Abstract
Background and Aims
Ramularia collo-cygni is an ascomycete fungus that colonizes barley primarily as a benign endophyte, although this interaction can become pathogenic, causing the disease Ramularia leaf spot (RLS). Factors, particularly reactive oxygen species, that resulted in the transition of the fungus from endophyte to necrotrophic parasite and the development of disease symptoms were investigated.
Methods
Disease development in artificially inoculated seedlings of barley varieties varying in partial resistance to RLS was related to exposure to abiotic stress prior to inoculation. Histochemical and molecular analysis determined the effect of R. collo-cygni colonization on accumulation of reactive oxygen species and antioxidant gene expression. Development of RLS on barley lines defective in antioxidant enzymes and with altered redox status or non-functional chloroplasts was compared with the accumulation of fungal biomass to determine how these factors affect disease symptom expression.
Key Results
Exposure to abiotic stress increased symptom development in all susceptible and most partially resistant barley varieties, in association with greater hydrogen peroxide (H2O2) levels in leaves. Decreased activity of the antioxidant enzymes superoxide dismutase and catalase in transgenic and mutant plants had no effect on the disease transition, whereas manipulation of H2O2 levels during asymptomatic growth of the fungus increased disease symptoms in most susceptible varieties but not in partially resistant plants. Barley mutants that undergo rapid loss of green leaf area when infected by R. collo-cygni or albino mutants with non-functional chloroplasts showed reduced development of RLS symptoms.
Conclusions
These results imply that in seedlings the pathogenic transition of the normally endophytic fungus R. collo-cygni does not result from senescence as such, but rather is promoted by factors that result in changes to host reactive oxygen species. Barley varieties vary in the extent to which these factors promote RLS disease.
Keywords: Ramularia collo-cygni, Ramularia leaf spot, Hordeum vulgare, hemibiotroph, senescence, antioxidant system, disease susceptibility, non-functional chloroplasts, endophyte, necrotroph
INTRODUCTION
Ramularia leaf spot (RLS) is a late season disease that has emerged as a major problem in barley production in Europe, South America and New Zealand over the last 20 years (Havis et al., 2015; McGrann and Havis, 2017). The pathogen, the Dothideomycete fungus Ramularia collo-cygni (McGrann et al., 2016), is transmitted both in infected seeds and by spore dispersal between plants (Stabentheiner et al., 2009; Havis et al., 2014). Ramularia collo-cygni grows initially as an endophyte and can remain in this asymptomatic state for several weeks but, after a long period of latent development, it can then undergo a developmental switch to become an aggressive necrotrophic pathogen (Kaczmarek et al., 2017). In diseased plants, RLS symptoms typically develop on leaves towards the end of the growing season after ear emergence, although symptoms can be observed earlier in the season if environmental conditions are especially conducive to the disease (Harvey, 2002; McGrann and Havis, 2017). The risk of RLS epidemics is predicted to increase as the global climate changes (West et al., 2012), but current understanding of what factors trigger the transition of R. collo-cygni from benign endophyte to necrotrophic pathogen is limited.
Host–endophyte interactions can be commensal, mutualistic or antagonistic (Saikkonen et al., 1998). External conditions that can trigger the switch from endophyte to parasite include an imbalance in nutrient exchange, leaf ageing and senescence, or when the host is under stress (Saikkonen et al., 1998; Schulz and Boyle, 2005; Rodriguez and Redman, 2008). As the agent of a late season disease, the shift of R. collo-cygni from endophyte to pathogen has been associated with changes in host development from vegetative to reproductive stages and with a decline in the host antioxidant system during monocarpic senescence (Schützendübel et al., 2008). However, as most RLS-susceptible elite barley varieties tend to senesce early as a result of high levels of disease, it is difficult to ascertain if there is a causal relationship between senescence and RLS (Oxley et al., 2008). Exposure to high light stress increased the severity of RLS symptoms in controlled-environment experiments on a range of spring barley varieties and the model grass Brachypodium distachyon (Makepeace et al., 2008; Peraldi et al., 2014), indicating that the fungal switch to the pathogenic phase may be intimately linked to the host response to its surrounding environment.
Interactions between host genotype and the local abiotic environment can also affect the outcome of an endophyte’s association with its host plant (Saikkonen et al., 1998). Transgenic barley plants which overexpressed Stressed-related NAC1 (SNAC1), encoding a transcription factor involved in drought tolerance in cereals, were more resistant to RLS (McGrann et al., 2015a). Reduced RLS was associated with delayed leaf senescence in plants overexpressing SNAC1, further suggesting a link between the host signalling pathways that regulate the abiotic stress response and the development of this disease. Additional host genetic components have been implicated in the pathogenic transition of R. collo-cygni from endophyte to aggressive necrotroph. The broad-spectrum barley powdery mildew resistance gene mlo, which also regulates a spontaneous lesion mimic phenotype (Wolter et al., 1993) and is associated with accelerated leaf senescence and host cell death (Piffanelli et al., 2002), is a major genetic factor conferring susceptibility to RLS (McGrann et al., 2014). Two genes required for mlo-mediated mildew resistance, ROR1 and ROR2, also control the expression of RLS symptoms but not the accumulation of fungal biomass, implicating the MLO pathway in the pathogenic transition of R. collo-cygni. Mis-regulated host cell death in barley lesion mimic mutants elicits diverse effects on the switch from a symptomless state to disease, and in some, but not all, cases increases growth of R. collo-cygni (McGrann et al., 2015b). Furthermore, a gain-of-function mutation of the cereal dwarfing gene Slender 1, encoding a DELLA protein, restricts cell death and increases RLS symptoms (Saville et al., 2012), whereas mutants of a second dwarfing gene Brassinosteroid insensitive 1 show no effect on RLS (Goddard et al., 2014).
Cell death, leaf senescence and exposure to abiotic stress factors increase levels of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (H2O2) through breakdown of the chloroplast, photorespiration in peroxisomes and loss of functional integrity of photosystem II. Altered ROS status causes an imbalance to the redox system, resulting in oxidative stress to the plant (Zimmermann and Zentgraf, 2005). Tight regulation of ROS levels through the action of antioxidant enzymes such as catalase, superoxide dismutase and ascorbate peroxidase, and non-enzymic antioxidants such as glutathione and ascorbate maintains redox balance and prevents cellular damage (Foyer and Noctor, 2005). ROS also play an important role in cellular signalling to enable plants to respond rapidly to changes in the external environment (Suzuki et al., 2012; Saxena et al., 2016). Whether or not oxidative stress has a direct effect on R. collo-cygni development is not understood, but ROS levels are affected by the functions of mlo (Piffanelli et al., 2002), ROR genes (Hückelhoven et al., 2000), DELLA proteins (Achard et al., 2000) and SNAC1 (You et al., 2013), all of which affect the biology of this pathosystem.
The research reported here investigated how factors that can cause endophytic fungi to become pathogenic, such as abiotic stress, senescence and ROS, influence the development of RLS symptoms in artificially inoculated seedlings. As in most plant diseases, resistance to RLS is quantitative, and breeding has selected varieties with higher partial resistance (Havis et al., 2015). Variation in the pathogenic transition between barley varieties which vary in susceptibility to RLS was investigated. Barley lesion mimic and albino mutants were used to examine how ROS, cell death and functional chloroplasts affect expression of RLS symptoms as R. collo-cygni makes its transition from a benign endophyte to an aggressive necrotrophic pathogen.
MATERIALS AND METHODS
Plant material
Host responses to RLS were investigated in a collection of spring barley varieties differing in their susceptibility to the disease. The variety Power has strong partial resistance to RLS, whilst Braemar is highly susceptible (Oxley et al., 2008, McGrann et al., 2014). Golden Promise, which is somewhat less susceptible than Braemar, was also included in these experiments. Other spring barley varieties used in this study are listed in Supplementary Data Table S1.
RPr 74/9 is a catalase-deficient mutant of the barley variety Maris Mink (Kendall et al., 1983). Growth inhibition and development of spontaneous necrotic lesions on the leaves were observed under standard glasshouse conditions, but were not visible on RPr 79/4 plants when grown under standard growth room light levels of 220 μmol m–2 s–1 used in the experiments described here. RNA interference (RNAi) line 161–7- 11 has reduced transcript levels of the barley Copper/Zinc superoxide dismutase 1 (CSD1; Lightfoot et al., 2017). The Bipolaris sorokiniana tolerant 1 (bst1) mutants have resistance to the spot blotch pathogen Cochliobolus sativus (anamorph Bipolaris sorokiniana; Persson et al., 2008, 2009). albostrians is a barley albino mutant that is blocked in chloroplast development. This recessive mutation is inherited maternally and produces plants that segregate in their leaf phenotype, with either completely green, completely white or green and white striped leaves, all of which are genetically identical except for plastid differentiation (Hess et al., 1994). White leaves contain only traces of chlorophyll and carotenoids, lack 70S ribosomes and are photosynthetically inactive (Hess et al., 1994).
Growth conditions and abiotic stress treatments
Plants were grown in Levington F2 compost (Scotts Professional, Ipswich, UK) with a 16/8 h day/night photoperiod at day/night temperatures of 18/12 °C in a controlled-environment room (Sanyo) supplemented with 220 μmol m–2 s–1 fluorescent lighting. Plants were watered as required to keep the potting medium moist but not saturated. High light stress was applied by growing plants in a growth cabinet (Snijders Scientific, Tilburg, The Netherlands) under the conditions described above except that 700 μmol m−2 s−1 fluorescent light was supplied for the entire growing period. Plants were waterlogged by placing plant pots into trays filled with 4–5 cm of water from 8 d post-sowing for 6 d until pathogen inoculation.
Ramularia collo-cygni inoculation
Ramularia collo-cygni isolate Rcc09B4 was maintained and inoculum prepared as previously described (Makepeace et al., 2008; Peraldi et al., 2014). Barley seedlings at GS11–12 (Zadoks et al., 1974) were inoculated evenly with a fine mist at a rate of 10 mL of inoculum on 50 plants (Makepeace et al., 2008). Following inoculation, plants were placed under plastic covers to maintain high relative humidity (80–100 %) in the dark for 48 h and then grown under light levels of 220 μmol m−2 s−1 for the duration of the experiment. Disease development was assessed over the duration of the experiment as previously described, and disease severity was summarized as the area under the disease progress curve (AUDPC) as a percentage of the maximum AUDPC possible (McGrann et al., 2014). RLS progression on Power, Braemar and Golden Promise was scored as the average disease level per pot from five pots in each experiment. Data on the effects of abiotic stress treatments on RLS was scored on ten plants per variety in each treatment. Disease was scored on at least five plants of each of the bst1 mutants per experiment. As the albostrians mutants segregated for leaf phenotype, 40 seeds were sown in to a 20 × 15 × 5 cm tray for inoculation. Across five independent inoculation experiments, 18.7 % of plants had green leaves, 69.7 % had green and white striped leaves and 11.6 % had white leaves. For all pathology experiments, disease data were collected from a minimum of three separate experiments with independent inoculations.
