Abstract
Background
Diagnostic biomarkers of major depressive disorder, bipolar disorder, and schizophrenia are urgently needed, because none are currently available.
Methods
We performed a comprehensive metabolome analysis of plasma samples from drug-free patients with major depressive disorder (n=9), bipolar disorder (n=6), schizophrenia (n=17), and matched healthy controls (n=19) (cohort 1) using liquid chromatography time-of-flight mass spectrometry. A significant effect of diagnosis was found for 2 metabolites: nervonic acid and cortisone, with nervonic acid being the most significantly altered. The reproducibility of the results and effects of psychotropic medication on nervonic acid were verified in cohort 2, an independent sample set of medicated patients [major depressive disorder (n=45), bipolar disorder (n=71), schizophrenia (n=115)], and controls (n=90) using gas chromatography time-of-flight mass spectrometry.
Results
The increased levels of nervonic acid in patients with major depressive disorder compared with controls and patients with bipolar disorder in cohort 1 were replicated in the independent sample set (cohort 2). In cohort 2, plasma nervonic acid levels were also increased in the patients with major depressive disorder compared with the patients with schizophrenia. In cohort 2, nervonic acid levels were increased in the depressive state in patients with major depressive disorder compared with the levels in the remission state in patients with major depressive disorder and the depressive state in patients with bipolar disorder.
Conclusion
These results suggested that plasma nervonic acid is a good candidate biomarker for the depressive state of major depressive disorder.
Keywords: biomarker, bipolar disorder, major depressive disorder, metabolomics, nervonic acid
Significance Statement
Plasma nervonic acid levels were increased in patients with major depressive disorder (MDD) compared with those in patients with bipolar disorder and healthy controls. Nervonic acid levels were higher in the depressive state in the patients with MDD compared with the remission state in the patients with MDD and the depressive state of patients with bipolar disorder, suggesting state-dependent alterations. Therefore, plasma nervonic acid is a good candidate diagnostic biomarker for MDD.
Introduction
Although a proper diagnosis and medical treatment are important for improving the prognosis of patients with mental illness (Post et al., 2010; Gaebel and Zielasek, 2015), diagnoses are difficult to make, especially when major depressive disorder (MDD) needs to be distinguished from the depressive state of bipolar disorder (BD). In addition, patients with schizophrenia (SZ) can also show depressive symptoms. Because treatment approaches differ among patients with MDD, BD, and SZ, diagnostic biomarkers that distinguish MDD, BD, and SZ are required to avoid misdiagnosis (Altamura et al., 2015) and obtain a better prognosis (Drancourt et al., 2013). Although candidate biomarkers have been studied in various ways (Singh and Rose, 2009), the results of previous studies lack reproducibility, sensitivity, and selectivity (Kunugi et al., 2015; Buoli et al., 2016).
The plasma levels of hydrophobic molecules, such as steroids and unsaturated fatty acids, have been studied as candidate biomarkers of MDD (Halbreich et al., 1985; Lin et al., 2010), BD (Chiu et al., 2003; Marx et al., 2006; Sublette et al., 2007), and/or SZ (Peet et al., 2004; Reddy et al., 2004; Freeman et al., 2006; Marx et al., 2006; Amminger et al., 2015). However, these candidate molecules were examined in patients taking medication, and the potential effects of medication cannot therefore be excluded.
In the present study, we performed a comprehensive metabolome analysis using liquid chromatography time-of-flight mass spectrometry (LC-TOFMS) to investigate the use of nonpolar or medium-polar metabolites as biomarkers in plasma samples of drug-free patients with MDD, BD, and SZ. The reproducibility of the results and the effects of psychotropic medication on nervonic acid, which was the most significantly altered metabolite, were verified in an independent sample set of mostly medicated patients. Here, we report that the levels of nervonic acid were increased in patients with MDD in 2 independent sample sets.
Materials and Methods
Participants
Cohort 1 of the samples used for LC-TOFMS [MDD (n=9), BD (n=6), SZ (n=17)] and matched healthy controls (n=19) was derived from the National Center of Neurology and Psychiatry (NCNP) Biobank (project no. NCNPBB-0002). The patients in cohort 1 had not taken any antipsychotic or antidepressant medications for at least 2 weeks. To measure the absolute concentrations of nervonic acid in cohort 1, gas chromatography TOFMS (GC-TOFMS) was performed, but only 30 samples [MDD (n=6), BD (n=4), SZ (n=13)] and controls (n=7) of the 51 samples of cohort 1 from the NCNP Biobank were available. A second cohort of samples [MDD (n=45), BD (n=71), SZ (n=115)] and controls (n=90) was obtained from the NCNP Biobank (project no. NCNPBB-0002-1). Cohort 2 also contained samples from Osaka City University Hospital and Hannan Hospital, where controls were recruited from among the healthy spouses of the patients. The control samples in the NCNP Biobank were collected by advertising in local free magazines and on websites.
