Abstract
Background
The continuing epidemic of methamphetamine addiction has prompted research aimed at understanding striatal dysfunctions potentially associated with long-term methamphetamine use.
Methods
Here, we investigated transcriptional and translational alterations in the expression of neurotrophic factors in the rat striatum at 30 days following methamphetamine self-administration and footshock punishment. Male Sprague–Dawley rats were trained to self-administer methamphetamine (0.1 mg/kg/injection, i.v.) or saline during twenty-two 9-hour sessions. Subsequently, rats were subjected to incremental footshocks for 13 additional methamphetamine self-administration sessions. This paradigm led to the identification of rats with shock-resistant and shock-sensitive phenotypes. Thirty days following the last footshock session, the dorsal striatum was dissected and processed for gene expression and protein analyses.
Results
PCR arrays revealed significant differences in neurotrophins and their receptors between the 2 phenotypes. Brain-derived neurotrophic factor and nerve growth factor protein levels were increased in the dorsal striatum of both shock-resistant and shock-sensitive rats. However, neurotrophic receptor tyrosine kinase 1 phosphorylation and nerve growth factor receptor protein expression were increased only in the shock-sensitive phenotype. Moreover, shock-sensitive rats showed increased abundance of several phosphorylated proteins known to participate in Ras/Raf/MEK/ERK signaling cascade including cRaf, ERK1/2, MSK1, and CREB.
Conclusions
These findings support the notion that animals with distinct phenotypes for methamphetamine intake in the presence of adverse consequences also display differential changes in an intracellular signaling cascade activated by nerve growth factor-TrkA/p75NTR interactions. Thus, the development of pharmacological agents that can activate nerve growth factor-dependent pathways may be a promising therapeutic approach to combat methamphetamine addiction.
Keywords: addiction, footshocks, neurotrophins, signal transduction
Significance Statement
Methamphetamine addiction is characterized by continued drug use despite adverse life events associated with its use. To mimic the human conditions, we used a self-administration model of methamphetamine (METH) intake performed in conjunction with footshocks as adverse consequences. This paradigm helped to discriminate shock-sensitive (SS) from shock-resistant (SR) rats, with SR rats being considered an addicted phenotype. Our main findings indicated that there were specific differences in the striatal expression of genes coding for neurotrophic factors between the 2 METH self-administration (SA) phenotypes. Importantly, nerve growth factor (NGF) and its downstream signaling pathway (NGF-TrkA and p75NTR/MAPK signaling) were found to be selectively increased in the SS rats. These observations suggest that METH abstinence may be associated with an upregulated NGF-signaling cascade. Our data may have important therapeutic implications in the treatment of patients at different stages of METH addiction.
Introduction
Addiction to methamphetamine (METH) is considered a chronic neuropsychiatric disorder characterized by striatal dysfunctions, cognitive deficits, and loss of control over drug consumption despite adverse consequences associated with its use (Scott et al., 2007; Rusyniak, 2013). METH addiction is also associated with a high prevalence of relapse to drug-taking behaviors even after years of sobriety (Brecht and Herbeck, 2014; Gowin et al., 2015). These clinical observations have implicated long-lasting neuroplastic changes in brain regions involved with both reward mechanisms and compulsive diatheses (Belin et al., 2013). In fact, several groups of investigators have reported significant changes in gene expression within mesolimbic reward circuitry following noncontingent injections of METH in rodents [for a review see (Cadet and Krasnova, 2009)]. Using the self-administration (SA) model of METH intake in rats, we and others have also documented substantial transcriptional and translational changes within the rodent dorsal striatum (Krasnova et al., 2013; Cadet et al., 2015; Li et al., 2015; Caprioli et al., 2017). The dorsal striatum is indeed a key structure involved with the habitual manifestations of addiction (Koob and Volkow, 2010; Belin et al., 2013). Together, these findings support the notion that the path from casual drug use to habitual drug taking may depend on long-lasting neuroadaptations that include a shift in control from ventral reward circuits to more dorsal striatal habit circuits (Feil et al., 2010; Everitt and Robbins, 2013).
We recently developed a rat model in which we use footshocks as adverse consequences during METH SA. This model has helped to dichotomize rats into 2 distinct addiction-related phenotypes (Cadet et al., 2017). Specifically, these procedures were able to segregate rats that continued to take METH despite footshock punishment (shock-resistant [SR]) from rats that reduce their METH intake with increasing shock intensity (shock-sensitive [SS]). Interestingly, SR rats were found to exhibit higher incubation of METH-seeking behavior compared with SS rats (Krasnova et al., 2017; Torres et al., 2017).
In the present study, we examined tissue from animals used in a previous behavioral study (Torres et al., 2017) to test whether neurobiological alterations in the striatum might reflect molecular adaptations that may distinguish SR from SS even after a prolonged period of drug abstinence. We focused on the dorsal striatum, because neuroadaptations within this structure might underlie the transition from casual to compulsive drug-seeking behavior (Everitt and Robbins, 2013, 2016). The importance of striatum is also highlighted by substantial transcriptional and translational changes following models of METH SA (Krasnova et al., 2013; Cadet et al., 2015; Li et al., 2015; D’Arcy et al., 2016). Because neurotrophins are known to participate in the generation of long-lasting plastic changes in the brain (Alder et al., 2003; Bekinschtein et al., 2008), we tested the possibility that alterations in their striatal expression might occur following prolonged abstinence from our METH SA procedures. Here, we provide evidence that the striatal NGF-TrkA/p75NTR/MAPK signaling pathway is selectively activated in SS rats at 30 days following METH SA and footshocks. Our results support the view that distinct signaling cascades may play important roles in mediating the long-lasting effects of METH taking depending on the level of control over drug consumption.
Methods
Animals and SA Procedures
Male Sprague-Dawley rats (Charles River) weighing 350 to 400 g were used in these experiments. Rats were anesthetized with a ketamine/xylazine mixture (50 and 5 mg/kg, i.p., respectively) and were inserted with catheters into the jugular vein. After recovery, rats were placed in Med Associates SA chambers and trained to self-administer METH (0.1 mg/kg/injection, i.v.) on an FR-1 schedule during three 3-hour sessions/d (9 h/d). Each 3-hour session was separated by a 30-minute time interval. Following SA training, rats were subjected to punishment where 50% of active lever-presses resulted in a mild foot-shock. Animals were classified as SS if they reduced their intake by 70%. Subgroups were divided into SR (n = 7), SS (n = 9), and saline controls (n = 5) as previously reported by Torres et al. (2017). Cue-induced drug-seeking behavior was assessed at 2 and 21 days post-shock essentially as described in Krasnova et al. (2014). Briefly, each test consisted of a 1-hour session without METH availability, during which time drug-associated lever presses resulted only in tone and light cues previously paired with METH infusions. All animal procedures were conducted following the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the NIDA Intramural Research Program.
