs vehicle-treated group. (D) Reinstatement tests of cocaine-CPP with treatment of CHX (60 mg/kg, s.c.) after saline reexposure. (E–F) Reinstatement tests of cocaine-CPP with post context-retrieval treatment of CHX (60 mg/kg, s.c.) or anisomycin (Ani, 150 mg/kg, i.p.). Values in the bar indicate number of mice per group.
Cocaine Retrieval Induced Neuronal Activation in the CeA
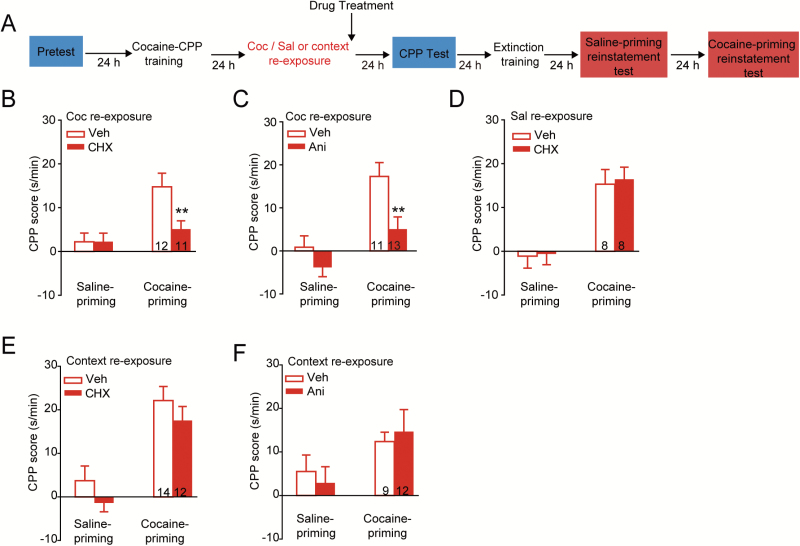
The above data indicated that the memory reconsolidation process induced by cocaine retrieval was distinct from that induced by context retrieval. We next investigated brain nuclei activation induced by cocaine reexposure or context reexposure. The optogenetic and pharmacogenetic studies have revealed that, during learning, c-Fos-positive neurons encode and store information (Liu et al., 2012; Liu et al., 2014b). c-Fos, the immediate-early gene, is induced in specific brain regions during neuronal activity associated with behavioral tasks (Morgan et al., 1987; Kang et al., 2001). Maximum expression levels of c-Fos in rodents were observed from 1 to 3 hours after sensory stimuli or behavioral tasks, returning to baseline values at 6 hours (Xiu et al., 2014; Barros et al., 2015). We measured the c-Fos expression levels 1 hour after memory retrieval (Figure 3A). The levels of c-Fos immunoreactivity in the brain sections of the control group (injected with saline at homecage) were used to determine the threshold for c-Fos positive cell counts for all groups. Our data showed that cocaine and context reexposure both induced a significant increase in c-Fos positive cell counts in the lateral amygdala (LA), the BLA, the prelimbic prefrontal cortex (PrL-PFC), and nucleus accumbens core (AcbC). c-Fos positive cell counts in the BLA, PrL-PFC, and AcbC were also significantly elevated in the cocaine reexposure group compared with the context reexposure group (Figure 3B, LA: F (2, 17) = 9.138, P=.002; Bonferroni posthoc test: t = 4.185, P=.002 cocaine vs saline, t = 2.582, P=.038, context vs saline; BLA: F (2, 17) = 15.228, P < .001; Bonferroni posthoc test: t = 5.518, P < .001, cocaine vs saline, t = 2.256, P = .037, context vs saline, t =2.978, P=.016, cocaine vs context; supplementary Figure 1, PrL-PFC: F (2, 10) = 25.126, P < .001; Bonferroni posthoc test: t =7.029, P<.001, cocaine vs saline, t=2.927, P=.045, context vs saline, t = 4.483, P=.004, cocaine vs context; IL-PFC: F (2, 9) = 3.346, P=.082; AcbC: F (2, 10) = 16.642, P<.001; Bonferroni posthoc test: t = 5.768, P<.001, cocaine vs saline, t = 2.928, P=.045, context vs saline, t = 3.152, P=.031, cocaine vs context, 1-way ANOVA). In addition, c-Fos positive cells significantly increased in the CeA only after cocaine reexposure, however, no significant increase of c-Fos positive cells in the CeA was detected after context reexposure (Figure 3B, CeA: F (2, 17) = 7.068, P = .006; Bonferroni posthoc test: t =3.624, P=.006, cocaine vs saline, t =2.785, P=.025, cocaine vs context, 1-way ANOVA). These results suggest that cocaine retrieval induced full activation of the amygdala, and the CeA played a distinct role from other subnuclei of the amygdala in cocaine retrieval triggered memory reconsolidation of cocaine-CPP.
Figure 3.
Cocaine retrieval-induced neuronal activation in the central amygdala (CeA). One hour after memory retrieval, mice were decapitated, and we examined the activation of brain regions induced by context or cocaine reexposure. (A) Experimental design. (B) Immunofluorescence staining of c-Fos (red) in the CeA, basolateral amygdala (BLA), and lateral amygdala (LA). *P < .05, **P < .01, and ***P < .001 vs control group. Scale bar: 100 µm. (C) mRNA expression of Adrb1 and Adrb2 in the CeA, BLA, and LA. Scale bar: 50 µm.