Light microscopy of Ramularia collo-cygni infection
For microscopic observation of R. collo-cygni development, inoculated prophyll leaves were placed in 70 % ethanol until the chlorophyll was cleared. Each leaf was cut into two sections (tip and base) and stained with aniline blue [0.1 % (w/v) in lactoglycerol; Sigma]. Fungal development was quantified using bright-field microscopy (Nikon 800 Eclipse; Nikon Precision Europe GmbH, Langen, Germany) under ×40 magnification. In each field of view where R. collo-cygni hyphae were visible, all stomata present were scored for the number of (1) stomata that were penetrated by hyphae; (2) stomata from which conidiophore structures were erupting; (3) penetrated stomata which were surrounded by cells that showed reddish-brown coloured necrosis; and (4) non-penetrated stomata. At least two leaves of each genotype at each time point were scored, with a minimum of 300 stomata in total assessed on each leaf. Samples for light microscopy were collected from Braemar, Power and Golden Promise plants in three independent R. collo-cygni inoculation experiments at 5, 10, 15, 18 and 21 days post-inoculation (dpi).
Histochemical staining of ROS production
To assess the production of ROS during RLS development, five leaves were sampled at 5, 10, 15, 18 and 21 dpi with each of two treatments, either inoculated with Rcc09B4 or mock-inoculated with potato dextrose broth. Leaves were stained with 3’,3-diaminobenzidine (DAB; Sigma) to detect peroxide precipitation (Thordal-Christensen et al., 1997) and with nitroblue tetrazolium (NBT; Sigma; Adam et al., 1989) for superoxide detection. After staining, leaves were cleared in ethanol:lactic acid:glycerol (3:1:1) until transparent and then photographed. The amount of brown or blue staining visible on each leaf following DAB or NBT treatment, respectively, was quantified from the photographs using ImageJ (Abramoff et al., 2004: https://www.fmhs.auckland.ac.nz/assets/fmhs/sms/biru/docs/Colour_Analysis_Tools_in_ImageJ.pdf) and expressed as a proportion of the total leaf area. Samples for both staining methods were collected from three independent R. collo-cygni inoculation experiments.
Quantitative PCR analysis of R. collo-cygni DNA and host transcript levels
Ramularia collo-cygni in planta DNA levels were quantified using quantitative PCR (qPCR) (Taylor et al., 2010). Changes in target gene transcript levels following R. collo-cygni inoculation or in uninoculated plants were assessed by quantitative reverse transcription–PCR (qRT–PCR) using gene-specific primers as previously described (McGrann et al., 2009, 2015a; Colebrook et al., 2012; Supplementary data Table S2). Samples for qPCR of fungal DNA levels and host transcriptional changes were collected 5, 10, 15, 18 and 21 dpi from at least three independent R. collo-cygni inoculation experiments. Five leaves per time point were collected for qPCR, whereas two replicates each consisting of two leaves were collected for qRT–PCR analysis at each time point.
In vitro fungal growth assays
Fungal growth inhibition was assessed by measuring culture diameter extending from a 5 mm agar plug of each fungus on PDA (potato dextrose agar) medium supplemented with 1, 5, 10, 20, 50 or 100 mm H2O2; 100 μm methyl viologen (Sigma); 5 mm CaCl2 (Sigma); 50 mm LiCl (Sigma); and 2000 U ml–1 catalase (Sigma); or 5 mm of the irreversible catalase inhibitor 3-amino-1,2,4-triazole (3-AT; Sigma). As each fungus grew at a different rate, the effects of the oxidative stress-inducing agents on each pathogen were assessed at the following time points: R. collo-cygni (isolate Rcc09B4) at 42 d, Fusarium culmorum (isolate Fu42) at 5 d, Magnaporthe oryzae (isolate Guy11) and Oculimacula yallundae (isolate P149) at 21 d and Botrytis cinerea (isolate B05:WT) at 3 d. A minimum of three independent experiments were performed for all in vitro growth assays, with fungal growth measured on six replicate plates per experiment.
Leaf infiltration with catalase, H2O2, 3-AT and water
To test the effect of H2O2 status on the development of RLS symptoms, prophyll leaves of barley genotypes were infiltrated with 100 μL of catalase (2000 U ml–1; Sigma), H2O2 (5 mm; Sigma), 3-AT (5 mm; Sigma) or water (control) at 5, 7 and 10 dpi with Rcc09B4. Leaves were infiltrated through a puncture wound at the distal end using a needleless syringe as described previously (Colebrook et al., 2012). Each compound was infiltrated into five leaves per variety at each time point. Disease progression was assessed as described above. Data were collected from three independent R. collo-cygni inoculation experiments.
ROS-induced cell death assays
Plants were grown under standard light levels (220 μmol m–2 s–1) for 14 d. The sensitivity of different barley genotypes to ROS was assessed using 200 mm alloxan (Sigma), which is an H2O2 donor, 100 mm menadione (Sigma), a mitochondrial superoxide donor, and 25 μm methyl viologen (Sigma), a chloroplastic superoxide donor, as described previously (McGrann et al., 2015a). Treated leaves were incubated in boxes at room temperature under constant light (15–20 μmol m–2 s–1) for 96 h. After incubation, each box was photographed and the lesion size measured using ImageJ. Lesion size was assessed on at least six leaves from a minimum of three independent experiments.
Dark-induced senescence assays
Plants were grown under standard light levels or at high light for 14 d. Dark-induced senescence was assessed through measurements of relative chlorophyll content of the prophyll leaves of each genotype using Chlorophyll Meter SPAD-502 (Konica Minolta, Warrington, UK). Measurements were taken from six leaves per variety in a minimum of three independent experiments at 0, 2, 4 and 6 d of dark treatment as described previously (McGrann et al., 2015a).
Data analysis
All data were analysed using GenStat version 14 (Payne et al., 2009). Unless stated otherwise, data were analysed by analysis of variance (ANOVA) using general linear models that accounted for the variation attributable to different factors used in each experiment. Disease data from all R. collo-cygni inoculation experiments were Logit+ transformed (McGrann et al., 2014) prior to analysis. Data from the microscopy and ROS staining experiments were analysed using generalized linear models. Each score category from the microscopy experiments was analysed separately as a proportion of the total number of stomata scored on each leaf using a binomial distribution as the link function. Data on DAB- and NBT-stained samples were log transformed prior to analysis. Dark-induced senescence data were analysed with a linear mixed model of repeated measures using the uniform correlation/split plot in time covariance matrix (McGrann et al., 2015a). Fixed factors were day, experiment, genotype and the interactions between them, with the leaf × day interaction term as the random factor. After modelling, treatments were compared using t-test probabilities and applying Fisher’s least significant difference to unplanned comparisons.
RESULTS
Development of Ramularia leaf spot in resistant and susceptible varieties
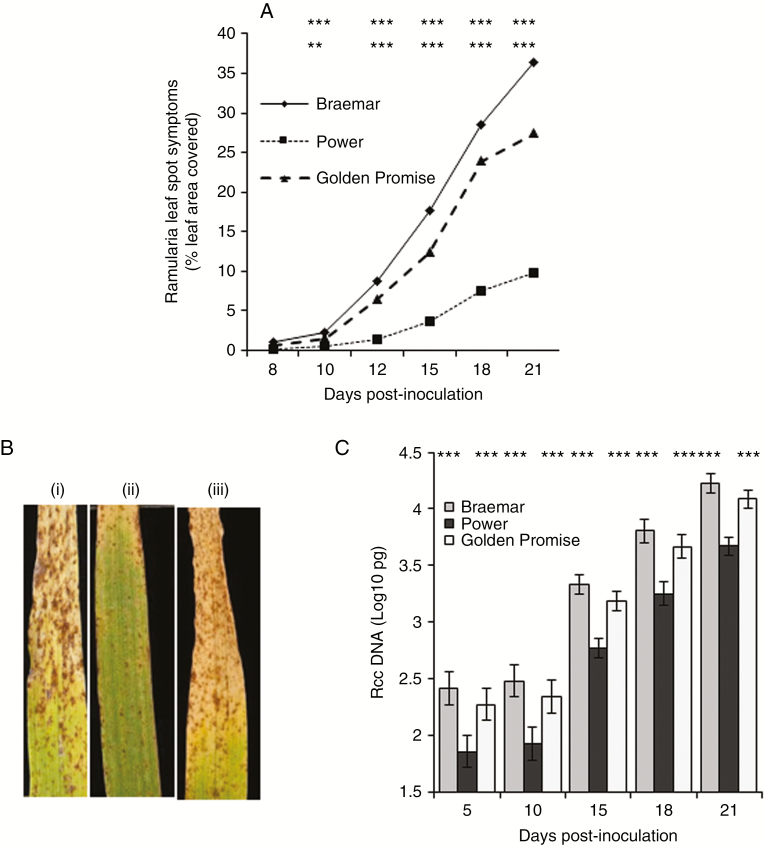
Symptoms of RLS were first visible as small brown pepper spots on the prophyll leaf of inoculated plants approx. 8–10 dpi. Subsequent development of the disease was rapid on the highly susceptible variety Braemar but much slower on Power, which has high partial resistance (Fig. 1A). RLS development on the variety Golden Promise was similar to that on Braemar, although final levels of disease were slightly lower (Fig. 1B). As the disease progressed, the small spots enlarged to form typical RLS lesions that began to coalesce from 15 dpi onwards. The later stages of RLS symptom development were associated with leaf senescence, particularly in susceptible varieties (Fig. 1B). Transcript levels of chlorophyll a/b-binding protein were reduced approx. 2-fold or more in R. collo-cygni-infected leaves of the susceptible varieties Braemar and Golden Promise, concurrent with the onset of disease symptom development at 10 dpi, but not in Power, which has strong partial resistance to RLS (Supplementary Data Fig. S1). Ramularia collo-cygni genomic DNA levels significantly increased with time after inoculation in all three varieties tested (P < 0.001; Fig. 1C). There was significantly more fungal DNA in the susceptible variety Braemar than in the resistant variety Power (P < 0.001).
Fig. 1.
Ramularia leaf spot development on the spring barley varieties Braemar, Power and Golden Promise. (A) Symptom development on prophyll leaves over the infection time course. (B) Typical disease symptoms on Braemar (i), Power (ii) and Golden Promise (iii) 21 days post-inoculation. (C) Ramularia collo-cygni DNA levels in prophyll leaves over the infection time course measured by qPCR. Error bars indicate ±1 s.e. Data were analysed by general linear modelling. ***P <0.001; **0.001 < P < 0.01; *0.01 < P < 0.05.