Tissue Collection and RNA Extraction
To measure potential differences in gene expression between the SR and SS phenotypes after a long period of abstinence, rats were killed 9 days following the second extinction test (30 days total after the last METH SA session). This time frame was based on our previous studies showing METH-induced changes in striatal expression of neurotrophins and genes involved in the CREB (cAMP responsive element binding protein) signaling pathway 1 month after METH SA (Krasnova et al., 2013, 2016). Following rapid decapitation, dorsal striatal tissue was dissected, immediately placed on dry ice, and stored at -80°C. Total RNA was then isolated using Qiagen RNeasy Mini kits and treated against genomic DNA contamination by using Qiagen RNase-free DNase kits (Qiagen) following the manufacturer’s protocol. RNA levels were then assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). RNA integrity numbers were also examined, as described by Schroeder et al. (2006), using an Agilent 2100 Bioanalyzer System in conjunction with the RNA LabChip assay (Agilent) with no sample showing signs of degradation.
RT2 Profiler PCR Array
The RT2 Profiler PCR Array (PARN-031Z, Qiagen) discovery platform was used to examine differences in striatal gene expression between SR and SS rats. Each array contained 84 gene-specific primers related to neurotrophic signaling, neuropeptides, and transcription factors. Total RNA (0.5μg) was treated with 5x buffer GE, incubated at 42°C for 5 minutes, and placed on ice. Purified RNA was then reverse-transcribed to cDNA by using the RT2 First Strand Kit (Qiagen) and RNase-free water following the manufacturer’s instructions. Each 96-well RT2 Profiler PCR Array was applied on a LightCycler 480 II PCR system (Roche Diagnostics) for each striatal sample. Each array plate contained a genomic DNA contamination control, 3 reverse transcription controls, 3 positive PCR controls, and 5 wells with different housekeeping genes (Beta-actin, Beta-2 microglobulin, Hypoxanthine phosphoribosyltransferase 1, Lactate dehydrogenase A, and Ribosomal Protein, Large, p1). Data analyses for threshold amplification cycle numbers (Tc) were performed using the complimentary web-based Qiagen software tools following the manufacturer’s protocol and normalized to the 4 housekeeping genes. To confirm primer specificity of each gene product, amplicon melting curves were recorded and analyzed after every array experiment.
Independent qRT- PCR Analysis
RT2 Profiler PCR Array data were verified by individual qRT-PCR comparisons. Briefly, unpooled total RNA (0.5μg) was reverse-transcribed to cDNA using oligo dT primer from the Advantage RT for PCR kit (Clontech). Sequences for rat gene-specific primers corresponding to PCR targets were then designed using the LightCycler Probe Design software version 1 (Roche Diagnostics) and synthesized by the Synthesis and Sequencing Facility of Johns Hopkins University. qRT-PCR reactions were carried out in a final volume of 12.5 μL consisting of reverse transcribed cDNA, gene-specific primers, nucleic-acid free water, and iQ SYBR Green Supermix (Bio-Rad). All qRT-PCR experiments were conducted using a LightCycler 480 II instrument (Roche). qRT-PCR primers were designed for the specific amplification of rat Bdnf (brain-derived neurotrophic factor), Ngf (nerve growth factor), TrkA (neurotrophic receptor tyrosine kinase 1), TrkB (neurotrophic receptor tyrosine kinase 2), Gfra2 (GDNF family receptor alpha 2), Crh (corticotrophin-releasing hormone), Crhr1 (Crh receptor 1), Crhr2 (Crh receptor 2), Crhbp (Crh binding protein), Ucn2 (urocortin 2), c-fos, fosb, Egr1 (early growth response 1), Egr2 (early growth response 2), and Egr3 (early growth response 3) (sequences are listed on supplementary Table 1). The purity of each amplicon was subsequently verified by melting curve analysis. Expression of mRNA levels for each gene was normalized to the reference gene beta-2-microglobulin. Results are reported as fold changes calculated by the ratios of normalized target gene products compared with the gene expression data of saline SA control group.
Western-Blot Analysis
Western-blot analyses were carried out essentially as previously described by our laboratory (Jayanthi et al., 2009). Briefly, striatal tissue was homogenized in ice-cold lysis buffer A (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, and 1% igepal) containing protease and phosphatase inhibitor cocktail tablets (Roche Diagnostics). After homogenization, samples were centrifuged at 14 000 g for 5 minutes at 4°C. Supernatant was collected and used as cytosolic protein fraction. Nuclear fractions were then suspended in buffer B (20 mM HEPES, 840 mM NaCl, 1.5 mM MgCl2, 4 mM EDTA, and 10% glycerol) containing protease and phosphatase inhibitor cocktail tablets (Roche Diagnostics). Individual protein concentrations were determined by utilizing the BCA assay kit (Thermo Fisher Scientific). Samples were then transferred on to PVDF membranes and incubated overnight at 4°C with a specific antibody raised against: Pro-BDNF (Millipore, #AB5613P), BDNF (Santa Cruz Biotechnology, #SC546), Pro-NGF and mature NGF (Santa Cruz, #SC365944), TrkB (Millipore, #07-225), pTrkB (Sigma-Aldrich, #SAB 4301317), TrkA (Cell Signaling, #2508), pTrkA (Cell Signaling, #9141), P75NTR (nerve growth factor receptor) (Cell Signaling, #2693), Sortilin (Thermo Fisher Scientific, #PA5-19481), p-cRaf (Cell Signaling, #9421), pMEK1/2 (mitogen activated protein kinase 1) (Cell Signaling, #9154), pErk1/2 (mitogen activated protein kinase 3) (Cell Signaling, #4370), pMSK1 (mitogen and stress activated protein kinase 1) (Cell Signaling, #9595), pCREB (Cell Signaling, #9198), H3ac (acetylated histone H3) (Active Motif, #39139), p-MeCP2 (methyl-CpG binding protein 2) (ActiveMotif, #39733), and p-mTOR (mammalian target of rapamycin) (Cell Signaling, #2971). To confirm equal protein loading, blots were reprobed with an antibody against α-Tubulin (Sigma) for 2 hours at room temperature. Optical densities were measured using the ChemiDoc MP Imaging system (Bio-Rad) and normalized using the signal intensity of α-tubulin. The results are reported as percentage of control changes calculated as the ratios of protein expression for each METH group compared with the saline SA control group.
Statistical Analysis
Profiler PCR Array data were analyzed using the Qiagen web-based software. qRT-PCR data were analyzed using 1-way ANOVAs followed by posthoc tests (Bonferroni) or planned comparisons (2-tailed Student tests) (SPSS 20). Western-blot data were also analyzed by 1-way ANOVAs followed by posthoc tests (Fisher’s LSD) or planned comparisons (StatView, SAS). Individual planned comparisons were used in specific cases where the difference between SR and SS rats was based on an empirical framework. Correlations between RT2 Profiler PCR Arrays and qRT-PCR data were assessed using linear regression (SPSS 20). All data are presented as means ± SEM and considered statistically significant when P ≤ .05.