We then explored the distribution of mRNAs encoding for β1-AR and β2-AR protein in the amygdala by performing high-resolution FISH on brain slices. Adrb1 and Adrb2 mRNAs were present in the LA, BLA, and CeA (Figure 3C). No FISH signal could be detected using the negative control probe.
β1-Adrenergic Blockade in the CeA Impaired Cocaine Retrieval-Induced, Not Context Retrieval-Induced, Memory Reconsolidation and the Subsequent Reinstatement of Cocaine-CPP
As c-Fos expression significantly increased in the CeA after cocaine reexposure, but not context reexposure, it is critical to confirm the role of the CeA in cocaine retrieval triggered memory reconsolidation.
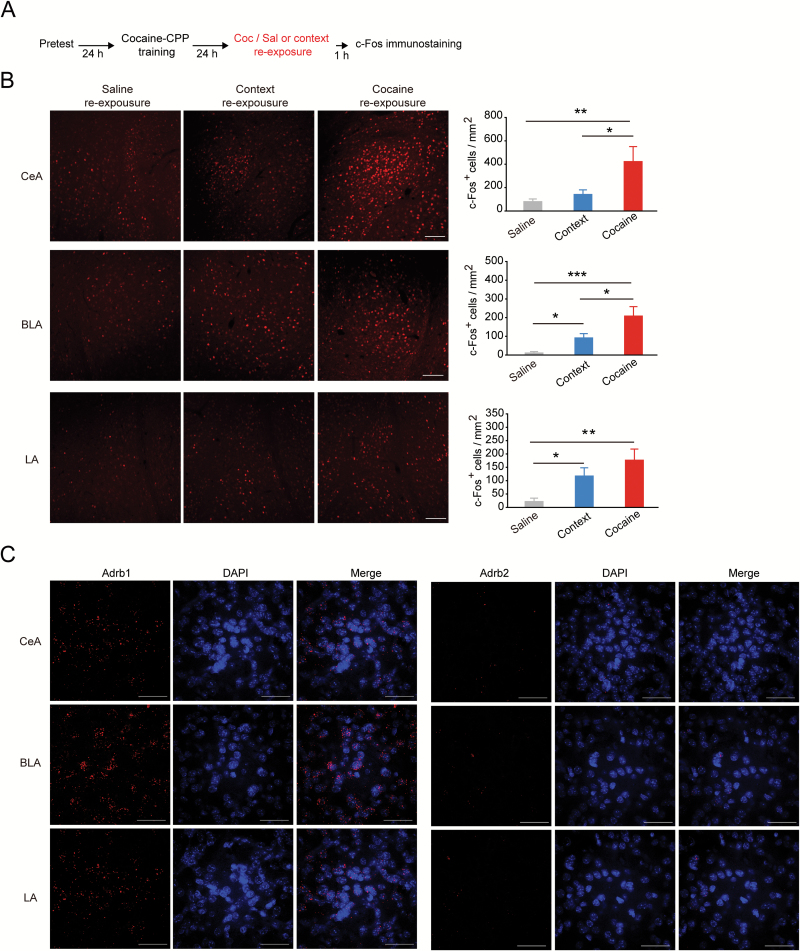
The inhibition of CPP memory reconsolidation was observed by CHX infusion in the CeA immediately after cocaine retrieval (Figure 4A-4C, Ftreatment × session (1, 18) = 11.010, P=.004; Bonferroni posthoc test: t = 3.928, P < .001, vs Veh in test, 2-way RM ANOVA). Cocaine priming-induced reinstatement was also inhibited after CPP extinction in the same cohort of mice (Figure 4D, Ftreatment × session (1, 18) = 4.449, P=.049; Bonferroni posthoc test: t = 3.233, P=.003, vs Veh in cocaine priming, 2-way RM ANOVA). The above data suggest the CeA was critically involved in cocaine retrieval-induced memory reconsolidation of cocaine-CPP.
Figure 4.
Protein synthesis inhibition or β1-AR blockade in the central amygdala (CeA) after cocaine retrieval impaired cocaine-conditioned place preference (CPP) memory reconsolidation and reinstatement. The 3-day cocaine-CPP training was performed 2 weeks after cannula implantation in CeA of C57 mice. The protein synthesis inhibitor was infused in the CeA after cocaine reexposure (A–D). (A) Experimental design. (B) Representative image of implanted cannula traces of drug infusion site in the CeA. (C) Memory retention tests of cocaine-CPP 24 hours after the cocaine retrieval followed by infusion of CHX (3.5 μg/side). (D) Reinstatement tests of cocaine-CPP with infusion of cycloheximide (CHX) after cocaine retrieval (3.5 μg/side). **P < .01 and ***P < .001 vs vehicle-treated group. After cocaine-CPP training, β-blocker was infused in the CeA after cocaine retrieval (E–G). (E) Memory retention tests of cocaine-CPP 24 hours after cocaine retrieval followed by propranolol injection (10 mg/kg, i.p.). (F) Memory retention tests of cocaine-CPP 24 hours after cocaine retrieval followed by infusion of β-blocker (Prop: 3 μg/side; Bet: 5 μg/side; ICI: 5 μg/side). *P < .05 and **P < .01 vs vehicle-treated group. (G) Reinstatement tests of cocaine-CPP with post cocaine retrieval infusion of β-blocker in the CeA. *P < .05 vs vehicle-treated group. After cocaine-CPP training, CHX or betaxolol was infused in the CeA after context retrieval (H–L). (H) Experimental design. (I) Memory retention tests of cocaine-CPP 24 hours after context retrieval followed by infusion of CHX (CHX: 3.5 μg/side). (J) Reinstatement tests of cocaine-CPP with post context retrieval infusion of CHX. (K) Memory retention tests of cocaine-CPP 24 hours after context retrieval followed by infusion of betaxolol (Bet: 5 μg/side). (L) Reinstatement tests of cocaine-CPP with post context retrieval infusion of betaxolol. Values in the bar indicate number of mice per group.