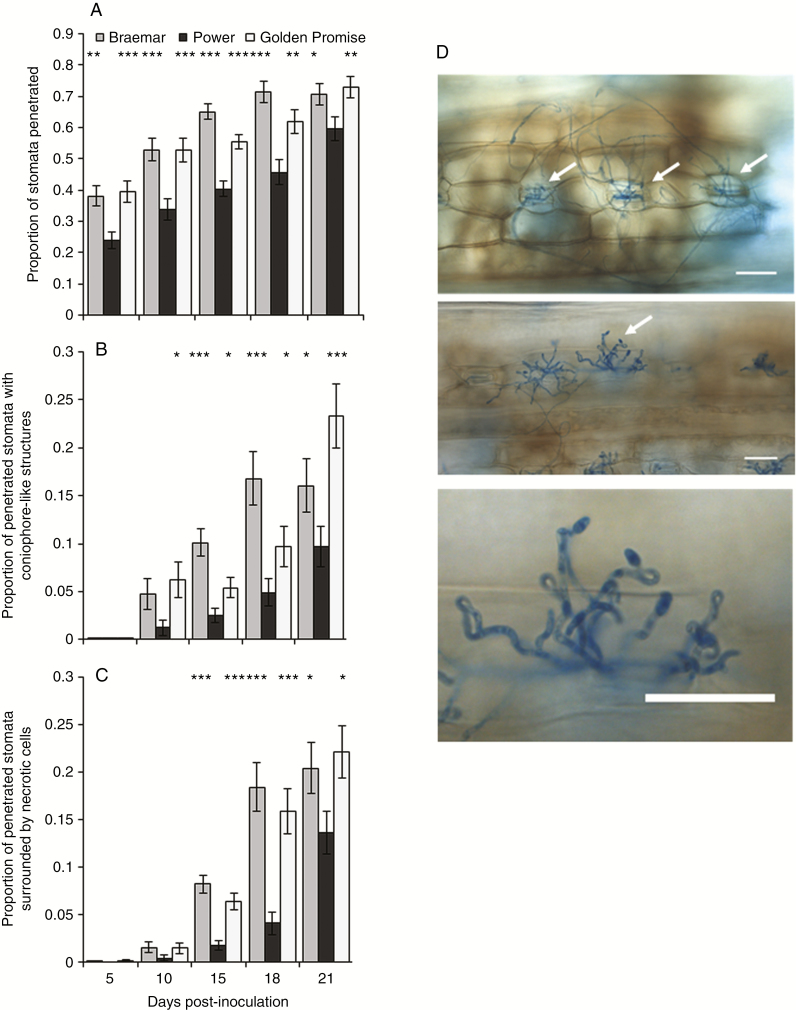
Ramularia collo-cygni development on partially resistant and susceptible varieties was visually assessed using light microscopy (Fig. 2A–D). The frequency of stomata penetrated by fungal hyphae increased over time in both varieties but was significantly higher in Braemar throughout the infection time course than in Power (Fig. 2A; P < 0.05). Emergence of conidiophore-like structures was rarely observed before 10 dpi but significant differences between resistant and susceptible varieties were recorded from 10 dpi onwards (Fig. 2B; P < 0.05). A few regions of reddish-brown cellular necrosis were observed at 5 dpi prior to the appearance of macroscopic disease lesions on the leaf. Larger areas of necrosis were scored as the symptoms developed, with significant differences between Power and Braemar from 15 dpi onwards (Fig. 2C; P < 0.05). Ramularia collo-cygni development in Golden Promise was similar to that observed in Braemar (Fig. 2A–D).
Fig. 2.
Microscopic comparison of Ramularia collo-cygni infection in the spring barley varieties Braemar, Power and Golden Promise during an infection time course. (A) Proportion of stomata penetrated by R. collo-cygni hyphae. (B) Proportion of penetrated stomata with conidiophore-like structures emerging. (C). Proportion of penetrated stomata surrounded by reddish-brown necrotic areas. Error bars indicate ±1 s.e.. (D) Images of microscopic development of R. collo-cygni infection under ×20 magnification. (i) Stomata penetrated by R. collo-cygni hyphae (arrows) surrounded by reddish-brown necrotic regions. (ii) Ramularia collo-cygni conidiophores (arrows) erupting from stomata. (iii) Close-up of R. collo-cygni conidiophore highlighting the characteristic swan-neck shape. Scale bar = 50 μm. Data were analysed using a generalized linear model of binomial proportions. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05.
Abiotic stress and Ramularia leaf spot development
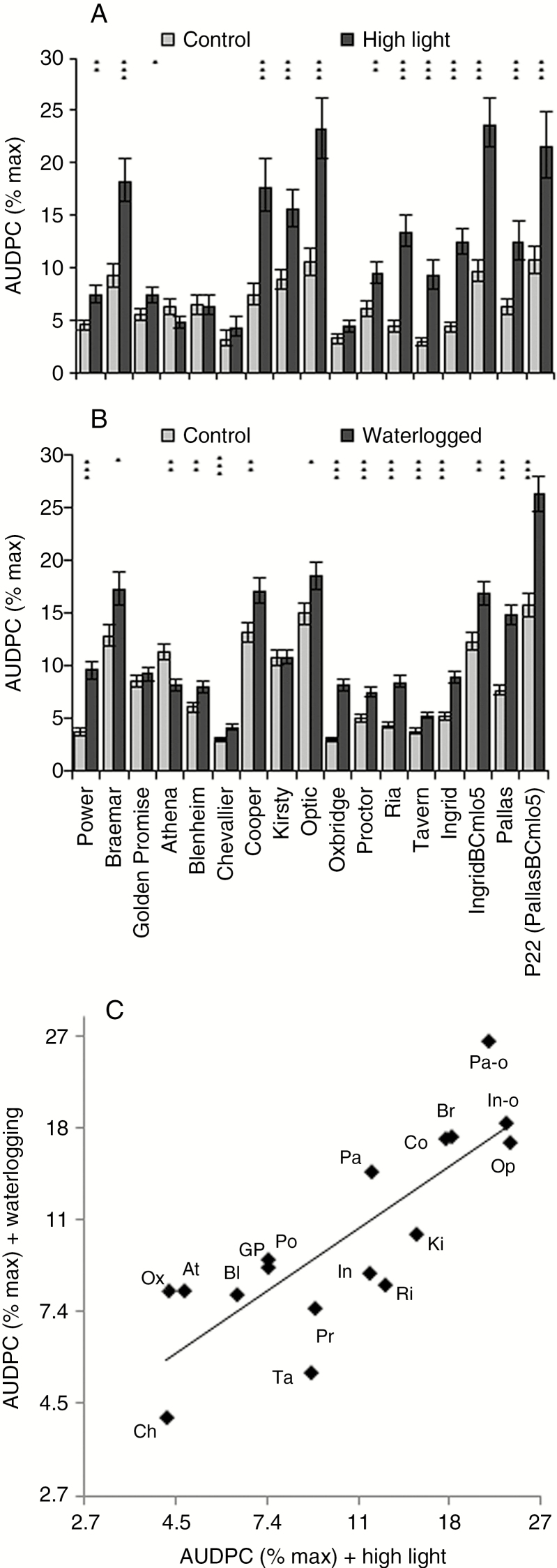
In a larger panel of barley varieties, exposure to high light levels before inoculation increased disease symptom development (P < 0.001) although there was a significant interaction between the stress treatment and variety (Fig. 3A; P < 0.001). To assess whether this stress-induced disease increase was specific to high light or a more general response to abiotic stress, a waterlogging treatment was also tested. Similar to the high light treatment, most varieties that had been waterlogged prior to inoculation showed significantly increased levels of RLS (Fig. 3B; P < 0.001). Pre-inoculation exposure to abiotic stresses increased disease severity in both RLS-susceptible and partially resistant varieties, with a high correlation between varieties’ susceptibility to RLS under different abiotic stresses [Fig. 3C; correlation (r) between mean logit-transformed RLS scores = 0.81, P < 0.001]. Varieties’ responses to the two stresses varied; notably, the resistant variety Athena exhibited reduced RLS symptoms following exposure to waterlogging or high light before inoculation (Fig. 3).
Fig. 3.
Effect of abiotic stress on Ramularia leaf spot development. Different spring barley varieties were grown under high light conditions (A) or waterlogged for 6 d (B) before inoculation with Ramularia collo-cygni. Disease development on abiotic stress-treated plants (dark grey bars) was compared with control plants (light grey bars) grown under normal controlled-environment room conditions. Disease is expressed as the area under the disease progress curve (AUDPC). Data were analysed by general linear modelling. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05 for comparisons between stress-treated and control plants. (C) Comparison of varieties’ disease scores under the two abiotic stresses. Codes are the first two letters of the varieties’ names; In-o, Pa-o: mlo5 near-isogenic lines of Ingrid and Pallas, respectively. Error bars indicate ± 1 s.e.
To assess if there was a link between high light stress, senescence and varietal susceptibility to RLS dark-induced senescence, a method for studying the physiological mechanisms of leaf senescence in plants (Gan and Amasino, 1997) was used. Leaves from plants grown under high light senesced significantly earlier than control leaves for all the varieties tested (P < 0.001; Supplementary Data Fig. S2). We also observed significant differences in dark-induced senescence between genotypes (P < 0.001) but no link between the rate of senescence and varietal susceptibility to RLS (Supplementary Data Fig. S2).
ROS accumulation during Ramularia leaf spot development
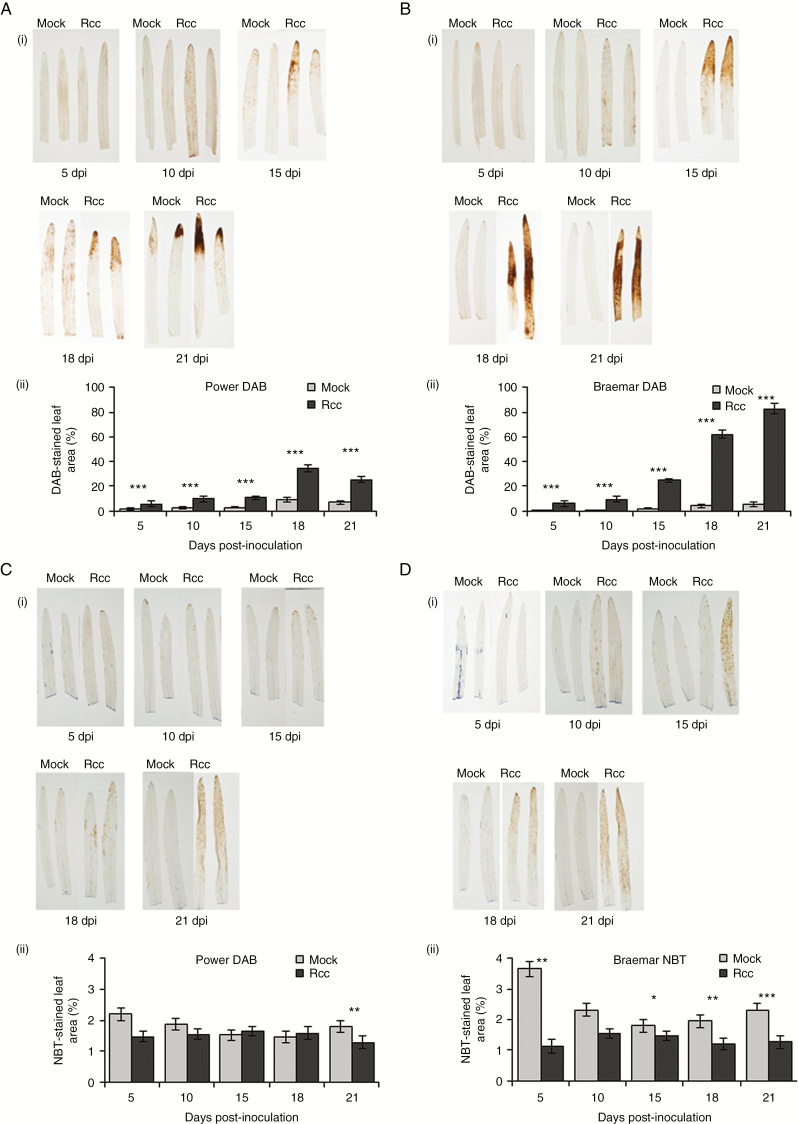
Staining with DAB and NBT was used to assess the production of H2O2 and superoxide, respectively, during RLS development in partially resistant and susceptible varieties. Rcc09B4 inoculation increased the proportion of DAB-stained leaf area over time in all genotypes tested (P < 0.001). More brown staining following DAB treatment to reveal peroxide was observed on inoculated leaves of the susceptible variety Braemar (Fig. 4B) than on the more resistant variety Power (Fig. 4A) from 15 dpi onwards (P < 0.001). Strong DAB staining, comparable with that on Braemar, was also observed on Golden Promise (Supplementary Data Fig. S3). Rcc09B4 inoculation slightly reduced the overall level of NBT staining on Braemar compared with mock-inoculated control leaves (P < 0.001; Fig. 4D). On Power, the reduction in NBT staining following inoculation was only statistically significant at 21 dpi (P = 0.005; Fig. 4D). Overall there was no significant difference in NBT staining between Power and Braemar (P = 0.065), but there was a significant three-way interaction of variety, treatment and time point, implying that the rate of accumulation of NBT differed between the two varieties (P <0.001). This was largely because mock-inoculated Braemar stained more heavily with NBT at 5 dpi although the area stained was much smaller in all cases than with DAB. NBT staining was also lowered by Rcc09B4 inoculation in Golden Promise, although this was only significant at 5 dpi (P = 0.008; Supplementary Data Fig. S3).