Results
Transcriptional Alterations in the Dorsal Striatum 30 Days after METH SA and Footshock Punishment
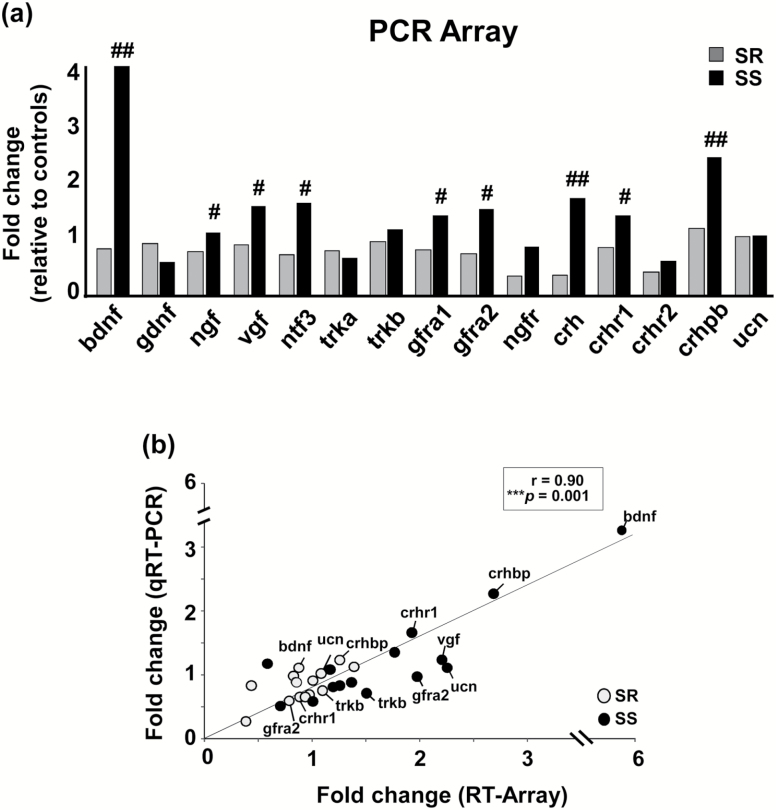
To identify differentially expressed neuroplasticity-related genes that might distinguish SR from SS rats, we used the unbiased discovery platform Neurotrophins and Receptors RT2 Profiler PCR Array system. This approach allowed us to potentially target a specific subset of genes that were found to be affected after cessation of METH SA experiments (Krasnova et al., 2013, 2016). Figure 1a illustrates METH-induced changes in the expression of neurotrophic and neuropeptide associated genes in the dorsal striatum. Specifically, mRNA expression for Bdnf, Ngf, Vgf, Trkb, Ntf3 (neurotrophin 3), Gfra1, and Gfra2 were upregulated in SS rats compared with SR rats at 30 days after METH SA and punishment. These findings may be related to the demonstration that METH exposure can increase synaptic spine density and structural plasticity in the dorsal striatum (Jedynak et al., 2007). We also found that Crh, Crhr1, and Crhbp mRNA levels were increased in SS rats relative to SR rats (P < .05). Array data were subsequently validated by running qRT-PCRs using the same RNA samples isolated from the dorsal striatum of these rats. We found a significant correlation (r = 0.90, P < .01) between the array and PCR data (Figure 1b).
Figure 1.
Transcriptional alterations 30 days after the punishment phase of methamphetamine (METH) self-administration (SA) in the dorsal striatum of shock-resistant (SR) and shock-sensitive (SS) rats. (a) The graph shows neurotrophic- and neuropeptide-associated genes that were differentially expressed between SR and SS rats as determined by RT2 Profiler PCR Arrays. (b) The figure shows transcriptional responses obtained by Profiler Arrays (“x” axis) and individual qRT-PCRs (“y” axis) for upregulated genes in the dorsal striatum of SR rats (in grey circles) and SS rats (in black circles). Data are presented as fold-changes relative to saline control rats. A significant correlation was observed between the 2 analyses (P = .001). Key to statistics: #P < .05, ##P < .01 compared with SR rats.
Differential Expression of Neurotrophins, Neuropeptides, and Immediate Early Genes (IEGs) in SR and SS Phenotypes
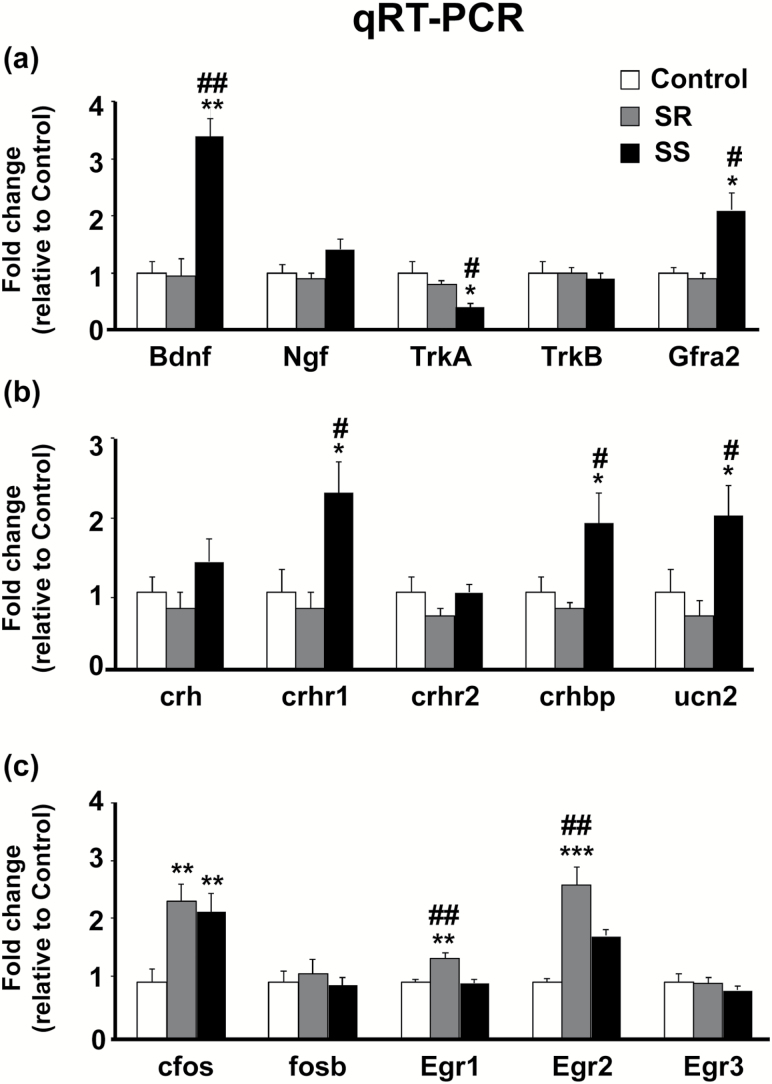
qRT-PCR analysis showed significant alterations in Bdnf mRNA levels [F(2, 17) = 7.86, P < .01], with SS rats showing significant increases compared with saline controls and SR rats (P < .01) (Figure 2a). Ngf mRNA levels were nonsignificantly higher in SS rats compared with SR rats [F(2, 17) = 1.78, P = .21]. Gfra2 mRNA levels were also increased [F(2, 18) = 7.97, P < .01] in SS rats relative to SR and saline control rats. Interestingly, SS rats displayed a significant decrease in Trka [F(2, 18) = 6.39, P < .01] mRNA levels compared with SR rats. Our PCR analyses also confirmed the upregulation of some, but not all, neuropeptide-associated genes. Crhr1 mRNA levels were increased in SS rats [F(2, 17) = 5.14, P < .05] (Figure 2b) but showed no changes in SR rats. Differential changes were also observed for Crhbp [F(2, 18) = 3.7, P < .05] and Ucn2 [F(2, 18) = 3.9, P < .05] mRNA levels, with SS rats displaying significant increases compared with saline control and SR rats (P < .05) (Figure 2b).