It has been hypothesized that β-adrenergic antagonism impairs memory reconsolidation via deactivation of second messengers that initiate gene transcription and translation of new proteins (Johansen et al., 2011). Our results suggest that β-AR was involved in and required for cocaine retrieval -induced reward memory reconsolidation, because systemic injection of propranolol after cocaine retrieval significantly impaired the preference for the cocaine paired side (Figure 4E, Ftreatment × session (1, 25) = 5.935, P=.022; Bonferroni posthoc test: t = 3.285, P=.002, vs Veh in test, 2-way RM ANOVA). To test the role of β-AR in the CeA in cocaine retrieval-induced memory reconsolidation, we injected a β-blocker in the CeA after cocaine retrieval. Our data showed that betaxolol, a β1 subtype-selective antagonist, as well as propranolol, infused in the CeA after cocaine retrieval significantly decreased the preference for the cocaine paired side in the memory retention test, but ICI 118, 551, a β2-AR selective antagonist, did not show the inhibitory effects on CPP expression (Figure 4F, Ftreatment × session (3, 39) = 3.837, P=.017; Bonferroni posthoc test: t = 3.677, P=.003, Prop vs Veh in test, t = 2.905, P=.029, Bet vs Veh in test, 2-way RM ANOVA). In addition, β1-AR blockade in the CeA post cocaine retrieval impaired cocaine priming-induced reinstatement (Figure 4G, Ftreatment × session (3, 39) = 3.204, P=.034; Bonferroni posthoc test: t = 2.821, P=.037, Prop vs Veh in Cocaine-priming, t = 2.884, P=.031, Bet vs Veh in Cocaine-priming, 2-way RM ANOVA). When CHX or betaxolol was infused in the CeA after context-retrieval (Figure 4H), memory reconsolidation and reinstatement were not significantly changed (Figure 4I-L, CHX/CPP test 2: Ftreatment × session (2, 38) = 0.426, P=.656; CHXreinstatement, Ftreatment × session (1, 19) = 0.073, P=.789; betaxolol / CPP test 2: Ftreatment × session (2, 32) = 0.118, P=.889; betaxolol/reinstatement: Ftreatment × session (1, 16) = 0.356, P=.559, 2-way RM ANOVA). Our data suggest that β1-AR activation in the CeA was specifically involved in US retrieval-induced, but not CS retrieval-induced, memory reconsolidation of cocaine-CPP, and the amnesia effect induced by β1-AR blockade in the CeA after US-retrieval was persistent.
β1-AR Deletion in the CeA Impaired Cocaine Retrieval-Induced Memory Reconsolidation and Reinstatement of Cocaine-CPP
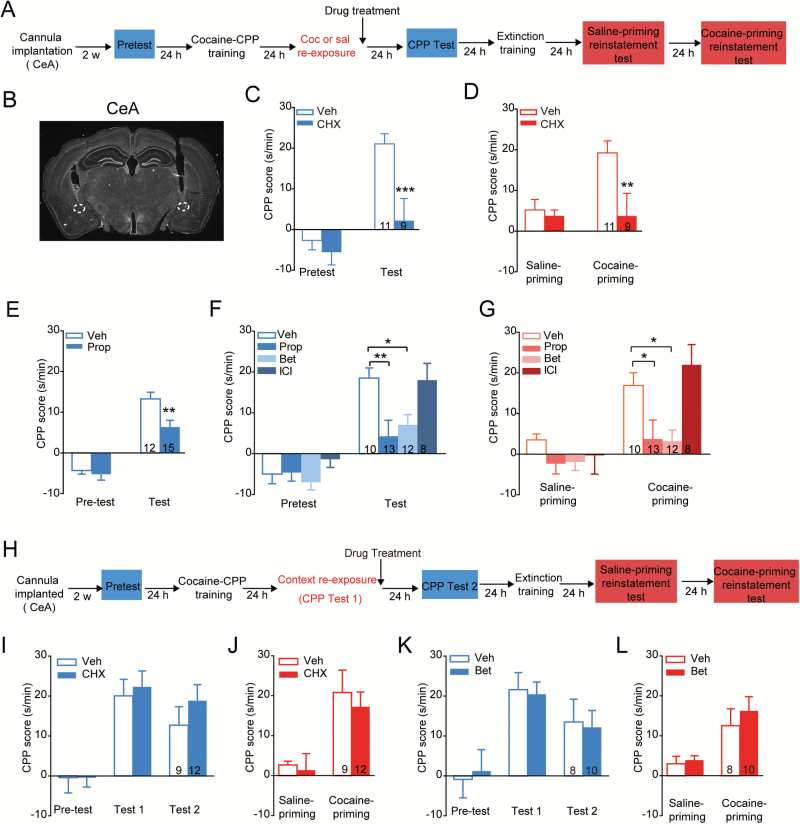
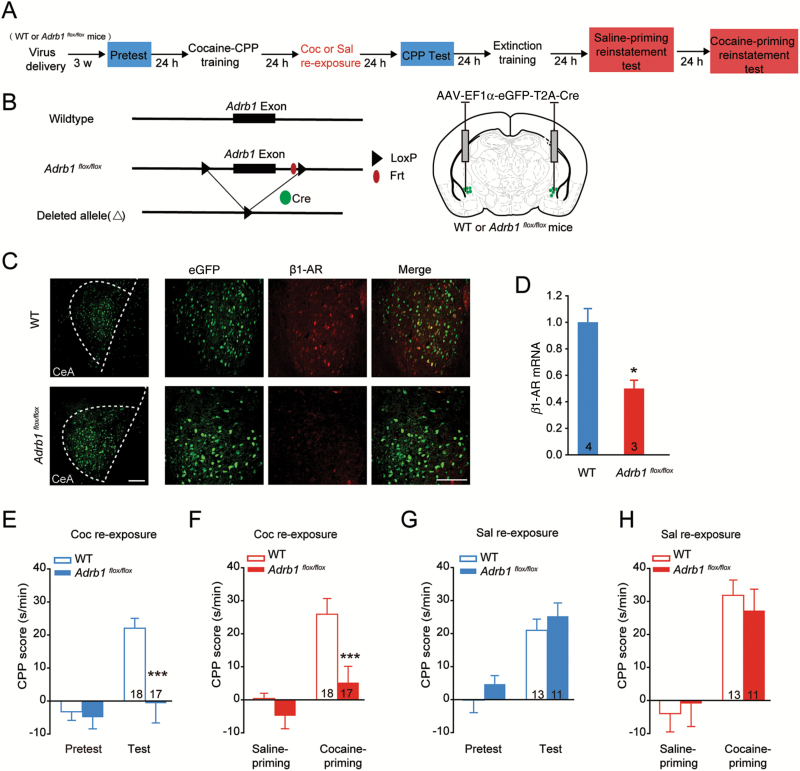
Furthermore, the role of β1-AR in cocaine retrieval-induced memory reconsolidation was further examined by β1-AR knockout in the CeA (Figure 5A-B). An AAV encoding Cre recombinase (AAV-EF1α:eGFP-T2A-Cre) was bilaterally infused into the CeA of Adrb1flox/flox mice to generate focal homozygous deletions of β1-AR in the CeA. As shown in Figure 5C, β1-AR focal knockout was confirmed by eGFP expression combined with β1-AR immunostaining. The deletion