Fig. 4.
Reactive oxygen species accumulation in Power and Braemar following Ramularia collo-cygni inoculation. 3',3-Diaminobenzidine (DAB) staining for peroxide accumulation in Power (A) and Braemar (B). Representative images of DAB-stained leaves (i) and proportion of leaves stained with DAB (ii) in mock- and R. collo-cygni-inoculated leaves. Nitroblue tetrazolium (NBT) staining for superoxide accumulation in Power (C) and Braemar (D). Representative images of NBT-stained leaves (i) and proportion of leaves stained with NBT (ii) in mock- and R. collo-cygni-inoculated leaves. Error bars indicate ±1 s.e. Data were analysed using a generalized linear model. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05 for differences between inoculated and mock-inoculated samples.
Ramularia collo-cygni tolerance to conditions inducing oxidative stress
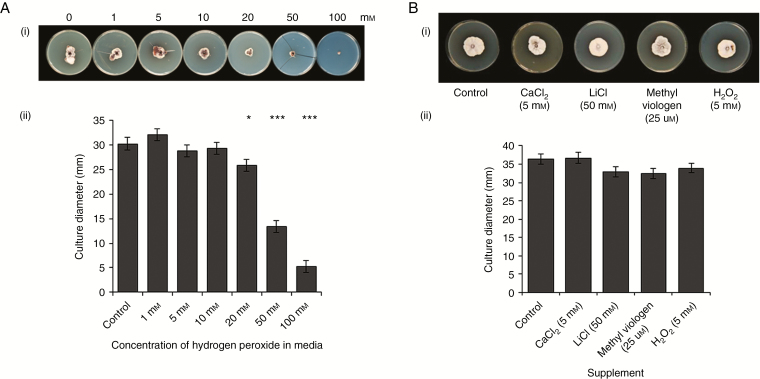
As elevated H2O2 levels coincided with the later stages of RLS symptom development, the tolerance of R. collo-cygni to this ROS was tested in an in vitro growth assay. Growth of Rcc09B4 was significantly inhibited only at H2O2 concentrations >10 mm (Fig. 5A). Furthermore, R. collo-cygni in vitro growth was not significantly inhibited compared with control plates by conditions expected to induce oxidative stress to the fungus including salinity and methyl viologen (Fig. 5B; P = 0.099 for comparisons of treatments with controls). In contrast, in vitro growth of the fungi M. oryzae, B. cinerea, F. culmorum and O. yallundae was inhibited by some or all of the stress-inducing compounds LiCl, CaCl2, methyl viologen and H2O2 (Supplementary Data Fig. S4).
Fig. 5.
Ramularia collo-cygni in vitro growth on oxidative stress-inducing media. (A) Growth of R. collo-cygni on potato dextrose agar (PDA) media supplemented with different concentrations of H2O2. Representative image (A) and measurements of R. collo-cygni culture diameter after 42 d growth. (B) Effect of oxidative stress-inducing media on in vitro growth of R. collo-cygni. Representative image (i) and measurements (ii) of R. collo-cygni culture diameter after 42 d growth on PDA media supplemented with CaCl2 (5 mm), LiCl (50 mm), H2O2 (5 mm) and methyl viologen (25 µm). Error bars indicate ±1 s.e. Data were analysed by general linear modelling. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05 for differences from control plates with no supplement.
Response of antioxidant and defence transcripts during R. collo-cygni infection of resistant and susceptible varieties
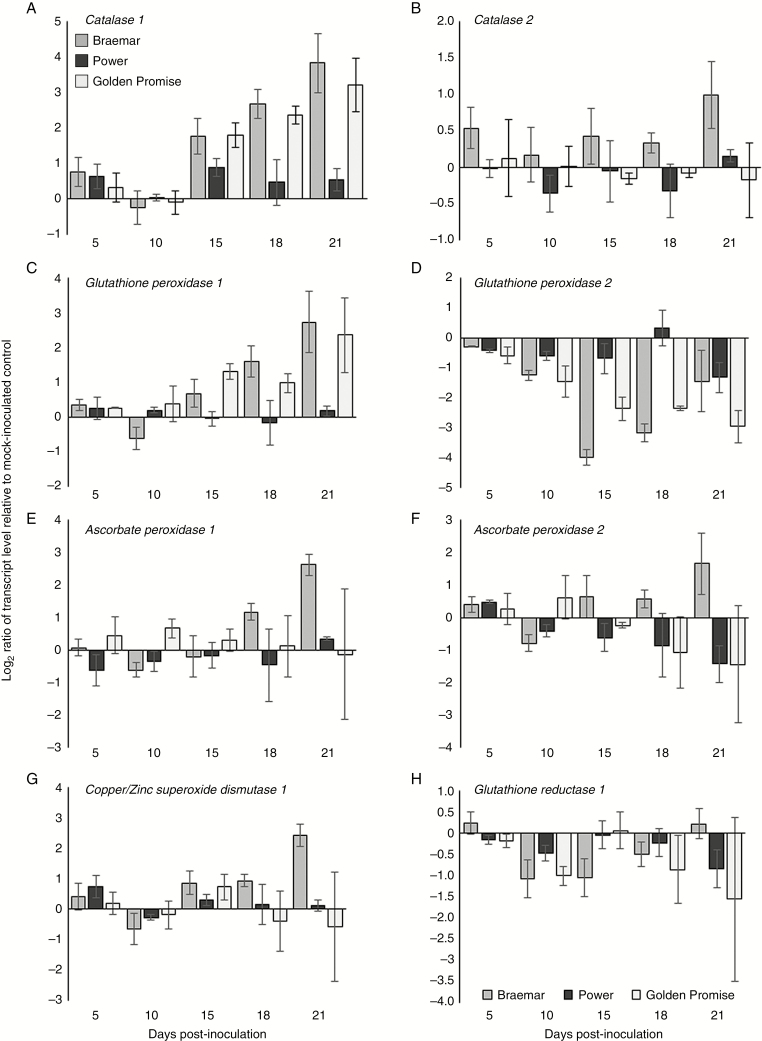
The antioxidant system acts to protect the plant from oxidative damage caused by high levels of toxic ROS (Foyer and Noctor, 2005). No change in expression of any gene involved in the antioxidant system was observed in the partially resistant variety Power (Fig. 6). Ramularia collo-cygni infection reduced Glutathione peroxidase 2 (GPX2) transcript accumulation from 10 dpi onwards in the susceptible varieties Braemar and Golden Promise (Fig. 6D). None of the other antioxidant genes was differentially expressed during asymptomatic development (5–10 dpi) in any of the cultivars tested (Fig. 6). There was increased expression of the major H2O2 and superoxide scavenger genes Catalase 1 (Cat1) from 15 dpi and Glutathione peroxidase 1 (GPX1) from 21 dpi in both Braemar and Golden Promise (Fig. 6A). Expression of CSD1 was also elevated in Braemar at 21 dpi (Fig. 6G).
Fig. 6.
Expression analysis of host antioxidant transcript levels during the Ramularia collo-cygni infection time course. Transcript accumulation of (A) Catalase 1, (B) Catalase 2, (C) Glutathione peroxidase 1, (D) Glutathione peroxidase 2, (E) Ascorbate peroxidase 1, (F) Ascorbate peroxidase 2, (G) Copper/zinc superoxide dismutase 1 and (H) Glutathione reductase 1 in Braemar, Power and Golden Promise. Log2-transformed data are presented. Error bars indicate ±1 s.e.
Transcript levels of Pathogenesis-related 1 (PR1) increased in all three varieties at 10 dpi and remained high in both the susceptible varieties, especially Braemar, but not in Power (Supplementary Data Fig. S5A). Expression of the cell death suppressor Bax-inhibitor 1 (BI-1) increased over time in the susceptible varieties Braemar and Golden Promise, but not significantly in Power (Supplementary Data Fig. S5B). Induction of Mitogen-activated protein kinase 3 (MPK3) has been linked to host-induced cell death involved in the susceptibility of wheat to the related pathogen Zymoseptoria tritici (Rudd et al., 2008). The MPK3 transcript was elevated at the later stage of infection in the highly susceptible variety Braemar but was not differentially expressed in either Power or Golden Promise (Supplementary Data Fig. S5C). There was no change in the expression of Mitogen-activated protein kinase 6 (MPK6) in any of the varieties (Supplementary Data Fig. S5D).