Figure 2.
Distinct neurotrophin, neuropeptide, and immediate early genes (IEGs) expression in the dorsal striatum of shock-resistant (SR) and shock-sensitive (SS) rats. (a) Independent qRT-PCR analyses for neurotrophin mRNA levels in the dorsal striatum with increased expression of Bdnf in both SR and SS rats, decrease TrkA levels in SS rats, and increased Gfra2 levels also in SS rats. (b) Differential expression neuropeptide-related genes and receptors in the dorsal striatum, with SS rats having increased mRNA levels for crhr1, crhbp, and unc2 compared with saline controls and SR rats. (c) Altered mRNA levels of IEGs in the dorsal striatum of SR and SS rats. Both SR and SS rats had increased expression of c-fos mRNA levels compared with saline controls, while only SR rats had increased egr1 and egr2 mRNA levels compared with saline and SS rats. Key to statistics: *P < .05, **P < .01, ***P < .001 compared with saline controls; #P < .05, ##P < .01 compared with SR rats.
To test if other genes known to be regulated by trophic factors were also altered between the SR and SS phenotypes, we measured mRNA levels of some IEGs (Figure 2c). We found significant increases in c-fos mRNA levels [F(2, 17) = 3.93, P < .05] in both SR and SS rats compared with controls. No changes in fosb mRNA levels [F(2, 18) = 0.30, P = .74] were observed. There were also significant increases in Egr1 [F(2, 18) = 9.0, P < .01] and Egr2 [F(2, 18) = 13.52, P < .01] mRNA levels in SR rats compared with SS rats and controls (Figure 2c). There were no significant changes in Egr3 mRNA levels [F(2, 18) = 0.53, P < .59].
Differential Expression of Neurotrophin-Related Proteins in the Dorsal Striatum of SR and SS Rats
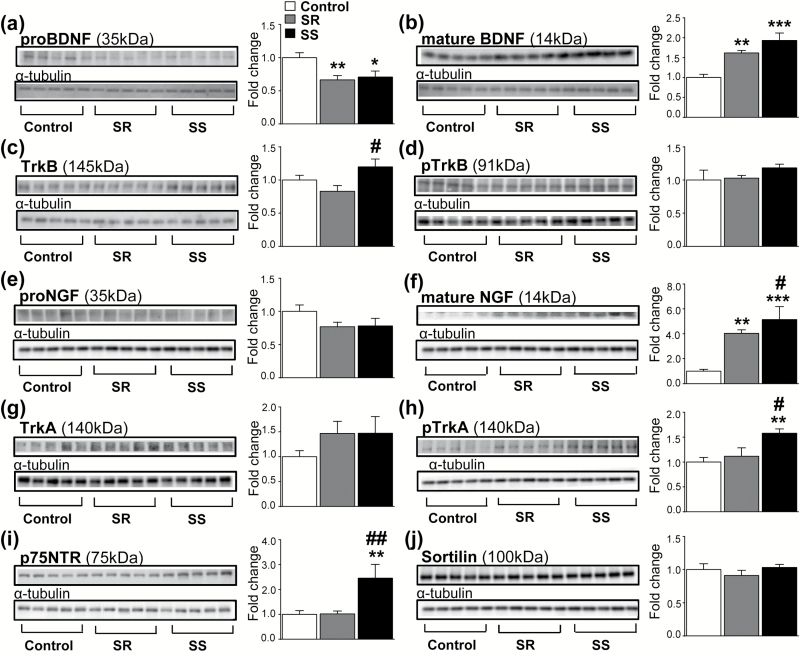
To better elucidate the neuroplastic bases for the SR from SS phenotypes, we tested if changes in the striatal expression of neurotrophin mRNA levels were also associated with corresponding changes in protein products. There were significant decreases in striatal proBDNF levels [F (2, 15) = 5.13, P < .05] in SR rats compared with controls (Figure 3a). Figure 3b shows that there were also increases in mature BDNF levels [F(2, 16) = 11.80, P < .001] in both SR and SS rats. There were also significant changes in the BDNF receptor, TrkB, protein levels [F(2, 15) = 3.63, P < .05] (Figure 3c) but no significant changes in the phosphorylated form, pTrkB [F(2, 16) = 1.58, P = .23] (Figure 3d) between the SR and SS rats (Figure 3c).
Figure 3.
Expression of BDNF- and NGF-related proteins in the dorsal striatum following 30 days after methamphetamine (METH) self-administration (SA). (a) Significantly decreased pro-BDNF levels in shock-resistant (SR) rats compared with saline controls. (b) Increased mature BDNF in SR and shock-sensitive (SS) rats compared with saline controls. (c) TrkB levels are slightly increased in SS rats compared with SR rats. (d) Similar expression of pTrkB protein levels in SR and SS phenotypes. (e) No changes in pro-NGF levels in SR and SS rats. (f) Increased mature NGF protein levels in both SR and SS rats, with SS showing larger increases. (g) Comparable upregulated TrkA levels in SR and SS rats. (h) Increased pTrkA expression in SS rats compared with saline controls. (i) Increased of p75NTR expression in SS rats compared with saline controls and SR rats. (j) Similar levels of sortilin expression between SR and SS rats. Values represent means ±SEM of fold changes relative to the controls. Key to statistics: *P < .05, **P < .01, ***P < .001 compared with saline controls; #P < .05, ##P < .01 compared with SR rats.