of Adrb1 was also confirmed by examination of its mRNA expression levels 3 weeks after surgery (Figure 5D, t(5) = 3.731, P=.0136, 2-tailed Student’s t test). After 3 weeks of AAV infection, daily conditioning and CPP tests were performed. The mice with selective deletion of β1-AR in the CeA showed a significantly decreased preference for the cocaine paired side 24 hours after cocaine retrieval compared with wild-type mice (Figure 5E, Fgenotype × session (1, 33) = 9.288, P = .005; Bonferroni posthoc test: t = 4.652, P < .001, vs wild type in test, 2-way RM ANOVA). CPP expression was not inhibited in β1-AR CKO mice with saline reexposure (Figure 5G, Fgenotype × session (1, 22) = 0.009, P = .923, 2-way RM ANOVA). Together, these results suggest that β1-adrenergic signaling in the CeA was required for cocaine retrieval-triggered memory reconsolidation of cocaine-CPP. In addition, β1-AR deletion in the CeA impaired cocaine priming-induced reinstatement (Figure 5F, cocaine reexposure: Fgenotype × session (1, 33)=6.011, P=.020; Bonferroni posthoc test: t=4.382, P<.001 vs WT in cocaine priming; Figure 5H, saline reexposure: Fgenotype × session (1, 22)=0.532, P=.473, 2-way RM ANOVA). Furthermore, β2-AR was also deleted in the CeA in Ardb2flox/flox mice (supplementary Figure 2A–C, t(6) = 3.865, P = .008, 2-tailed Student’s t test), and no significant suppression of memory reconsolidation or reinstatement was detected in β2-AR conditional knockout mice (supplementary Figure 2D, CPP test: Ftreatment × session (1, 22)=2.546, P=.125; reinstatement: Ftreatment × session (1, 22)=2.304, P=.143, 2-way RM ANOVA). Our data suggest that β1-AR in the CeA was required for US retrieval-triggered memory reconsolidation of cocaine-CPP.
Figure 5.
β1-AR deletion in the central amygdala (CeA) impaired cocaine retrieval-induced memory reconsolidation and reinstatement of cocaine- conditioned place preference (CPP). (A) Experimental design. AAV-EF1α:eGFP-2A-Cre was injected in CeA of Adrb1flox/flox mice 3 weeks before cocaine-CPP training. (B) Generation of β1-AR CKO mice using the Cre-loxp system. The Adrb1flox/flox mice contain loxp sites flanking the region of the exon to be deleted. The loxp sites can be recognized by Cre recombinase, resulting in the deletion of the exon of Adrb1. (C) Immunofluorescence staining of β1-AR (red) in the CeA of Adrb1flox/flox mice injected with AAV-EF1α:eGFP-T2A-Cre. Scale bar: 100 μm. (D) β1-AR mRNA levels significantly decreased 3 weeks after AAV injection in the CeA of Adrb1flox/flox mice. Number of mice per group is indicated in the bar. *P < .05 vs wild-type mice. Memory retention tests (E) and reinstatement tests (F) of cocaine-CPP with cocaine retrieval in β1-AR CKO mice. ***P < .001 vs wild-type group. Memory retention tests (G) and reinstatement tests (H) of cocaine-CPP in β1-AR CKO mice without cocaine retrieval. Values in the bar indicate number of mice per group.