Effect of manipulation of the host antioxidant system on the development of Ramularia leaf spot
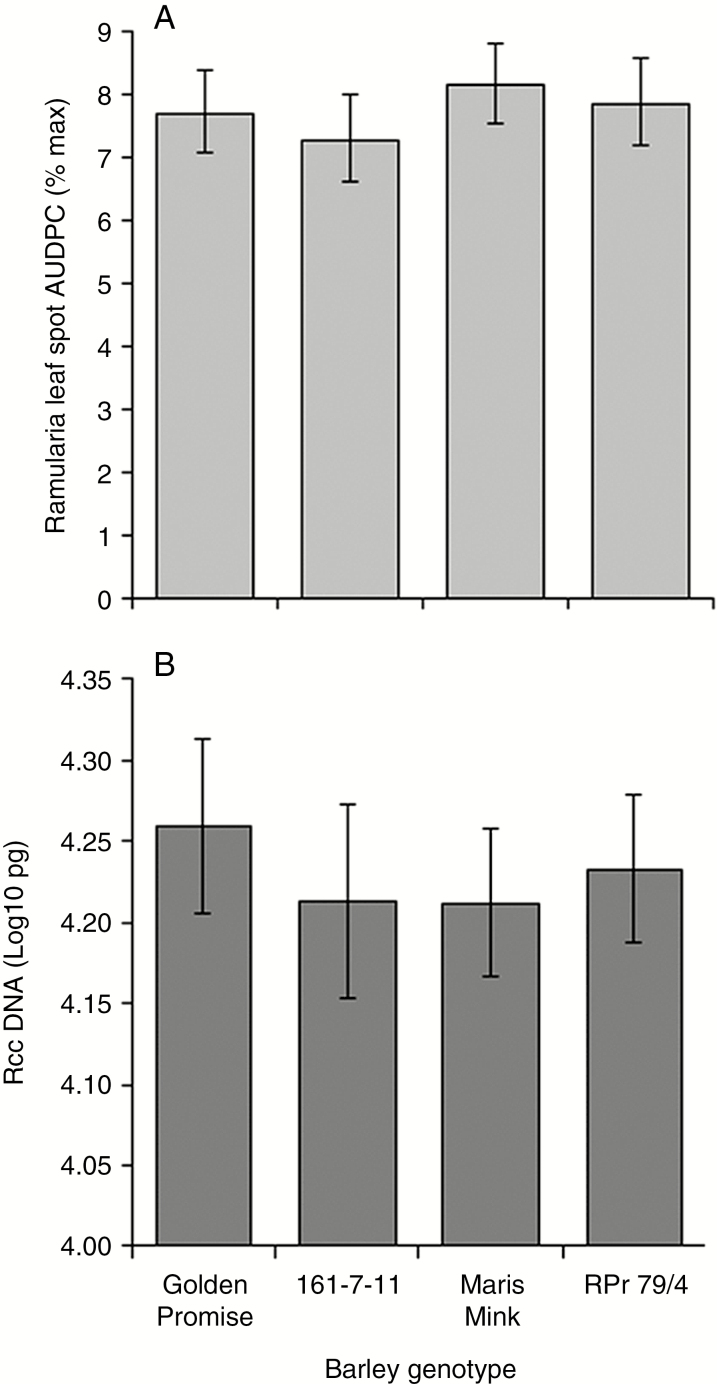
The effect of reduced host antioxidant capacity on the development of RLS was investigated using barley lines deficient in major ROS-scavenging enzymes. The transgenic RNAi line, 161-7-11, has an approx. 4-fold reduction in transcript levels of CSD1 (Lightfoot et al., 2017), whereas the mutant RPr 79/4 has an approx. 90 % reduction in leaf catalase activity (Kendall et al., 1983). No significant differences in RLS symptom development were observed in either 161-7-11 or RPr 79/4 compared with their respective wild-type lines (Fig. 7A). Similar levels of R. collo-cygni DNA were recorded in both line 161-7-11 and RPr 79/4 compared with their respective wild-type lines at 21 dpi (Fig. 7B).
Fig. 7.
Development of Ramularia leaf spot on spring barley lines with impaired antioxidant systems. (A) Disease development on transgenic Golden Promise RNAi line 161-7-11 with reduced Copper/zinc superoxide dismutase 1 transcript levels and the Maris Mink mutant RPr 79/4 with reduced catalase activity. Disease is expressed as the area under the disease progress curve (AUDPC). (B) Ramularia collo-cygni DNA levels in prophyll leaves 21 d post-inoculation measured by qPCR. Error bars indicate ±1 s.d. Data were analysed by general linear modelling. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05.
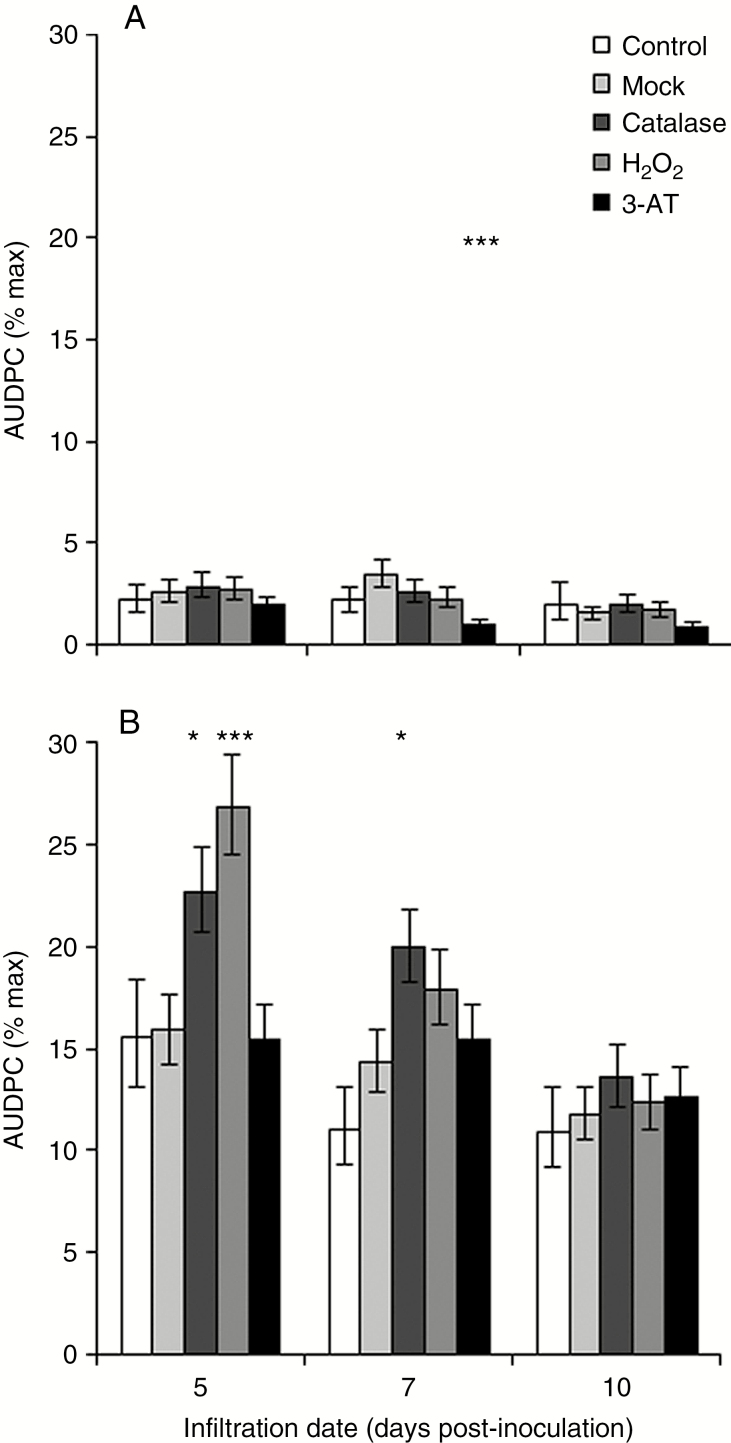
Next the effect of manipulating foliar H2O2 on RLS symptom development was tested. Neither catalase nor H2O2 infiltration affected disease development in Power significantly. Although the catalase inhibitor 3-AT significantly reduced RLS in Power at 7 dpi (Fig. 8A), 3-AT has a fungistatic effect in vitro (P < 0.001; Supplementary Data Fig. S6). Infiltration of catalase into the leaf apoplast at 5 or 7 dpi significantly (P < 0.05) increased disease severity in Braemar (Fig. 8B), as did infiltration of H2O2 at 5 dpi (P < 0.001), but no significant effect was seen 10 dpi. The partially RLS-resistant varieties Chevallier and Proctor also showed no significant RLS response to catalase or H2O2 infiltration, but neither did the moderately susceptible variety Optic (Supplementary Data Fig. S7). Catalase infiltration 5 dpi significantly increased RLS susceptibility in Ingrid (P < 0.05) and in IngridBCmlo5 (Supplementary Data Fig. S7; P < 0.01). Golden Promise showed significantly increased RLS following infiltration with catalase (P < 0.01) or H2O2 (P < 0.05) at 5 dpi (Supplementary Data Fig. S7). However, the infiltration process itself appeared to affect disease development in this variety as infiltrated control leaves showed higher levels of disease than water-infiltrated mock-inoculated leaves (Supplementary Data Fig. S7; P < 0.01). Disease levels were also lower in Golden Promise leaves infiltrated with any treatment at 7 dpi. Infiltration with 3-AT reduced disease severity at one time point or more in all the varieties tested except Braemar (Fig. 8; Supplementary Data Fig. S7).
Fig. 8.
Effect of manipulation of H2O2 in barley leaves infected with Ramularia collo-cygni. Prophyll leaves of Power (A) and Braemar (B) were infiltrated with water (Control), 2000 U mL–1 catalase, 5 mm H2O2 or 5 mm 3-amino-1,2,4-triazole (3-AT), or uninfiltrated (control) at 5, 7 and 10 d post-inoculation with R. collo-cygni and the effect on disease development assessed by calculating the area under the disease progress curve (AUDPC). Error bars indicate ±1 s.e. Data were analysed using general linear modelling. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05 for comparisons with mock-inoculated leaves.
Changes to the host antioxidant systems appeared to affect disease development in RLS-susceptible varieties (Fig. 8; Supplementary Data Fig. S7). As R. collo-cygni produces toxins that induce ROS and tissue death (Heiser et al., 2003), the sensitivity of barley varieties to ROS-induced lesion development was tested. The size of lesions formed by alloxan (P < 0.001), menadione (P < 0.001) and methyl viologen (P < 0.001) varied between barley varieties (Supplementary Data Fig. S8) but was not correlated with varietal susceptibility to RLS [correlation (r) between alloxan lesion size and mean logit-transformed RLS scores = 0.11, P = 0.8; menadione r = 0.67, P = 0.07; methyl viologen r = 0.34, P = 0.4].
The effect of the bst1 lesion mimic mutants on Ramularia leaf spot development
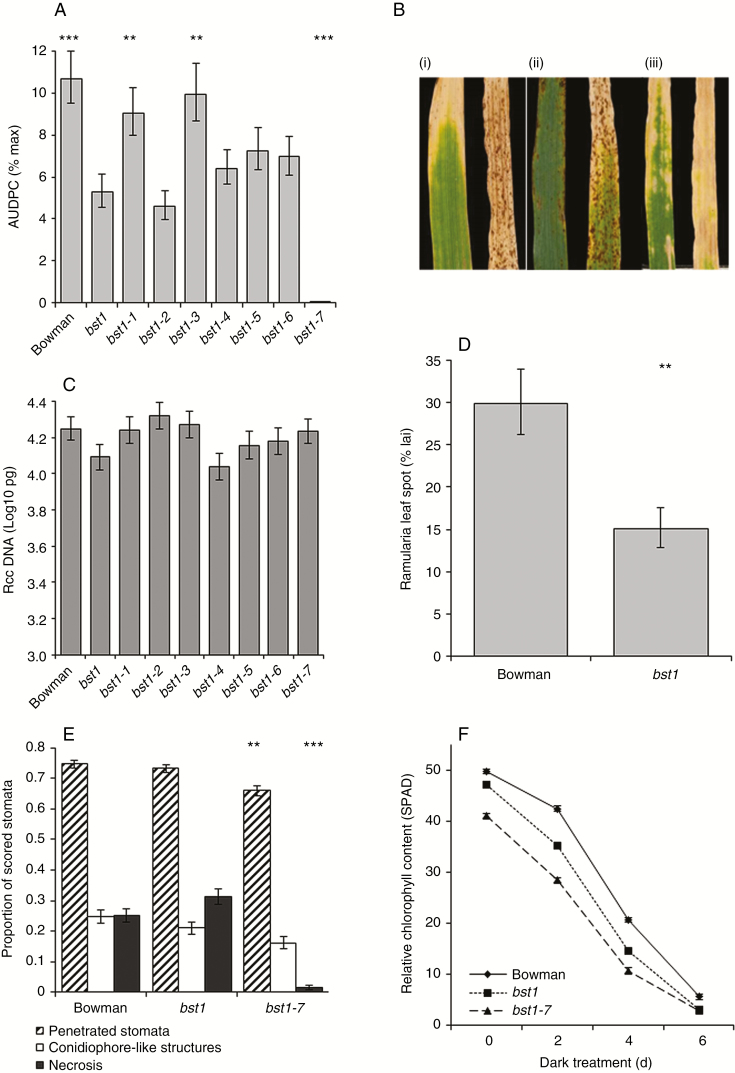
The role of redox status and senescence in the development of RLS was further evaluated using the bst1 lesion mimic mutant which has lower H2O2 levels during pathogen infection (Persson et al., 2008, 2009). When inoculated with R. collo-cygni, the bst1 mutant showed significantly reduced development of RLS symptoms compared with wild-type Bowman (Fig. 9A, B; P < 0.001) but not a significantly lower amount of R. collo-cygni DNA 21 dpi (Fig. 9C, P = 0.2). Despite the two lines having similar fungal DNA levels 21 dpi, bst1 had fewer disease symptoms than Bowman (Fig. 9B, D; P = 0.002).