We next focused our attention on proNGF, NGF, and TrkA protein levels. We detected no significant changes in proNGF levels [F(2, 15) = 1.72, P = .21] (Figure 3e) but found significant increases in mature NGF levels [F(2, 16) = 42.45, P < .001] in both SR and SS groups compared with the control group (Figure 3f). However, mature NGF protein levels were significantly higher in the SS rats compared with the SR rats (P < .05) (Figure 3f). There were no significant differences in TrkA protein levels between the groups (Figure 3g). However, there was increased abundance of phosphorylated TrkA protein [F(2, 15) = 5.10, P < .05] only in the SS phenotype (Figure 3h). Because NGF can also interact with the p75NTR receptor (Bucci et al., 2014), we tested the possibility that there might be differential protein expression of this receptor in the 2 phenotypes. Figure 3i shows that there were indeed significant changes in p75NTR protein levels [F(2, 15) = 6.13, P < .05], with the SS rats showing increased expression compared with SR and control rats. Given that p75NTR can interact with sortilin to cause cellular damage (Nykjaer and Willnow, 2012) and because METH is also a known toxicant (Cadet and Krasnova, 2009), we measured striatal sortilin levels. Figure 3j shows that there were no significant changes in sortilin levels [F(2, 16) = 0.81, P = .46] in the 2 phenotypes (Figure 3j), supporting the idea that increased p75NTR may be related to enhanced neuroplasticity in the SS phenotype but not to the reported toxic effects of METH SA (Krasnova et al., 2010).
Activation of the Ras/Raf/MEK/ERK Pathway in the Dorsal Striatum of SS Rats
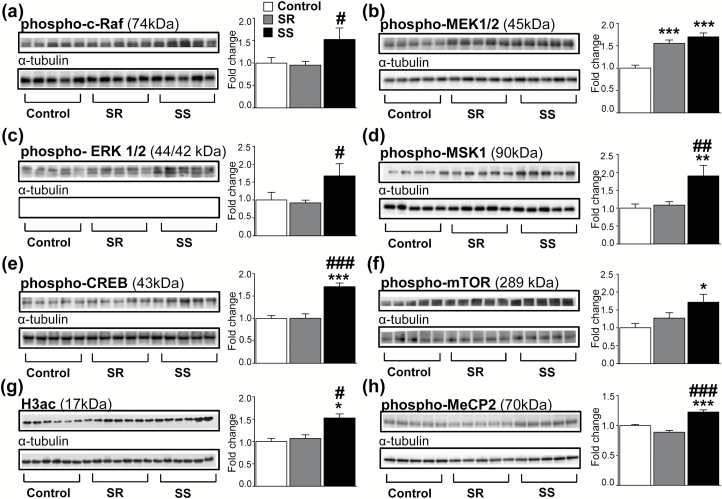
Neurotrophins exert their functions by activation of the Ras/Raf/MEK/ERK intracellular signaling cascade through phosphorylation of several proteins in that cascade (Reichardt, 2006; Cargnello and Roux, 2011). Importantly, it is known that p75NTR can interact with TrkA to potentiate activation of this cascade (Chao et al., 2006; Reichardt, 2006; Matusica et al., 2013), which is known to participate in the modulation of gene expression in response to neuronal activity (Flavell and Greenberg, 2008). We thus thought it likely that the SS phenotype might also show increased phosphorylation of proteins of the MAPK cascade. Indeed, Figure 4a showed a trend [F(2, 15) = 3.51, P = .06] of increased p-Raf abundance in SS rats, with planned comparisons revealing significant increases compared with SR rats (P < .05). Figure 4b also shows significant increases in pMEK1/2 abundance [F(2, 16) = 18.87, P < .001] in both SR and SS rats relative to controls. Figure 4c illustrates a trend [F(2, 15) = 3.34, P = .06] towards increased pERK abundance, with planned comparisons revealing significant increases in the SS rats compared with SR rats (P < .05). There were significant increases [F(2, 15) = 6.78, P < .01] in pMSK1 abundance in SS rats compared with SR and control rats (Figure 4d). Similarly, CREB phosphorylation was significantly [F(2, 16) = 22.55, P < .01] increased only in the SS phenotype (Figure 4e).
Figure 4.
Activation of the MAPK/ERK intracellular signaling cascade in the dorsal striatum of shock-sensitive (SS) rats. (a) p-cRaf expression in SS rats compared with controls and SR rats. (b) Increased pMEK1/2 abundance in both SR and SS rats compared with saline controls. (c) Increased pERK 1/2 expression in SS rats compared with SR rats. (d) Increased pMSK1 expression in SS rats compared with saline controls and SR rats. (e) Increased pCREB expression in SS rats compared with saline controls and SR rats. (f) Increased p-mTOR levels in SS rats compared with control rats. (g) Increased H3ac levels in SS rats compared with saline controls and SR rats. (h) Increased p-MeCP2 expression in SS rats compared with saline controls and SR rats. Key to statistics: *P < .05, **P < .01, ***P < .001 compared with saline controls; #P < .05, ##P < .01, ###P < .001 compared with SR rats.
Neurotrophins are also known to increase activation of mTOR complexes via mTOR phosphorylation with secondary increase in translation processes (Laplante and Sabatini, 2012). Because such an increase in translation may provide a partial explanation for the increased p75NTR protein expression observed only in the sensitive phenotype, we measured mTOR phosphorylation and found significant increases in phosphorylated mTOR abundance [F(2, 13) = 4.22, P < .05] in SS rats compared with controls (Figure 4f). We also measured the abundance of total histone 3 acetylation (H3ac) in the dorsal striatum and found significant increases in H3ac abundance [F(2, 16) = 4.67, P < .05] in SS rats compared with SR and saline controls (Figure 4g). Phosphorylated MeCP2 protein levels were also increased in SS rats compared with SR and controls (Figure 4h).
Discussion
Recent work in our laboratory has indicated that, even in rats that had escalated their intake of METH during SA procedures, footshock punishment can lead to the segregation of distinct subgroups of rats that either continue to take the drug compulsively or suppress their responding for the drug (Cadet et al., 2017; Krasnova et al., 2017; Torres et al., 2017). Here, we report that SS rats showed increased activation of multiple proteins involved with the NGF-TrkA/p75NTR/MAPK signaling cascade in the dorsal striatum after 30 days of withdrawal from METH SA and punishment (Figure 5). SS rats also showed increases in the expression of neuropeptide and neurotrophic genes compared with SR rats. Specifically, neuropeptide-related genes that were increased in SS rats included Crhr, Crhbp, and Ucn2, whereas neurotrophic-associated genes that showed upregulation included Bdnf and Gfra2. These observations are interesting given that UCN and its receptors serve to integrate physiological stress responses that might have served as triggers for the appearance of the SS phenotype in reaction to negative stimuli.