The sex ratios and ages did not significantly differ among the groups in cohort 1 (Table 1). Among the groups in cohort 2, the sex ratios did not significantly differ, while age did (Table 2). The age of participants in the MDD and control groups was higher than that of SZ (P=.000046 and .000004, respectively). ANCOVA was used for the analysis of age.
Table 1.
Demographic and Clinical Data for the Participants in Cohort 1
| SZ | MDD | BD | Control | Statistics | |
|---|---|---|---|---|---|
| Number (male/female) | 17 (8/9) | 9 (3/6) | 6 (1/5) | 19 (10/9) | P=.41 a |
| Age, years (mean±SD) | 33.6±15.7 | 39.1±10.2 | 41.8±13.3 | 36.1±12.9 | P=.57 b |
| Duration of illness, years (mean±SD) c | 9.1±13.7 | 4.8±2.9 | 7.8±6.3 | N.A. | P =.81 b |
| HAMD-21, score (mean±SD) | N.A. | 11.2±8.5 | 14.4±5.9 | N.A. | N.A |
| PANSS positive, score (mean±SD) d | 21.4±5.4 | N.A. | N.A. | N.A. | N.A. |
| PANSS negative, score (mean±SD) d | 22.4±6.4 | N.A. | N.A. | N.A. | N.A. |
| PANSS general pathology, score (mean±SD) d | 47.3±11.4 | N.A. | N.A. | N.A. | N.A. |
| YMRS, score (mean±SD) | N.A. | N.A. | 6.3±4.0 | N.A. | N.A. |
Abbreviations: BD, bipolar disorder; HAMD-21, 21-item Hamilton Depression Rating Scale; MDD, major depressive disorder; N.A., not applicable; PANSS, Positive and Negative Symptom Scale; SZ, schizophrenia; YMRS, Young Mania Rating Scale.
aChi-squared test.
bANOVA.
cMissing data for 5 individuals in the MDD patient group and 1 individual in the BD patient group.
dMissing data for 4 individuals in the SZ patient group.
Table 2.
Demographic and Clinical Data for the Participants in Cohort 2
| SZ | MDD | BD | Control | Statistics | |
|---|---|---|---|---|---|
| Number (male/female) | 115 (59/56) | 45 (19/26) | 71 (37/34) | 90 (46/54) | P=.72 a |
| Age, years (mean±SD) | 39.0±13.6 | 50.4±16.0 | 45.2±13.3 | 49.2±15.0 | P=.000046 b |
| Number of drug-free patients (male/female) | 7 (5/2) | 4 (3/1) | 0 | N.A. | N.A. |
| Number of patients with depressed state | N.A. | 34 | 25 | N.A. | N.A. |
| Number of patients with remitted state | N.A. | 11 | 34 | N.A. | N.A. |
| Number of patients with manic state | N.A. | N.A. | 12 | N.A. | N.A. |
| HAMD-21, score (mean ±SD) | N.A. | 17.0±8.6 | 9.2±8.1 | N.A. | N.A |
| PANSS positive, score (mean ±SD) | 17.7±5.6 | N.A. | N.A. | N.A. | N.A. |
| PANSS negative, score (mean±SD) | 18.7±6.2 | N.A. | N.A. | N.A. | N.A. |
| PANSS general pathology, score (mean ±SD) | 36.0±8.7 | N.A. | N.A. | N.A. | N.A. |
| YMRS, score (mean ±SD) | N.A. | N.A. | 5.7±7.4 | N.A. | N.A. |
Abbreviations: BD, bipolar disorder; HAMD-21, 21-item Hamilton Depression Rating Scale; MDD, major depressive disorder; N.A., not applicable; PANSS, Positive and Negative Symptom Scale; SZ, schizophrenia; YMRS, Young Mania Rating Scale.
aChi-squared test.
bANOVA.
All participants provided written informed consent after the entire study was explained to them. This study was conducted in accordance with the Declaration of Helsinki and approved by the following: the Ethics Committee of NCNP, the Ethics Committee of Hannan Hospital, the Research Ethics Committee of Osaka City University, and Wako First Research Ethics Committee of RIKEN.
Diagnosis and Assessment of Symptoms
Trained psychiatrists or psychologists conducted structured interviews of the participants using a Japanese version of the Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998), and the results were used to make the diagnoses according to the criteria of the DSM-IV (American Psychiatric Association, 1994). In cohort 2, we divided the patients with MDD into remitted and depressive state based on M.I.N.I. We also divided the patients with BD into manic, remitted, and depressive states based on M.I.N.I. Patients with any comorbid axis I disorders, histories of central nervous system diseases, substance abuse/dependence, or severe head trauma were excluded. Depressive symptoms were assessed using the 21-item Hamilton Depression Rating Scale (HAMD-21) (Hamilton, 1960). The Positive and Negative Syndrome Scale (PANSS) (Kay and Fiszbein, 1987) was used to measure the symptom severity of the patients with SZ. The Young Mania Rating Scale (YMRS) (Young et al., 1978) was utilized to measure the severity of the manic episodes in patients with BD.