Fig. 9.
Effect of Bipolaris sorokiniana tolerant 1 (bst1) mutations on Ramularia leaf spot development. (A) Disease development on bst1 mutants. (B) Representative images of uninoculated (left) and Ramularia collo-cygni-inoculated wild-type Bowman (i), bst1 (ii) and bst1-7 (iii) leaves 21 d post-inoculation (dpi). Disease is expressed as the area under the disease progress curve (AUDPC). (C) R. collo-cygni DNA levels in prophyll leaves 21 dpi measured by qPCR. (D) Final levels of Ramularia leaf spot symptoms expressed as percent leaf area infected (% lai) on Bowman and bst1 mutants 21 dpi. (E) Microscopic comparison of R. collo-cygni infection between Bowman, bst1 and bst1-7 at 21 dpi. (F) Dark-induced senescence assays for Bowman, bst1 and bst1-7. Error bars indicate ±1 s.e. Pathology and qPCR data were analysed by general linear modelling. Dark-induced senescence data were analysed using a linear mixed model of repeated measures. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05 for comparisons with bst1 (A and E) or Bowman (D).
How the bst1 mutation influences RLS development was further examined using a set of bst1 double mutants that had undergone a second round of mutagenesis resulting in variation in the size, shape and number of spontaneous necrotic spots produced by each lesion mimic (Persson et al., 2008). Some bst1 double mutation lines displayed a differential response to RLS compared with the bst1 mutant. The lines bst1-2, bst1-4, bst1-5 and bst1-6 all had disease levels comparable with bst1 (Fig. 9A). bst1-1 and bst1-3 both showed significantly more RLS symptoms than bst1, similar to those on the wild-type line Bowman (Fig. 9A). No RLS symptoms were observed on the line bst1-7, but rather inoculated leaves of this line turned yellow and appeared to senesce completely in the absence of fungal symptoms (Fig. 9B). No significant difference in R. collo-cygni DNA levels was observed between the bst1 mutant or any of the bst1 double mutation lines, including bst1-7 which did not exhibit any RLS symptoms (Fig. 9C; P = 0.2). Microscopic analysis of R. collo-cygni development 21 dpi indicated no significant differences in fungal penetration between the bst1 mutant and Bowman (P = 0.5), but stomatal penetration was lower in the double mutant (P = 0.002). There was no significant difference in the production of conidiophore-like structures between bst1 and either Bowman (P = 0.2) or bst1-7 (P = 0.1; Fig. 9E). bst1-7 mutants had much less cellular necrosis than bst1 plants (Fig. 9E; P < 0.001). More necrosis was scored on the bst1 mutants than on Bowman, but this was not statistically significant (Fig. 9E; P = 0.09). These results suggest that R. collo-cygni biomass accumulates normally on bst1-7 plants, but the triggers required for changes in fungal development and to enter the necrotrophic, pathogenic phase are altered.
Both the bst1 and bst1-7 mutants exhibited a ≥2-fold reduction in expression of the antioxidant genes APX1, APX2, GPX2 and GR1, and a >2-fold increase in expression of Cat2 (Supplementary Data Fig. S9A) relative to wild-type plants. bst1 and bst1-7 plants also showed a very large increase in expression of the defence-related gene PR1 (Supplementary Data Fig. S9B) but only a small reduction in expression of the cell death-related gene BI-1 (Supplementary Data Fig. S9C) compared with Bowman. Dark-induced senescence assays were used to examine differences in leaf senescence between the two bst mutants and Bowman. Both bst1 (P < 0.01) and bst1-7 (P < 0.001) leaves had significantly lower SPAD readings at day 0, suggesting that these lines have a reduced chlorophyll content relative to the wild type, a trend that continued throughout the senescence time course (Fig. 9F).
The role of functional chloroplasts in expression of Ramularia leaf spot symptoms
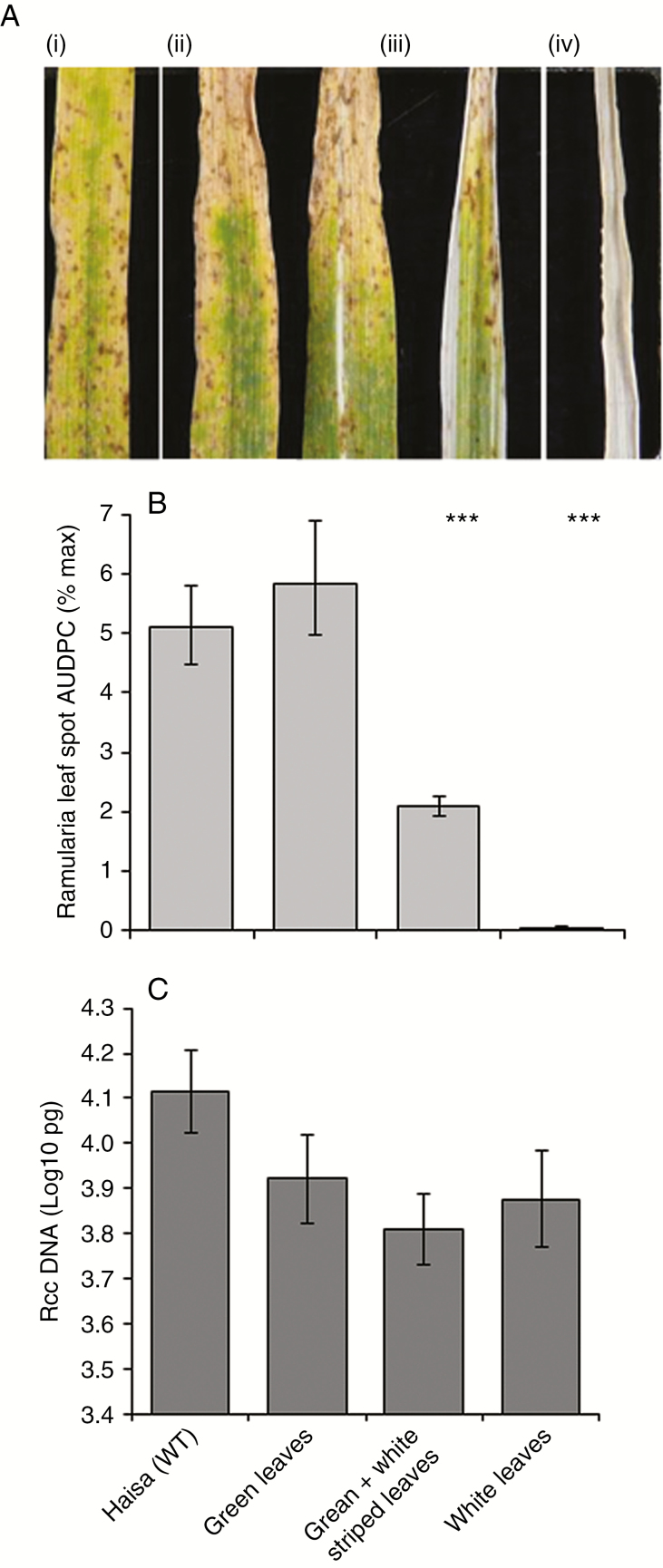
The rapid onset of leaf chlorosis of bst1-7-inoculated leaves in the absence of RLS symptoms suggested that functional chloroplasts are required for R. collo-cygni to enter its pathogenic phase. This hypothesis was tested using the albostrians mutants which have defective chloroplast development (Hess et al., 1994). Typical RLS lesions formed on wild-type Haisa [Fig. 10A (i)] and green albostrians [Fig. 10A (ii)] leaves and there was no significant difference in disease development between these lines (Fig. 10B). Classic RLS symptoms also formed on green and white striped leaves [Fig. 10A (iii)] but disease development was significantly lower on these leaves than on green leaves (Fig. 10B; P < 0.001). No RLS symptoms were observed on white leaved mutant plants [Fig. 10A (iv), 10B; P <0.001]. Despite the reduced development of disease symptoms in the green and white striped leaves and the complete absence of disease symptoms in plants with white leaves, there was no significant difference in the level of R. collo-cygni genomic DNA detected 21 dpi between any of the mutant lines (Fig. 10C; P = 0.1). Together these data suggest that plants with defective chloroplasts affect the ability of R. collo-cygni to cause disease.
Fig. 10.
Development of Ramularia leaf spot on barley albostrians mutants. (A) Typical disease symptoms observed on the mother line Haisa (i), green-leafed albostrians mutants (ii), green and white-leafed albostrians mutants (iii) and white-leafed albostrians mutants (iv). (B) Disease development was measured as the area under disease progress curve (AUDPC). (C) Ramularia collo-cygni DNA levels in prophyll leaves 21 d post-inoculation measured by qPCR. Error bars indicate ±1 s.e. All data were analysed by general linear modelling. ***P < 0.001 for comparisons with green-leaved albostrians mutants.
DISCUSSION
Ramularia leaf spot has become an important disease of barley in Europe and other temperate regions of the world in the last 20 years, but the fungus responsible for the disease, R. collo-cygni, has been associated with barley at least as far back as the 1800s (Fountaine and Fraaije, 2009; Havis et al., 2015). Like its relatives within the Mycosphaerellaceae, R. collo-cygni has a prolonged latent growth phase on its host, with symptoms only observed late in the growing season, usually after flowering; in some years, disease symptoms fail to develop at all. This, together with horizontal transmission of the fungus through seed (Havis et al., 2014) and other features of fungal development (Kaczmarek et al., 2017), has led to the proposal that R. collo-cygni is an endophyte that only causes disease under specific circumstances (McGrann and Havis, 2017). This prompts the question of what factors cause R. collo-cygni to become a destructive pathogen. While RLS is typically most severe in the top two leaves of naturally infected, adult barley plants in field conditions (Schützendübel et al., 2008), artificial inoculation of seedlings with Rcc was used in the experiments reported here as a tractable model system for investigating factors that induce symptom development.