β1-Adrenergic Blockade Impaired Cocaine Retrieval-Induced Memory Reconsolidation and Reinstatement of Cocaine-SA
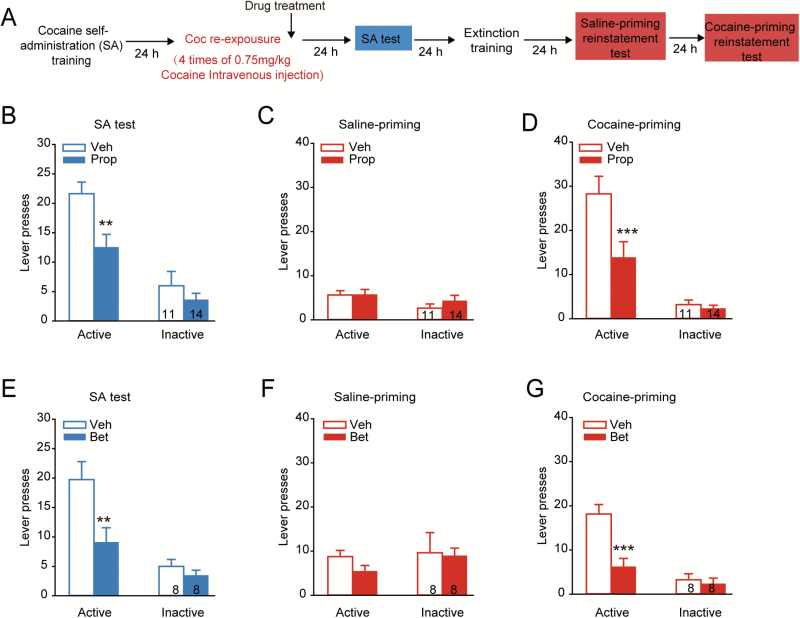
To further determine whether the treatment combined with US retrieval has translational potential, we used a cocaine-SA procedure in this study. With a 10-day cocaine-SA training, stable lever pressing (approximately 40 times within 4 hours) was developed in all rats (supplementary Figure 3). Then the rats were injected with a β-blocker immediately after i.v. cocaine injections without levers and cues. Memory retention tests were performed 24 hours later (Figure 6A). The data showed that administration of propranolol immediately after cocaine retrieval significantly decreased lever presses for cocaine (Figure 6B, Ftreatment × lever (1, 23) = 4.315, P = .049; Bonferroni posthoc test: t = 3.316, P = .002, vs Veh in active lever press, 2-way RM ANOVA). When propranolol was injected after saline reexposure, the lever presses for cocaine were not significantly different from those of control group in the memory retention tests (supplementary Figure 4A, Ftreatment × lever (1, 14) = 1.638, P = .221, 2-way RM ANOVA). To address whether β-blocker treatment after cocaine retrieval might affect cocaine relapse, we tested reinstatement after extinction training in the same cohort of rats (Figure 6A). Extinction training was performed after the memory retention test in all groups to obtain a low number of lever presses in response to cues (extinction criteria: no more than 10 active lever presses for the last 2 days). One day after the extinction session, rats showed no spontaneous recovery with saline-priming (Figure 6C, Ftreatment × lever (1, 23) = 0.434, P = .516, 2-way RM ANOVA). Then, in reinstatement tests with cocaine priming, propranolol injection after cocaine retrieval significantly inhibited drug seeking behavior (Figure 6D, Ftreatment × lever (1, 23) = 6.121, P = .021; Bonferroni posthoc test: t = 3.728, P < .001, vs Veh in active lever press, 2-way RM ANOVA). A significant inhibitory effect on memory reconsolidation was also detected by administration of betaxolol, but not ICI 118, 551, after cocaine retrieval (Figure 6E, Bet: Ftreatment × lever (1, 14) = 5.327, P = .037; Bonferroni posthoc test: t = 3.602, P = .001, vs Bet in active lever press; supplementary Figure 4B, ICI: Ftreatment × lever (1, 21) = 0.505, P = .485, 2-way RM ANOVA). Treatment of betaxolol after cocaine retrieval also suppressed relapse to cocaine in the reinstatement test (Figure 6F–G, saline priming: Ftreatment × lever (1, 14) = 0.257, P = .620; cocaine priming: Ftreatment × lever (1, 14) = 10.898, P = .005, Bonferroni posthoc test: t = 4.976, P < .001, vs Veh in active lever press, 2-way RM ANOVA). Our data suggest that activation of β-AR, particularly β1-AR, was required for US retrieval-induced memory reconsolidation of cocaine-SA. In agreement with the results of cocaine-CPP, this impaired memory reconsolidation of cocaine-SA by β1-AR blockade during the time window after US retrieval also led to decreased relapse to cocaine.
Figure 6.
β1-AR blockade post cocaine retrieval impaired memory reconsolidation and reinstatement of cocaine-self-administration (SA). With 10-day cocaine-SA training, 4 i.v. infusions of cocaine (0.75 mg/kg/infusion) were given as cocaine retrieval 24 hours after cocaine-SA training, followed by the treatment of β-blocker. The cocaine-SA memory retention test was carried out at the time indicated. After the memory retention test, the extinction training without cocaine delivery was carried out for 7 days followed by the reinstatement tests. (A) Experimental design. (B,E) Counts of lever presses in cocaine-SA memory retention tests 24 hours after cocaine retrieval followed by treatment of propranolol (Prop: 10 mg/kg i.p.) or betaxolol (Bet: 5 mg/kg i.p.). **P < .01 vs vehicle-treated group. (C–D, F–G) Counts of lever presses in cocaine-SA reinstatement tests with treatment of propranolol or betaxolol after cocaine retrieval ***P < .001 vs vehicle-treated group. Values in the bar indicate number of rats per group.