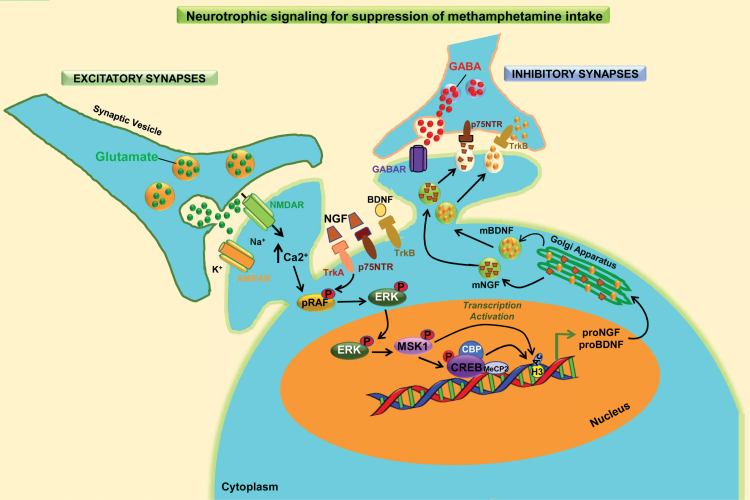
Figure 5.
Preferential activation of the NGF signaling pathway in shock-sensitive methamphetamine (METH) self-administering (SA) rats. The figure indicates that METH SA is accompanied by increased signaling in excitatory synapses in all rats. Yet contingent footshocks led to the identification of 2 METH SA phenotypes: rats that continued to press an active lever to receive METH (shock-resistant, SR) and those that reduced their lever pressing, thereby reducing their METH intake (shock-sensitive, SS). Both sets of rats showed increased BDNF, whereas rats that reduce their lever pressing exhibited increased mature nerve growth factor (NGF) levels and greater abundance of phopshorylated TrkA, a receptor for NGF. Importantly, the levels of P75NTR, another NGF receptor, were also increased only in the genetically prone shock-sensitive rats. In addition, several phospho-proteins that participate in the NGF/RAF/ERK/MSK cascade were activated only in the shock-sensitive rats. Moreover, the shock-sensitive rats showed increased CREB phosphorylation in the dorsal striatum. Phosphorylated CREB is known to recruit the histone acetyl-transferase, CREB binding protein (CBP), an event that leads to increased H3 histone acetylation, as observed in the shock-sensitive rats. These series of molecular events could have led to increased GABAergic tone in the dorsal striatum and subsequent suppression of METH SA in the shock-sensitive animals. Therefore, the development of molecules that can specifically activate this striatal NGF-mediated phosphorylation cascade may be of therapeutic benefit to METH-addicted individuals.
Our behavioral studies are in line with the reports of other authors who have developed SA methods to identify rats with persistent drug-seeking behaviors despite adverse consequences (Deroche-Gamonet et al., 2004; Pelloux et al., 2007; Chen et al., 2013) or despite environmental signals of potential adversity (Vanderschuren and Everitt, 2004) to drug taking. Our studies have used this approach to identify distinct molecular profiles between persistent drug-seeking rats and those that reduce their METH intake. For example, we have previously demonstrated that SS rats are characterized by differential changes in DNA hydroxymethylation in the nucleus accumbens (Cadet et al., 2017). Within this same region, we also demonstrated that SS rats show increased mRNA levels of various histone deacetylases that participate in gene regulation (Cadet et al., 2016a). In a subsequent report, we also documented that SR rats exhibited increases in nucleus accumbens proenkephalin and prodynorphin mRNA levels (Cadet et al., 2016b). Herein, we expand on our previous work by demonstrating that, in the dorsal striatum, neurotrophins and activation of their downstream signaling cascade distinguishes SS from SR phenotypes following prolonged abstinence from METH intake.
Neurotrophins play important roles in regulating diverse neuronal processes including synaptic plasticity, cell survival, differentiation, and neuronal growth (Alder et al., 2003; Bekinschtein et al., 2008). Our observations of increased mature BDNF protein levels in SR and SS rats are consistent with previous reports showing increased BDNF expression in rats that self-administered METH chronically (Krasnova et al., 2013; Li et al., 2015). These observations suggest that increased BDNF expression may be a consequence of long-term METH exposure but do not necessarily reflect molecular adaptations that would help to distinguish compulsive from controlled drug-taking behaviors. This conclusion is supported by our findings that there were no differential changes in TrkB phosphorylation between the two phenotypes.
Because BDNF and TrkB protein levels did not account for neuronal differences between SS and SR rats, the possibility arose that NGF and its receptor, TrkA, might play a more prominent role in discriminating the 2 phenotypes. Indeed, we observed increases in NGF protein levels accompanied by selective increases in phosphorylated TrkA and p75NTR levels in the SS phenotype compared with the SR rats. Although NGF was originally described as a neurotrophic factor required for cell proliferation (Cohen et al., 1954), it is now clear that NGF can also participate in a number of neurobiological events (Conner et al., 2009; Manni et al., 2013). For example, within the striatum NGF is produced by GABAergic interneurons (Bizon et al., 1999) and has significant protective and plastic effects on striatal cholinergic neurons under normal and pathological conditions (Fischer et al., 1998; Gratacos et al., 2001). Additionally, NGF can cause hypertrophy of striatal cholinergic neurons, increased levels of choline acetyltransferase mRNA, and reduced spontaneous neuronal activity (Forander et al., 1996). Moreover, the biological effects of NGF occur through its binding to TrkA and p75NTR, with distinct functional and structural interactions between the 2 receptors (Matusica et al., 2013; Bucci et al., 2014; Covaceuszach et al., 2015). For instance, p75NTR can co-precipitate along with TrkA (Bibel et al., 1999), with TrkA affinity for NGF increasing in the presence of p75NTR (Esposito et al., 2001). p75NTR can also prolong cell-surface TrkA-dependent signaling (Makkerh et al., 2005), thereby enhancing TrkA signaling capacity (Verdi et al., 1994; Epa et al., 2004). Interestingly, knockdown of p75NTR expression significantly reduced excessive alcohol intake in rats (Darcq et al., 2016), whereas our own findings suggest that increased p75NTR might be involved in suppressing METH-seeking behavior, since the SS rats also show less cue-induced drug seeking (Torres et al., 2017). This statement hints to the possibility that SS rats might have a better ability to learn and establish memory for adverse events through the activation of the NGF signaling cascade. In support of this hypothesis, a previous report suggested that enhancement of contextual fear memory in rodents is mediated by neurotrophic-dependent activation of the Erk1/2(MAPK) pathway (Revest et al., 2014). Similarly, increased neurotrophic factors in the dorsal striatum appear to be associated with enhanced performance on the lever-press escape/avoidance paradigm in rats (Albeck et al., 2005).