Endophytes develop a balanced interaction with their host, but can become pathogenic when that balance is disturbed (Saikonnen, 2005; Schulz and Boyle, 2005). Almost all barley varieties tested showed increased RLS symptoms if plants had been subjected to abiotic stress before fungal infection, whether caused by high light or by waterlogging. The exceptions concerned two of the most resistant varieties, as Athena plants had lower symptoms following either form of abiotic stress while Blenheim plants exposed to high light prior to inoculation showed no change in RLS. This indicates that stress to the host alters the relationship with the R. collo-cygni fungus and elicits more extensive development of RLS symptoms, especially in susceptible genotypes. Increased RLS expression has been associated with stress caused by high light intensity (Makepeace et al., 2008; Peraldi et al., 2014) in seedlings and the developmental shift to flowering (Schützendübel et al., 2008) in field-grown adult plants, both of which result in accelerated senescence (Supplementary Data Fig. S2) and altered ROS balance within the host (Zimmermann and Zentgraf, 2005; Jajic et al., 2015). Light intensity affects the transition to disease of many fungal pathogens in the Mycosphaerellaceae, including symptom expression by Z. tritici, Pseudocercospora fijienesis and Dothistroma septosporum (Bradshaw, 2004; Keon et al., 2007; Churchill, 2011).
In vitro, R. collo-cygni is tolerant of conditions that induce oxidative stress (Fig. 5) such as high H2O2 levels, which are associated with the later stages of disease (Figs 2 and 4; Supplementary Data Fig. S3). The role of H2O2 in RLS may, again, be similar to that in other diseases caused by fungi in the Mycosphaerellaceae. The development of Septoria tritici blotch symptoms in wheat caused by the fungus Z. tritici, which is closely related to R. collo-cygni (McGrann et al., 2016), was defined by an accumulation of H2O2, PR proteins and fungal biomass (Yang et al., 2013), similar to that observed in RLS (Figs 1C, 2 and 4; Supplementary Data Figs S3 and S5). Ramularia collo-cygni has an abundance of chloroperoxidase proteins which use H2O2 as a substrate, a common trait within the Mycosphaerellaceae (do Amaral et al., 2012). Many chloroperoxidase genes are upregulated during asymptomatic development of Z. tritici (Rudd et al., 2015), suggesting that they may be important in allowing the pathogen to develop in environments high in H2O2 such as plants under oxidative stress. It is feasible that R. collo-cygni uses a similar system to cope with high levels of this ROS during disease development.
It is not known why NBT staining was lower in Rcc09B4-inoculated leaves than in mock-inoculated leaves. At earlier time points (5–10 dpi), reduced NBT in inoculated material may indicate that the fungus acts to delay ROS production in the host during endophytic growth, whereas at later times it may reflect conversion of superoxide to H2O2, noting the strong DAB staining in susceptible varieties at these times (Fig. 4; Supplementary Data Fig. S3).
Expression of RLS symptoms appears to be linked to ROS status within the leaf. Mutations in genes such as mlo, ror1, ror2, nec1 and bst1 (Hückelhoven et al., 2000; Piffanelli et al., 2002; Persson et al., 2009; McGrann et al., 2014; McGrann et al., 2015b) that result in mis-regulation of ROS all affect the transition from endophyte to necrotroph in barley seedlings. It is likely, however, that accumulation of H2O2 either is not responsible for the transition to RLS disease or acts together with other factors to elicit symptoms. On the one hand, the bst1 mutant showed lower RLS levels than the wild type (Fig. 9) and accumulated reduced levels of H2O2 in response to pathogen challenge (Persson et al., 2009). On the other hand, barley nec1 mutants, which also show reduced RLS symptoms (McGrann et al., 2015b), increased H2O2 levels in response to pathogen inoculation (Keisa et al., 2011). Furthermore, no effect on RLS symptoms was observed in the catalase-deficient mutant RPr 79/4 (Kendall et al., 1983) or the HvCSD1 RNAi line (Lightfoot et al., 2017; Fig. 7), both of which have reduced antioxidant levels potentially affecting foliar H2O2 levels.
Infiltration of R. collo-cygni-inoculated barley leaves with catalase or H2O2 prior to symptom formation increased symptom expression in most of the susceptible varieties tested, but not the partially resistant varieties (Fig. 8; Supplementary Data Fig. S7). This trend was not as consistent across varieties as the effect of abiotic stress treatments on disease expression (Fig. 3), possibly indicating that varieties vary in their ability to cope with a change in H2O2 status and thus in the development of RLS. In Septoria tritici blotch, in contrast to RLS, infiltration of wheat leaves with catalase increased susceptibility to Z. tritici whereas H2O2 infiltration increased resistance, demonstrating that manipulating the redox status in cereal leaves can alter the host response to this pathogen (Shetty et al., 2007), although not necessarily in the same direction as R. collo-cygni. Note that Z. tritici was more sensitive to lower concentrations of H2O2 in the assays of Shetty et al. (2007) than R. collo-cygni (Fig. 5), which may explain why R. collo-cygni can remain asymptomatic throughout its life cycle in the field despite experiencing oxidative stress.
These data on the interaction of R. collo-cygni with the plant redox system suggest that expression of disease symptoms in seedlings is not associated with elevated ROS levels as such, but rather with changes in ROS status. This is consistent with the concept that in the endophytic stage of its life cycle, R. collo-cygni has a finely balanced interaction with its host plant, but a change in that balance in either direction may cause it to become a necrotrophic parasite. RLS development is associated with an increase in either H2O2 accumulation or catalase gene transcription, which is expected to accelerate reduction of H2O2, since infiltration with either of these reagents promotes symptom expression in more susceptible barley varieties. As such, disease caused by R. collo-cygni may reflect miscommunication between the pathogen and its host caused by changes in barley ROS levels rather than aggressive pathogenicity, as has been suggested for other endophytes (Rodriguez and Redman, 2008).
In the field, symptoms of RLS are typically observed in the later part of the growing season after the crop has flowered. Symptom expression in seedlings and adult plants has previously been linked with leaf senescence (McGrann et al., 2015a), a general decline in leaf antioxidant status (Schützendübel et al., 2008) and a concomitant increase in ROS (Fig. 4; Supplementary Data Fig. S3). Abiotic stress, such as high light, accelerates the onset of senescence in seedlings (Szechyńska-Hebda and Karpiński, 2013), but the data shown here indicate that there is not a simple correlation between the rate of senescence and susceptibility to RLS, implying that the rate of varietal senescence is not sufficient to account for variation in disease severity (Supplementary Data Fig. S2).
Gradual breakdown of the chloroplast and the resulting changes in ROS levels may therefore have key roles as signals to trigger the transition of R. collo-cygni to pathogenic development. In support of this hypothesis, when senescence was promoted but chloroplast degradation occurred slowly following abiotic stress treatments (Supplementary Data Fig. S2), disease symptom expression was enhanced in all but the most RLS-resistant barley varieties (Fig. 3). Conversely, when chloroplast degradation occurred rapidly, as in the bst1-7 mutant (Fig. 9), or did not occur, as in the albostrians mutants that have undifferentiated plastids (Fig. 10), R. collo-cygni remained in an endophytic stage and did not cause disease. This mechanism may be important in pathogenesis of other Mycosphaerellaceae fungi. Wheat plants in which defective chloroplast function was produced by silencing the carotenoid or chlorophyll biosynthesis genes Phytoene desaturase and Magnesium chelatase H subunit had reduced symptom production and less asexual reproduction by Z. tritici (Lee et al., 2015). They also showed accelerated pathogen-induced ROS production, further suggesting an important role for chloroplast function and ROS in the transition of Mycosphaerellaceae fungi to necrotrophic parasitism.
The data presented here suggest that abiotic stress, senescence and elevated levels of ROS are not the proximate causes of the transition of R. collo-cygni from endophyte to necrotroph in artificially inoculated barley seedlings. Rather, ROS-mediated processes that lead to senescence may initiate the pathogenic transition of R. collo-cygni and thus RLS symptom development. Further insights into mechanisms that determine host susceptibility to RLS in seedlings will provide a better understanding of the factors that contribute to the aetiology of RLS in the field. This may lead to novel strategies for reducing losses of yield and grain quality caused by this aggressive new disease, including methods of selecting RLS-resistant barley varieties.
SUPPLEMENTARY DATA
Supplementary data are available at https://academic.oup.com/aob and consist of the following. Table S1: barley varieties used in pathology experiments. Table S2: qRT-PCR primers used in this study. Figure S1: expression of chlorophyll a/b-binding protein in spring barley varieties Braemar, Power and Golden Promise following Ramularia collo-cygni inoculation. Figure S2: dark-induced senescence of prophyll leaves of the spring barley varieties Power, Braemar, Golden Promise, Chevallier, Optic, Proctor, Ingrid and IngridBCmlo5 grown under standard controlled environment room conditions or under high light conditions. Figure S3: ROS accumulation in Golden Promise following Ramularia collo-cygni inoculation. Figure S4: effect of oxidative stress-inducing media on in vitro growth of Magnaporthe oryzae, Fusarium culmorum, Oculimacula yallundae and Botrytis cinereal. Figure S5: expression analysis of defence-related gene transcript levels during the Ramularia collo-cygni infection time course. Figure S6: effect of reagents used to manipulate leaf H2O2 status on in vitro growth of Ramularia collo-cygni. Figure S7: effect of manipulation of H2O2 in barley leaves infected with Ramularia collo-cygni. Figure S8: lesion development caused by the reactive oxygen species donors alloxan, menadione and methyl viologen in different spring barley varieties. Figure S9: expression analysis of antioxidant- and defence-related gene transcript levels in Bowman, Bipolaris sorokiniana tolerant 1 (bst1) and bst1-7.
ACKNOWLEDGEMENTS
J.K.M.B. conceived the original research plans. G.R.D.M. designed and performed the experiments. G.R.D.M. and J.K.M.B. analysed the data and wrote the manuscript. We thank Amanda Able (University of Adelaide), Graham Noctor (Institute of Plant Sciences Paris-Saclay) and Thomas Börner (Humboldt-Universität zu Berlin) for providing seeds of the barley lines 161-7-11, RPr 74/9 and albostrians along with their parent varieties. We also thank Andrew Davis for taking photographs and Lisa McGrann for technical assistance. This research was supported by BBSRC, RESAS, AHDB-HGCA and a consortium of ten companies (BASF, Bayer, KWS, Lantmännen, Limagrain, LS, Saaten-Union, Secobra, Sejet and Syngenta) through Sustainable Arable LINK, and grant BB/G024006/1 from BBSRC.