Discussion
The present results demonstrated that cocaine triggered protein synthesis-dependent memory reconsolidation of cocaine-CPP or cocaine-SA. The drug memory reconsolidation triggered by cocaine reexposure, not by context reexposure, was found to be mediated by β1-AR in the CeA. Furthermore, the manipulation post cocaine retrieval also decreased cocaine relapse in the reinstatement tests, in contrast to the drug-seeking behavior observed in the cocaine priming-induced reinstatement test with treatment after context retrieval. These findings demonstrate that US retrieval triggered memory reconsolidation process is distinct from the CS retrieval, or US retrieval is more efficient than CS retrieval, thus offering a potential manipulation strategy by which the persistent inhibition of addiction memory might be obtained.
In this study, we demonstrated that a low dose of cocaine (1.5 mg/kg) was sufficient to trigger reconsolidation of reward memory, because protein synthesis inhibitors or β-blockers, the classic amnestic agents for CS retrieval-induced memory reconsolidation (Nader et al., 2000b; Debiec et al., 2002; De Jaeger et al., 2014), blocked cocaine reexposure-induced memory reconsolidation of cocaine-CPP or cocaine-SA. The similar US retrieval procedure was also performed with lower dose of nicotine or cocaine injection (compared with the dose for training) as UCS retrieval (Luo et al., 2015; Xue et al., 2017b). Furthermore, we tested other doses of cocaine. Cocaine-induced memory reconsolidation at a dose of 3 mg/kg, but not 5 mg/kg or more, could be inhibited by propranolol (data not shown). In this study, the appropriate dose of cocaine for US retrieval (1.5 mg/kg for cocaine-CPP memory retrieval; 4 i.v. injections for cocaine-SA memory retrieval) was critical to induce a labile state of reward memory leading to de novo protein synthesis-dependent memory reconsolidation. It is also possible that the dose of propranolol or protein synthesis inhibitor did not produce an adequate long-lasting effect on memory reconsolidation. The result that a higher dose of cocaine may not induce memory restabilization indicates that there is a nonmonotonic relationship between US reexposure and memory restabilization, which needs further investigation.
In the same cohort of mice, our data showed that reinstatement was impaired by post US retrieval treatment, but not post CS retrieval treatment, thus indicating that memory impairment induced by pharmacological treatment after US retrieval was persistent. The finding that treatment after US retrieval impairs reconsolidation is consistent with studies showing that anisomycin injection in the BLA after US retrieval of fear memory can impair multiple CS-US associations to the same US. Similarly, in studies of fear memory reconsolidation, memory retrieval by a single CS leads to a selective amnesia for that specific CS when multiple CSs are associated with the US. It is likely targeting of a different component of the association that underlies the difference. In addition, the pharmacological blockade after contextual reexposure impaired long-term memory as indicated by CPP test, while memories reinstated after extinction session as indicated by cocaine priming-induced reinstatement test, suggesting that memories were possibly not erased by pharmacological intervention but were temporally inhibited. It might be that CS induces memory trace partially reactivated, and systemic treatment after CS retrieval partially inhibited memory reconsolidation. Thus, manipulation post US retrieval may destabilize more memory trace of CS-US associations than CS retrieval. Our data suggest that US retrieval might be more efficient than CS retrieval, and manipulations after US retrieval might have an advantage for treating substance addiction by preventing drug relapse.
In this study, memory reconsolidation of cocaine-CPP was impaired by systemic treatment or CeA infusion of protein synthesis inhibitor or β-blocker after US-retrieval, but was intact with local injection in the CeA after CS-retrieval. In addition, immunofluorescence staining showed that c-Fos expression significantly increased in the CeA after cocaine reexposure, but not context reexposure. These data suggest that CeA might be involved in the US, but not CS, -induced memory reconsolidation process.
Our current study suggested that β-adrenergic activation might participate in the US retrieval-triggered memory reconsolidation process, adding new evidence to previous findings, and demonstrates that memory reconsolidation triggered by US retrieval is also dependent on β-AR mediated signaling. Previous research has revealed that activation of β-AR recruits ERK and/or mammalian target of rapamycin signaling, thereby facilitating protein synthesis-dependent long-term potentiation (Gelinas and Nguyen, 2005; Gelinas et al., 2007). In this study, systemic or intra-CeA injection of propranolol and a protein synthesis inhibitor impaired reconsolidation of cocaine reward memory, thus suggesting that β-blockers or protein synthesis inhibitors might induce a common cellular process responsible for the failure of US retrieval-induced memory reconsolidation. However, these 2 manipulations may also potentially drive behavior through 2 independent cellular processes, given that a recent study has shown that CHX, not propranolol, blocks cue-induced memory reconsolidation of cocaine-SA (Dunbar and Taylor, 2016). β1-AR and β2-AR are 2 subtypes of β-AR that are distributed widely in the central nervous system. Although β1-AR are in much higher levels than β1-AR within forebrain structures, isoproterenol, which has equal affinity for both subtypes, induces much greater adenylyl cyclase activity upon stimulation of β2-AR than β1-AR. Then effects of both β1-AR and β2-AR on memory reconsolidation were examined in this study by using pharmacological treatment or conditional knockout strategy, respectively. Our data suggested that β1-AR, rather than β2-AR, in the CeA was required for reconsolidation of cocaine reward memory induced by cocaine retrieval. However, previous work has demonstrated that postretrieval long-term memory of cocaine-CPP might be mediated by β2-AR but not β1-AR (Bernardi et al., 2009). The disagreement between our findings and these previous results might be due to differences in experimental designs and targeted brain regions. The negative results of ICI 118,551 infusion or β2-AR conditional knockout in the CeA could also attribute to its low expression levels. Future studies should determine the precise cellular and β-adrenoceptor-mediated signaling mechanisms of this phenomenon.