Plasma Sampling
Blood samples were collected between 9:00 am and 4:00 pm with or without overnight fasting. Information on meals was not available and not considered in the statistical analyses. Blood samples were drawn from a peripheral vein, collected in ethylenediaminetetraacetic acid-2Na-containing vacuum blood collection tubes (VENOJECT II, Terumo Corporation), and immediately placed on ice. Within 30 minutes of the blood collection, plasma samples were isolated via centrifugation at 2500×g at 4°C for 10 minutes and stored at –80°C until use.
LC-TOFMS Analysis
Plasma samples (500 µL) were added to 1200 µL of 1% formic acid/acetonitrile containing an internal standard solution (Solution ID: H3304-1002, Human Metabolome Technologies, Inc.) at 0°C. The solution was thoroughly mixed and centrifuged at 2300×g and 4°C for 5 minutes. The supernatant was filtered through Hybrid SPE-Phospholipid (product no. 55261-U; Sigma-Aldrich Corporation). The filtrate was desiccated and then dissolved with 80 µL of isopropanol and Milli-Q (1:1, v/v) for the LC-TOFMS analysis. The detection limit was determined at a signal-to-noise ratio>3. The candidate peaks were assigned a specific metabolite identity based on their m/z (±10 ppm) and retention time (±0.5 minutes) determined by TOFMS. The absolute concentrations were not determined, and the relative peak areas were used in the comparisons. The metabolome measurements were conducted at Human Metabolome Technologies, Inc.
GC-TOFMS Analysis
We measured the levels of nervonic acid using GC-TOFMS according to a protocol described in a previous report (Quehenberger et al., 2011) with slight modifications. Oleic acid d9 (2.5 ng) (Avanti Polar Lipids, Inc) was added to each plasma sample (100 µL) as an internal standard. The plasma sample was added to a mixture of 400 µL methanol, 25 µL 1N HCl, and 1000 µL isooctane. The solution was thoroughly mixed and centrifuged at 800×g for 2 minutes at room temperature. Next, 920 µL of supernatant (upper organic phase) was dispensed in a conical glass insert for autosampler vials, dried in vacuo, and added to a mixture of 40 µL of pyridine, 40 µL of N-(tert-butyldimethylsilyl)-N-methyl trifluoroacetamide, and 30 µL of acetonitrile to obtain tert-butyldimethylsilyl (TBDMS) esters from the free fatty acids. The sample solutions were placed in sealed autosampler vials, vortexed, and maintained at 60°C for at least 60 minutes before the analysis. Absolute concentrations were quantified using a series of standards of known concentrations of nervonic acid (Sigma-Aldrich, Japan K.K.) mixed with the internal standard. All other regents were obtained from Wako Pure Chemical Industries, Ltd.
We used a JMS-T100GCV time-of-flight mass spectrometer (JEOL Ltd) equipped with a 7890A GC (Agilent Technologies, Inc.) and 7693 autosampler (Agilent Technologies, Inc.). Each TBDMS-derivatized sample solution was injected (2 µL) in the splitless injection mode up to a temperature of 320°C. We used a Zebron ZB-1 MS column (30 m×0.32 mm id; film thickness 0.25µm, Phenomenex, Inc.). The carrier gas was helium, and it was applied at a constant flow-rate of 1.5 mL/min. The GC oven temperature was maintained at 120°C for 4 minutes, ramped up linearly from 120°C to 360°C at 30°C/min, and then maintained at 360°C for 0.5 minutes. The outlet of the column was directly connected to a 70-eV electron ionization source of the mass spectrometer, with a resolving power >6000 (typical full width at half-maximum mass resolution, 9000). The typical mass accuracies were <5 ppm after the application of single-point internal mass drift compensation using the column bleed peak (m/z, 281.0517). Peak areas of high-resolution accurate-mass extracted ion chromatograms for the characteristic fragment ions at [M - 57]+ from TBDMS-derivatized analyte and internal standards were used for quantitation. Examples of chromatograms of nervonic acid (m/z, 423.3658±0.005; retention time, 11.50 minutes) and oleic acid d9 (m/z, 348.32842±0.005; retention time, 10.00 minutes) are shown in supplementary Figure 1.