LITERATURE CITED
- Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42. [Google Scholar]
- Achard P, Renou JP, Berthome R, Harberd NP, Genschik P. 2008. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Current Biology 18: 656–660. [DOI] [PubMed] [Google Scholar]
- Adam A, Farkas T, Somlya G, Hevesi M, Kiraly Z. 1989. Consequence of O2·– generation during a bacterially induced hypersensitive reaction in tobacco – deterioration of membrane-lipids. Physiological and Molecular Plant Pathology 34: 13–26. [Google Scholar]
- do Amaral AM, Antoniw J, Rudd JJ, Hammond-Kosack KE. 2012. Defining the predicted protein secretome of the fungal wheat leaf pathogen Mycosphaerella graminicola. PLoS One 7: e49904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw RE. 2004. Dothistroma (red-band) needle blight of pines and the dothistromin toxin: a review. Forest Pathology 34: 163–185. [Google Scholar]
- Churchill AC. 2011. Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Molecular Plant Pathology 12: 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebrook EH, Creissen G, McGrann GRD, Dreos R, Lamb C, Boyd LA. 2012. Broad-spectrum acquired resistance in barley induced by the Pseudomonas pathosystem shares transcriptional components with Arabidopsis systemic acquired resistance. Molecular Plant-Microbe Interactions 25: 658–667. [DOI] [PubMed] [Google Scholar]
- Fountaine JM, Fraaije BA. 2009. Development of QoI resistant alleles in populations of Ramularia collo-cygni. Aspects of Applied Biology 92: 123–126. [Google Scholar]
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell 17: 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan SS, Amasino RM. 1997. Making sense of senescence – molecular genetic regulation and manipulation of leaf senescence. Plant Physiology 113: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard R, Peraldi A, Ridout C, Nicholson P. 2014. Enhanced disease resistance caused by BRI1 mutation is conserved between Brachypodium distachyon and barley (Hordeum vulgare). Molecular Plant-Microbe Interactions 27: 1095–1106. [DOI] [PubMed] [Google Scholar]
- Harvey IC. 2002. Epidemiology and control of leaf and awn spot of barley caused by Ramularia collo-cygni. New Zealand Plant Protection 55: 331–335. [Google Scholar]
- Havis ND, Nyman M, Oxley SJP. 2014. Evidence for seed transmission and symptomless growth of Ramularia collo-cygni in barley (Hordeum vulgare). Plant Pathology 63: 929–936. [Google Scholar]
- Havis ND, Clemente G, Brown JKM et al. 2015. Ramularia collo-cygni – an emerging pathogen of barley crops. Phytopathology 105: 895–904. [DOI] [PubMed] [Google Scholar]
- Heiser I, Sachs E, Liebermann B. 2003. Photodynamic oxygen activation by rubellin D, a phytotoxin produced by Ramularia collo-cygni (Sutton et Waller). Physiological and Molecular Plant Pathology 62: 29–36. [Google Scholar]
- Hess WR, Hubschmann T, Borner T. 1994. Ribosome-deficient plastids of albostrians barley – extreme representatives of nonphotosynthetic plastids. Endocytobiosis and Cell Research 10: 65–80. [Google Scholar]
- Hückelhoven R, Trujillo M, Kogel KH. 2000. Mutations in Ror1 and Ror2 genes cause modification of hydrogen peroxide accumulation in mlo-barley under attack from the powdery mildew fungus. Molecular Plant Pathology 1: 287–292. [DOI] [PubMed] [Google Scholar]
- Jajic I, Sarna T, Strzalka K. 2015. Senescence, stress, and reactive oxygen species. Plants 4: 393–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek M, Piotrowska MJ, Fountaine JM et al. 2016. Infection strategy of Ramularia collo-cygni and development of ramularia leaf spot on barley and alternative graminaceous hosts. Plant Pathology 66: 45–55. [Google Scholar]
- Keisa A, Kanberga-Silina K, Nakurte I, Kunga L, Rostoks N. 2011. Differential disease resistance response in the barley necrotic mutant nec1. BMC Plant Biology 11: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall AC, Keys AJ, Turner JC, Lea PJ, Miflin BJ. 1983. The isolation and characterization of a catalase-deficient mutant of barley (Hordeum vulgare L). Planta 159: 505–511. [DOI] [PubMed] [Google Scholar]
- Keon J, Antoniw J, Carzaniga R et al. 2007. Transcriptional adaptation of Mycosphaerella graminicola to programmed cell death (PCD) of its susceptible wheat host. Molecular Plant-Microbe Interactions 20: 178–193. [DOI] [PubMed] [Google Scholar]
- Lee WS, Devonshire BJ, Hammond-Kosack KE, Rudd JJ, Kanyuka K. 2015. Deregulation of plant cell death through disruption of chloroplast functionality affects asexual sporulation of Zymoseptoria tritici on wheat. Molecular Plant-Microbe Interactions 28: 590–604. [DOI] [PubMed] [Google Scholar]
- Lightfoot DJ, McGrann GRD, Able AJ. 2017. The role of a cytosolic superoxide dismutase in barley–pathogen interactions. Molecular Plant Pathology 18: 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makepeace JC, Havis ND, Burke JI, Oxley SJP, Brown JKM. 2008. A method of inoculating barley seedlings with Ramularia collo-cygni. Plant Pathology 57: 991–999. [Google Scholar]
- McGrann GRD, Havis ND. 2017. Ramularia leaf spot: a newly important threat to barley production. Outlooks on Pest Management 28: 65–69. [Google Scholar]
- McGrann GRD, Townsend BJ, Antoniw JF, Asher MJC, Mutasa-Goettgens ES. 2009. Barley elicits a similar early basal defence response during host and non-host interactions with Polymyxa root parasites. European Journal of Plant Pathology 123: 5–15. [Google Scholar]
- McGrann GRD, Stavrinides A, Russell J et al. 2014. A trade off between mlo resistance to powdery mildew and increased susceptibility of barley to a newly important disease, Ramularia leaf spot. Journal of Experimental Botany 65: 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrann GRD, Steed A, Burt C et al. 2015. a Contribution of the drought tolerance-related Stress-responsive NAC1 transcription factor to resistance of barley to Ramularia leaf spot. Molecular Plant Pathology 16: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrann GRD, Steed A, Burt C, Nicholson P, Brown JKM. 2015b Differential effects of lesion mimic mutants in barley on disease development by facultative pathogens. Journal of Experimental Botany 66: 3417–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrann GRD, Andongabo A, Sjökvist E et al. 2016. The genome of the emerging barley pathogen Ramularia collo-cygni. BMC Genomics 17: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley SJP, Havis ND, Brown JKM, Makepeace JC, Fountaine J. 2008. Impact and interactions of Ramularia collo-cygni and oxidative stress in barley. HGCA Project Report 100 https://cereals.ahdb.org.uk/publications/2008/july/01/impact-and-interactions-of-ramularia-collo-cygni-and-oxidative-stress-in-barley.aspx. [Google Scholar]
- Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM. 2009. GenStat for Windows (12th Edition) Introduction. Hemel Hempstead, UK: VSN International. [Google Scholar]
- Peraldi A, Griffe LL, Burt C, McGrann GRD, Nicholson P. 2014. Brachypodium distachyon exhibits compatible interactions with Oculimacula spp. and Ramularia collo-cygni, providing the first pathosystem model to study eyespot and Ramularia leaf spot diseases. Plant Pathology 63: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M, Rasmussen M, Falk A, Dixelius C. 2008. Barley mutants with enhanced level of resistance to Swedish isolates of Bipolaris sorokiniana, casual agent of spot blotch. Plant Breeding 127: 639–643. [Google Scholar]
- Persson M, Falk A, Dixelius C. 2009. Studies on the mechanism of resistance to Bipolaris sorokiniana in the barley lesion mimic mutant bst1. Molecular Plant Pathology 10: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffanelli P, Zhou FS, Casais C et al. 2002. The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant Physiology 129: 1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R, Redman R. 2008. More than 400 million years of evolution and some plants still can’t make it on their own: plant stress tolerance via fungal symbiosis. Journal of Experimental Botany 59: 1109–1114. [DOI] [PubMed] [Google Scholar]
- Rudd JJ, Keon J, Hammond-Kosack KE. 2008. The wheat mitogen-activated protein kinases TaMPK3 and TaMPK6 are differentially regulated at multiple levels during compatible disease interactions with Mycosphaerella graminicola. Plant Physiology 147: 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd JJ, Kanyuka K, Hassani-Pak K et al. 2015. Transcriptome and metabolite profiling of the infection cycle of Zymoseptoria tritici on wheat reveals a biphasic interaction with plant immunity involving differential pathogen chromosomal contributions and a variation on the hemibiotrophic lifestyle definition. Plant Physiology 167: 1158–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikkonen K, Faeth SH, Helander M, Sullivan TJ. 1998. Fungal endophytes: a continuum of interactions with host plants. Annual Review of Ecology and Systematics 29: 319–343. [Google Scholar]
- Saville RJ, Gosman N, Burt CJ et al. 2012. The ‘Green Revolution’ dwarfing genes play a role in disease resistance in Triticum aestivum and Hordeum vulgare. Journal of Experimental Botany 63: 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena I, Srikanth S, Chen Z. 2016. Cross talk between H2O2 and interacting signal molecules under plant stress response. Frontiers in Plant Science 7: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schützendübel A, Stadler M, Wallner D, von Tiedemann A. 2008. A hypothesis on physiological alterations during plant ontogenesis governing susceptibility of winter barley to Ramularia leaf spot. Plant Pathology 57: 518–526. [Google Scholar]
- Schulz B, Boyle C. 2005. The endophytic continuum. Mycological Research 109: 661–686. [DOI] [PubMed] [Google Scholar]
- Shetty NP, Mehrabi R, Lutken H et al. 2007. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytologist 174: 637–647. [DOI] [PubMed] [Google Scholar]
- Stabentheiner E, Minihofer T, Huss H. 2009. Infection of barley by Ramularia collo-cygni: scanning electron microscopic investigations. Mycopathologia 168: 135–143. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Koussevitzky S, Mittler R, Miller G. 2012. ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell and Environment 35: 259–270. [DOI] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Karpiński S. 2013. Light intensity-dependent retrograde signalling in higher plants. Journal of Plant Physiology 170: 1501–1516. [DOI] [PubMed] [Google Scholar]
- Taylor JMG, Paterson LJ, Havis ND. 2010. A quantitative real-time PCR assay for the detection of Ramularia collo-cygni from barley (Hordeum vulgare). Letters in Applied Microbiology 50: 493–499. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. The Plant Journal 11: 1187–1194. [Google Scholar]
- West JS, Townsend JA, Stevens M, Fitt BDL. 2012. Comparative biology of different plant pathogens to estimate effects of climate change on crop diseases in Europe. European Journal of Plant Pathology 133: 315–331. [Google Scholar]
- Wolter M, Hollricher K, Salamini F, Schulze-Lefert P. 1993. The mlo resistance alleles to powdery mildew infection in barley trigger a developmentally controlled defense mimic phenotype. Molecular and General Genetics 239: 122–128. [DOI] [PubMed] [Google Scholar]
- Yang F, Li W, Jorgensen HJ. 2013. Transcriptional reprogramming of wheat and the hemibiotrophic pathogen Septoria tritici during two phases of the compatible interaction. PLoS One 8: e81606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Zong W, Li X et al. 2013. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. Journal of Experimental Botany 64: 569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research 14: 415–421. [Google Scholar]
- Zimmermann P, Zentgraf U. 2005. The correlation between oxidative stress and leaf senescence during plant development. Cellular and Molecular Biology Letters 10: 515–534. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.