The amygdala plays an essential part in processing both fearful and rewarding environmental stimuli (Janak and Tye, 2015). The amygdala is comprised of multiple interconnected nuclei such as the BLA, LA, and CeA. The BLA has the status of a “cortical-level structure” that contains most of glutamatergic principal neurons (Heimer, 2003). The BLA is critically involved in CS retrieval-induced reconsolidation process of drug reward memories (Milton and Everitt, 2010; Torregrossa and Taylor, 2013), and the BLA neuronal activity is critical for both CS and US exposure-triggered reconsolidation of nicotine reward memories (Xue et al., 2017b). In this study, we showed the similar results that both CS retrieval and US retrieval induced BLA activation, while US retrieval induced greater c-Fos expression than CS retrieval did, indicating that US retrieval may reactivate more memory traces than CS retrieval did in BLA. The CeA has the status as a “striatal-level structure” that consists of primarily GABAergic neurons (Heimer, 2003). The CeA has primarily been studied as the site for negative behaviors and is considered to maintain a general representation of the motivational significance of an outcome (Balleine and Killcross, 2006). It is possible that the negative emotions or the suppressed motivation induced by β-blockade in the CeA could inhibit the memory reconsolidation. The causal role for CeA circuits underlying appetitive behaviors was demonstrated (Kim et al., 2017). The CeA can positively modulate motivation of drug taking for cocaine- and sucrose-related reward behaviors (Seo et al., 2016; Warlow et al., 2017). The CeA is also implicated in strengthening the tone-light cue-triggered craving for addictive drugs during incubation in animals (Lu et al., 2005, 2007; Li et al., 2015). Considering c-Fos induction in CeA was observed only after US retrieval, but not CS retrieval in this study, we speculate CeA is a critical region for reinforcing effects of drugs and positively regulates US retrieval-triggered reward memory reconsolidation. Moreover, the CeA receives noradrenergic innervation from the ventral noradrenergic bundle (Moore and Bloom, 1979), which has been implicated in psychological diseases (Itoi and Sugimoto, 2010). Therefore, we hypothesize that β1-AR in the CeA can regulate US retrieval-triggered memory reconsolidation.
The present study demonstrated a potentially useful behavioral procedure, cocaine reexposure, which effectively triggers drug memory reconsolidation dependent on β1-AR in the CeA. Moreover, manipulation after US retrieval disrupted the reconsolidation of cocaine reward memories and suppressed cocaine relapse in reinstatement, thus suggesting a potential treatment strategy to prevent relapse in drug-addicted individuals.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (grant nos. 31430033, 91632307, and 31421091 to L.M.; 31371136, 31571036, and 31771176 to X.L.) and the Ministry of Science and Technology (grant no. 2015CB553500 and 2014CB942801 to L.M.).
Statement of Interest
None.
References
- Arguello AA, Hodges MA, Wells AM, Lara H 3rd, Xie X, Fuchs RA(2014) Involvement of amygdalar protein kinase A, but not calcium/calmodulin-dependent protein kinase II, in the reconsolidation of cocaine-related contextual memories in rats. Psychopharmacology 231:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross S(2006)Parallel incentive processing: an integrated view of amygdala function. Trend Neurosci 29:272–279. [DOI] [PubMed] [Google Scholar]
- Barros VN, Mundim M, Galindo LT, Bittencourt S, Porcionatto M, Mello LE(2015)The pattern of c-Fos expression and its refractory period in the brain of rats and monkeys. Front Cell Neurosci 9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Ryabinin AE, Berger SP, Lattal KM(2009)Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn Mem 16:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boening JA.(2001)Neurobiology of an addiction memory. J Neural Transm (Vienna) 108:755–765. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM(2014)Neuropathology of substance use disorders. Acta neuropathologica 127:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jaeger X, Courtey J, Brus M, Artinian J, Villain H, Bacquie E, Roullet P(2014)Characterization of spatial memory reconsolidation. Learn Memory 21:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE(2004)Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129:267–272. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K(2002)Cellular and systems reconsolidation in the hippocampus. Neuron 36:527–538. [DOI] [PubMed] [Google Scholar]
- Debiec J, Diaz-Mataix L, Bush DEA, Doyere V, LeDoux JE(2010)The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci 13:536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Mataix L, Debiec J, LeDoux JE, Doyere V(2011)Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J Neurosci 31:9538–9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar AB, Taylor JR(2016)Inhibition of protein synthesis but not beta-adrenergic receptors blocks reconsolidation of a cocaine-associated cue memory. Learn Mem 23:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar AB, Taylor JR(2017)Garcinol blocks the reconsolidation of multiple cocaine-paired cues after a single cocaine-reactivation session. Neuropsychopharmacology 42:1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint RW Jr, Valentine S, Papandrea D Jr(2007)Reconsolidation of a long-term spatial memory is impaired by cycloheximide when reactivated with a contextual latent learning trial in male and female rats. Neuroscience 148:833–844. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV(2005)Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci 25:3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV(2007)ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem 282:27527–27535. [DOI] [PubMed] [Google Scholar]
- Heimer L.(2003)The legacy of the silver methods and the new anatomy of the basal forebrain: implications for neuropsychiatry and drug abuse. Scand J Psychol 44:189–201. [DOI] [PubMed] [Google Scholar]