Given that stimulation of TrkA by NGF induces auto-phosphorylation of this receptor and promotes ERK1/2 activation (Diolaiti et al., 2007), we thought it likely that NGF/TrkA interactions might differentially activate downstream signaling between the 2 phenotypes. This supposition was confirmed by our findings that only striatal tissues from the SS rats showed increased phosphorylation of proteins associated with the MAPK/MEK/ERK intracellular cascade. NGF-TrkA interactions are known to active multiple intracellular signaling pathways including the Ras/MAPK, PI3K, and PLC γ cascades (Marlin and Li, 2015). However, the Ras/MAPK cascade has received much attention due to the prominent role it plays in neuronal plasticity (Bruel-Jungerman et al., 2007; Cargnello and Roux, 2011; Correa et al., 2012), memory formation (Adams and Sweatt, 2002), and transcriptional responses (Davis et al., 2000). In neuronal cells, the binding of mature NGF onto TrkA activates the adaptor GRB2-SOS protein complex, which increases the rate of GDP-GTP exchange on Ras, leading to Ras activation (Bucci et al., 2014; Marlin and Li, 2015). Activation of Ras induces a sequential activation of Raf, a MAPK kinase kinase. Activation of Raf subtypes induces phosphorylation of MEK that then promotes the phosphorylation of ERK1/2 (Plotnikov et al., 2011; Ciccarelli and Giustetto, 2014). Translocation of pERK1/2 into the cell nucleus results in the phosphorylation of MSK1 (Plotnikov et al., 2011). Phosphorylated-MSK1 directly causes the phosphorylation of CREB (Arthur and Cohen, 2000) that binds onto cAMP response elements. Phosphorylated-CREB then recruits histone acetyltransferases that promote histone acetylation and increase accessibility of the genome for molecular machinery to initiate transcription (Nestler, 2012). This discussion is compatible with our observations of increased phosphorylation of Raf, MEK, ERK, and MSK1 with subsequent increases in CREB phosphorylation in SS rats. In addition, our finding of increased H3ac in SS rats agrees with the above discussion and postulates a selective activation of long-lasting plasticity events that enhance memory formation in this group of animals. The finding that phospho-mTOR is increased in SS, but not SR, rats is also of interest, since NGF is known to activate this protein (Zhang and Ma, 2014; Wang et al., 2017) that can regulate translation rates, metabolic processes, and synaptic plasticity in neurons (Cho, 2011). Furthermore, the observations that pMeCP2 levels were selectively increased in SS rats support our notion of enhanced learning in this group of rats, since mice deficient in pMeCP2 show memory deficits (Moretti et al., 2006). Although MeCP2 is known as gene silencer, recent evidence suggests it also binds to methylated non-CG sites (Chen et al., 2015) and can function as a transcriptional activator by association with pCREB (Chahrour et al., 2008). However, it is important to note a recent study that reported decreased METH SA after virally mediated knockdown of MeCP2 in the nucleus accumbens (Lewis et al., 2016). These findings implicate potentially distinct roles of this protein in various brain regions following METH SA. Taken together, our findings and those of others suggest that increased neurotrophic factors and activation of the Ras/Raf/MEK/ERK pathway may serve to prevent the development of plastic changes that promote compulsive METH intake.
In conclusion, we found that rats that reduce their METH intake in the presence of punishment show significant increases in striatal NGF levels, TrkA phosphorylation, p75NTR expression, and phosphorylation of the Ras/Raf/MEK/ERK signaling cascade. These molecular adaptations implicate neurotrophin-mediated signaling pathways during late stages of withdrawal from METH SA in a brain region associated with habitual and compulsive behaviors. Thus, our punishment-based model highlights the possibility of identifying specific molecular alterations that may help to distinguish rats with distinct profiles of drug intake after periods of drug withdrawal when a propensity to relapse might be pronounced. Therefore, the use of such models may be quite useful in the development of therapeutic modalities against drug addiction.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
This research was supported by funds of the Intramural Research Program of the DHHS/NIH/NIDA.
Statement of Interest
None.
References
- Adams JP, Sweatt JD(2002)Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42:135–163. [DOI] [PubMed] [Google Scholar]
- Albeck DS, Beck KD, Kung LH, Sano K, Brennan FX(2005)Leverpress escape/avoidance training increases neurotrophin levels in rat brain. Integr Physiol Behav Sci 40:28–34. [DOI] [PubMed] [Google Scholar]
- Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB(2003)Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci 23:10800–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JS, Cohen P(2000)MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett 482:44–48. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH(2008)BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA 105:2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ(2013)Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol 23:564–572. [DOI] [PubMed] [Google Scholar]
- Bibel M, Hoppe E, Barde YA(1999)Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J 18:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Lauterborn JC, Gall CM(1999)Subpopulations of striatal interneurons can be distinguished on the basis of neurotrophic factor expression. J Comp Neurol 408:283–298. [PubMed] [Google Scholar]
- Brecht ML, Herbeck D(2014)Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend 139:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Laroche S (. 2007) Brain plasticity mechanisms and memory: a party of four. Neuroscientist 13:492–505. [DOI] [PubMed] [Google Scholar]
- Bucci C, Alifano P, Cogli L(2014)The role of rab proteins in neuronal cells and in the trafficking of neurotrophin receptors. Membranes (Basel) 4:642–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN(2009)Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol 88:101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Jayanthi S, Krasnova IN(2015)Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat. Mol Neurobiol 51:696–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Ladenheim B, Krasnova IN, Jayanthi S (2016a) Differential Expression of mRNAs coding for histone deacetylases (HDACs) in the nucleus accumbens of compulsive methamphetamine takers and abstinent rat. J Drug Alc Res 5doi: 10.4303/jdar/235998. [Google Scholar]
- Cadet JL, Krasnova IN, Walther D, Brannock C, Ladenheim B, McCoy MT, Collector D, Torres OV, Terry N, Jayanthi S (2016b) Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats. Sci Rep 6:37002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Krasnova IN, Jayanthi S, Ladenheim B, McCoy MT, Walther D, Godino A, Pirooznia M, Lee RS(2017)Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol Psychiatry 22:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, Shaham Y(2017)Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci 37:1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP(2011)Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY(2008)MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320:1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS(2006)Neurotrophin signalling in health and disease. Clin Sci (Lond) 110:167–173. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A(2013)Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496:359–362. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen K, Lavery LA, Baker SA, Shaw CA, Li W, Zoghbi HY(2015)MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc Natl Acad Sci USA 112:5509–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH.(2011)Frontier of epilepsy research - mTOR signaling pathway. Exp Mol Med 43:231–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli A, Giustetto M(2014)Role of ERK signaling in activity-dependent modifications of histone proteins. Neuropharmacology 80:34–44. [DOI] [PubMed] [Google Scholar]
- Cohen S, Levi-Montalcini R, Hamburger V(1954)A nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proc Natl Acad Sci USA 40:1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Franks KM, Titterness AK, Russell K, Merrill DA, Christie BR, Sejnowski TJ, Tuszynski MH(2009)NGF is essential for hippocampal plasticity and learning. J Neurosci 29:10883–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SA, Hunter CJ, Palygin O, Wauters SC, Martin KJ, McKenzie C, McKelvey K, Morris RG, Pankratov Y, Arthur JS, Frenguelli BG(2012)MSK1 regulates homeostatic and experience-dependent synaptic plasticity. J Neurosci 32:13039–13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaceuszach S, Konarev PV, Cassetta A, Paoletti F, Svergun DI, Lamba D, Cattaneo A(2015)The conundrum of the high-affinity NGF binding site formation unveiled?Biophys J 108:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcy C, Luevano JE, Miranda-Arango M, Pipkin JA, Jackson JA, Castaneda E, Gosselink KL, O’Dell LE(2016)Extended access to methamphetamine self-administration up-regulates dopamine transporter levels 72 hours after withdrawal in rats. Behav Brain Res 296:125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Morisot N, Phamluong K, Warnault V, Jeanblanc J, Longo FM, Massa SM, Ron D(2016)The neurotrophic factor receptor p75 in the rat dorsolateral striatum drives excessive alcohol drinking. J Neurosci 36:10116–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S(2000)The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci 20:4563–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV(2004)Evidence for addiction-like behavior in the rat. Science 305:1014–1017. [DOI] [PubMed] [Google Scholar]
- Diolaiti D, Bernardoni R, Trazzi S, Papa A, Porro A Bono F, Herbert JM, Perini G, Della Valle G(2007)Functional cooperation between TrkA and p75(NTR) accelerates neuronal differentiation by increased transcription of GAP-43 and p21(CIP/WAF) genes via ERK1/2 and AP-1 activities. Exp Cell Res 313:2980–2992. [DOI] [PubMed] [Google Scholar]
- Epa WR, Markovska K, Barrett GL(2004)The p75 neurotrophin receptor enhances TrkA signalling by binding to Shc and augmenting its phosphorylation. J Neurochem 89:344–353. [DOI] [PubMed] [Google Scholar]
- Esposito D, Patel P Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL(2001)The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem 276:32687–32695. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW(2013)From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37:1946–1954. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW(2016)Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol 67:23–50. [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL(2010)Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev 35:248–275. [DOI] [PubMed] [Google Scholar]
- Fischer HP, Marksteiner J, Ransmayr G, Saria A, Humpel C(1998)NGF but not GDNF or neurturin enhance acetylcholine tissue levels in striatal organotypic brain slices. Int J Dev Neurosci 16:391–401. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME(2008)Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31:563–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forander P, Soderstrom S, Humpel C, Stromberg I(1996)Chronic infusion of nerve growth factor into rat striatum increases cholinergic markers and inhibits striatal neuronal discharge rate. Eur J Neurosci 8:1822–1832. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Ball TM, Wittmann M, Tapert SF, Paulus MP(2015)Individualized relapse prediction: personality measures and striatal and insular activity during reward-processing robustly predict relapse. Drug Alcohol Depend 152:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratacos E, Perez-Navarro E, Tolosa E, Arenas E, Alberch J(2001)Neuroprotection of striatal neurons against kainate excitotoxicity by neurotrophins and GDNF family members. J Neurochem 78:1287–1296. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Beauvais G, Ladenheim B, Gilmore K, Wood W 3rd, Becker K, Cadet JL(2009)Methamphetamine induces dopamine D1 receptor-dependent endoplasmic reticulum stress-related molecular events in the rat striatum. PLoS One 4:e6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE(2007)Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci 25:847–853. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND(2010)Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL(2010)Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PloS one 5:e8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E, Kobeissy FH, Gold MS, Becker KG, Goldberg SR, Cadet JL(2013)CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis 58:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, Shaham Y, Cadet JL(2014)Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology 39:2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Cadet JL(2016)Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways. Psychopharmacology (Berl) 233:1945–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Gerra MC, Walther D, Jayanthi S, Ladenheim B, McCoy MT, Cadet JL(2017)Compulsive methamphetamine taking in the presence of punishment is associated with increased oxytocin expression in the nucleus accumbens of rats. Scientific Reports 7:8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM(2012)mTOR signaling in growth control and disease. Cell 149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Bastle RM, Manning TB, Himes SM, Fennig P, Conrad PR, Colwell J, Pagni BA, Hess LA, Matekel CG, Newbern JM, Olive MF(2016)Interactions between early life stress, nucleus accumbens mecp2 expression, and methamphetamine self-administration in male rats. Neuropsychopharmacology 41:2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ, Shaham Y(2015)Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci 35:8232–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkerh JP, Ceni C, Auld DS, Vaillancourt F, Dorval G, Barker PA(2005)p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep 6:936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni L, Rocco ML, Bianchi P, Soligo M, Guaragna M, Barbaro SP, Aloe L(2013)Nerve growth factor: basic studies and possible therapeutic applications. Growth Factors 31:115–122. [DOI] [PubMed] [Google Scholar]
- Marlin MC, Li G(2015)Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol 314:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusica D, Skeldal S, Sykes AM, Palstra N, Sharma A, Coulson EJ(2013)An intracellular domain fragment of the p75 neurotrophin receptor (p75(NTR)) enhances tropomyosin receptor kinase A (TrkA) receptor function. J Biol Chem 288:11144–11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY(2006)Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci 26:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ.(2012)Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci 10:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE(2012)Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci 35:261–270. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A(2007)Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 194:127–137. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Zehorai E, Procaccia S, Seger R(2011)The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta 1813:1619–1633. [DOI] [PubMed] [Google Scholar]
- Reichardt LF.(2006)Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361:1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, Le Roux A, Roullot-Lacarriere V, Kaouane N, Vallee M, Kasanetz F, Rouge-Pont F, Tronche F, Desmedt A, Piazza PV(2014)BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol Psychiatry 19:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak DE.(2013)Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am 36:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T(2006)The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I(2007)Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275–297. [DOI] [PubMed] [Google Scholar]
- Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, Cadet JL(2017)Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav Brain Res 326:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ(2004)Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305:1017–1019. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Birren SJ, Ibanez CF, Persson H, Kaplan DR, Benedetti M, Chao MV, Anderson DJ(1994)p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron 12:733–745. [DOI] [PubMed] [Google Scholar]
- Wang ZG, Li H, Huang Y, Li R, Wang XF, Yu LX, Guang XQ, Li L, Zhang HY, Zhao YZ, Zhang C, Li XK, Wu RZ, Chu MP, Xiao J(2017)Nerve growth factor-induced Akt/mTOR activation protects the ischemic heart via restoring autophagic flux and attenuating ubiquitinated protein accumulation. Oncotarget 8:5400–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma WY(2014)Nerve growth factor regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes via PI3K/mTOR pathway. Genet Mol Res 13:9324–9335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.