- Hyman SE.(2005)Addiction: a disease of learning and memory. Am J Psychiatry 162:1414–1422. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC(2001)Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N(2010)The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol 22:355–361. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM(2015)From circuits to behaviour in the amygdala. Nature 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE(2011)Molecular mechanisms of fear learning and memory. Cell 147:509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Bukoski E, Nadel L, Fellous JM(2012)Remaking memories: reconsolidation updates positively motivated spatial memory in rats. Learn Mem 19:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S(2017)Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93:1464–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND(2016)Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Yan B, Yu X, Li Y, Song H, Zhu H, Hou W, Ma D, Wu F, Zhou Y, Ma L(2017)Drug-seeking motivation level in male rats determines offspring susceptibility or resistance to cocaine-seeking behaviour. Nat Commun 8:15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ(2005)Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47:795–801. [DOI] [PubMed] [Google Scholar]
- Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y(2015)The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacol 40:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Venniro M, Shaham Y(2016)Translational Research on Incubation of Cocaine Craving. JAMA Psychiatry 73:1115–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S(2012)Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Zhao LY, Xue YX, Shi J, Suo L, Luo YX, Chai BS, Yang C, Fang Q, Zhang Y, Bao YP, Pickens CL, Lu L(2014)An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biol Psychiat 76:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma L, Li HH, Huang B, Li YX, Tao YZ, Ma L(2015)beta-Arrestin-biased signaling mediates memory reconsolidation. P Natl Acad Sci USA 112:4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery RL, Majewska AK(2010)Intracranial injection of adeno-associated viral vectors. J Vis Exp doi: 10.3791/2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y(2005)Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8:212–219. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y(2007)Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry 61:591–598. [DOI] [PubMed] [Google Scholar]
- Luo YX, Xue YX, Liu JF, Shi HS, Jian M, Han Y, Zhu WL, Bao YP, Wu P, Ding ZB, Shen HW, Shi J, Shaham Y, Lu L(2015)A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nat Commun 6:7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE(1996)Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behavioural pharmacology 7:754–763. [PubMed] [Google Scholar]
- Milton AL, Everitt BJ(2010)The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci 31:2308–2319. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ(2008)Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learn Mem 15:88–92. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ(1968)Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160:554–555. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE(1979)Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci 2:113–168. [DOI] [PubMed] [Google Scholar]
- Nadel L, Land C(2000)Memory traces revisited. Nat Rev Neurosci 1:209–212. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O(2009)A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10:224–234. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE (2000a) The labile nature of consolidation theory. Nat Rev Neurosci 1:216–219. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE (2000b) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726. [DOI] [PubMed] [Google Scholar]
- Otis JM, Dashew KB, Mueller D(2013)Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci 33:1271–1281a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ(2004)The mouse brain in stereotaxic coordinates. Compact 2nd ed. Amsterdam, Boston: Elsevier Academic Press. [Google Scholar]
- Przybyslawski J, Sara SJ(1997)Reconsolidation of memory after its reactivation. Behav Brain Res 84:241–246. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ortiz CJ, Bermudez-Rattoni F(2007)Memory reconsolidation or updating consolidation? In: Neural plasticity and memory: from genes to brain imaging (Bermudez-Rattoni F, ed). Boca Raton (FL): CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Seo DO, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, McCall JG, Krashes M, Sparta DR, Bruchas MR(2016)A GABAergic projection from the centromedial nuclei of the Amygdala to ventromedial prefrontal cortex modulates reward behavior. J Neurosci 36:10831–10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y(2002)Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR(2013)Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology 226:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J(2006)Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Memory 13:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR(2007)Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8:262–275. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR(2006)Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9:167–169. [DOI] [PubMed] [Google Scholar]
- Wang L, Lv ZG, Hu ZY, Sheng J, Hui B, Sun J, Ma L(2010)Chronic cocaine-induced H3 acetylation and transcriptional activation of camkii alpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacol 35:913–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow SM, Robinson MJF, Berridge KC(2017)Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J Neurosci 37:8330–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA(2013)Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacol 38:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Koob GF(2014)The development and maintenance of drug addiction. Neuropsychopharmacol 39:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu J, Zhang Q, Zhou T, Zhou TT, Chen Y, Hu H(2014)Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat Neurosci 17:1552–1559. [DOI] [PubMed] [Google Scholar]
- Xue YX, Deng JH, Chen YY, Zhang LB, Wu P, Huang GD, Luo YX, Bao YP, Wang YM, Shaham Y, Shi J, Lu L (2017a) Effect of selective inhibition of reactivated nicotine-associated memories with propranolol on nicotine craving. JAMA Psychiatry 74:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Chen YY, Zhang LB, Zhang LQ, Huang GD, Sun SC, Deng JH, Luo YX, Bao YP, Wu P, Han Y, Hope BT, Shaham Y, Shi J, Lu L (2017b) Selective inhibition of amygdala neuronal ensembles encoding nicotine-associated memories inhibits nicotine preference and relapse. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.