Statistical Analysis
The data are presented as mean±SD. The means were compared using Welch t test, 1-way ANOVA followed by posthoc Tukey’s test, or Kruskal-Wallis test. Any missing values for the relative area were replaced with machine epsilon (≃0). In the full Institute of Electrical and Electronics Engineers system, the spacing was 2×10- 52. ANCOVA that controlled for age and sex was used to evaluate the effects of age and sex on the nervonic acid levels. Categorical variables were compared using chi-squared test. The threshold for statistical significance was set at P<.05 for all analyses, and multiple comparisons were corrected for using the false discovery rate. Statistical power and effect size were calculated using G-Power version 3.1 (Faul et al., 2007). The statistical analyses were conducted using R version 3.2.5 (https://www. r-project.org/) and IBM Statistical Package for Social Sciences Statistics 23, Japanese version (IBM Japan).
Results
Metabolome Analysis in Cohort 1
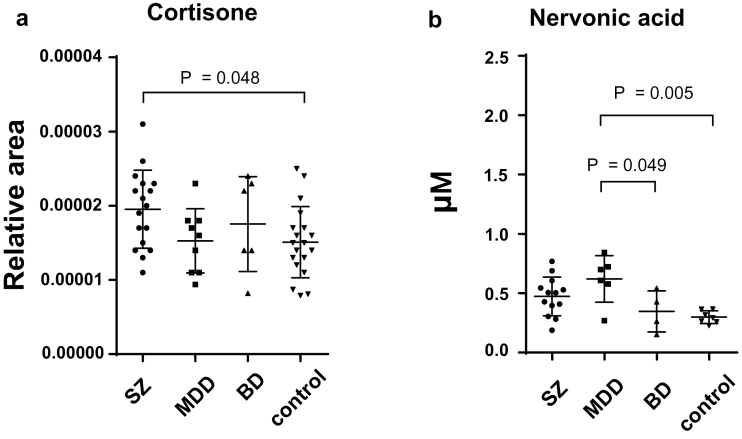
The LC-TOFMS metabolome analysis detected 126 candidate peaks in the cohort 1 plasma samples (supplementary Table 1). We used an ANOVA or Kruskal-Wallis test to identify the peaks that significantly differed among the 4 sample groups (MDD, BD, SZ, and controls). A nominally significant effect of diagnosis on the relative peak area was found for 2 peaks, which were assigned to cortisone (F=2.851, P=.047, f=0.42) and nervonic acid (χ2=13.41, P=.0038). A nominally significant increase was found in cortisone, a precursor of cortisol, in patients with SZ compared with controls (P=.048) (Figure 1a). None of these differences were statistically significant after correcting for multiple comparisons. For nervonic acid, a Kruskal-Wallis test showed nominally significant effect of diagnosis. However, over one-half of the nervonic acid measures were below the detection limit of LC-TOFMS. To determine the groups that showed significantly different levels of nervonic acid, we reanalyzed the samples for nervonic acid using GC-TOFMS, which is more sensitive than LC-TOFMS, to obtain more precise data. We measured the absolute concentrations of plasma nervonic acid in the 30 available samples [MDD (n=6), BD (n=4), SZ (n=13)] and controls (n=7) of the 51 subjects of cohort 1. We were not able to measure the nervonic acid in the remaining 21 subjects, because sufficient sample volumes were not available. We detected plasma nervonic acid in all 30 samples. The inter-assay CV for nervonic acid (n=12) was 9.2%. Patients with MDD had significantly increased levels of nervonic acid (0.62±0.20 µM) compared with controls (0.29±0.053 µM, P=.005) and patients with BD (0.35±0.17 µM, P=.049) (Figure 1b). To evaluate the effects of potential confounding factors on the plasma levels of nervonic acid, we applied an ANCOVA with a dependent variable of the metabolite levels, an independent variable of diagnosis, and the covariates of age and sex. The effects of diagnosis on the nervonic acid levels remained significant (P=.009). The nervonic acid levels were not significantly correlated with age (P=.082) or sex (P=.42).
Figure 1.
Plasma level of metabolites with significant differences in the cohort 1 samples. The bars indicate the mean of each group. The error bars represent the SDs. Comparison of the plasma levels of metabolites among the patients with schizophrenia (SZ), major depressive disorder (MDD), and bipolar disorder (BD), and healthy controls (1-way ANOVA with posthoc Tukey’s test). (a) Cortisone was measured using liquid chromatography time-of-flight mass spectrometry (LC-TOFMS) in 51 samples. The Y-axis shows the relative concentrations. (b) The levels of nervonic acid were measured using gas chromatography time-of-flight mass spectrometry (GC-TOFMS) in the 30 available samples of cohort 1. The y-axis shows the absolute concentrations. The data on the relative concentrations of nervonic acid are listed in supplementary Table 1.
Increased Levels of Nervonic Acid Level in Patients with MDD
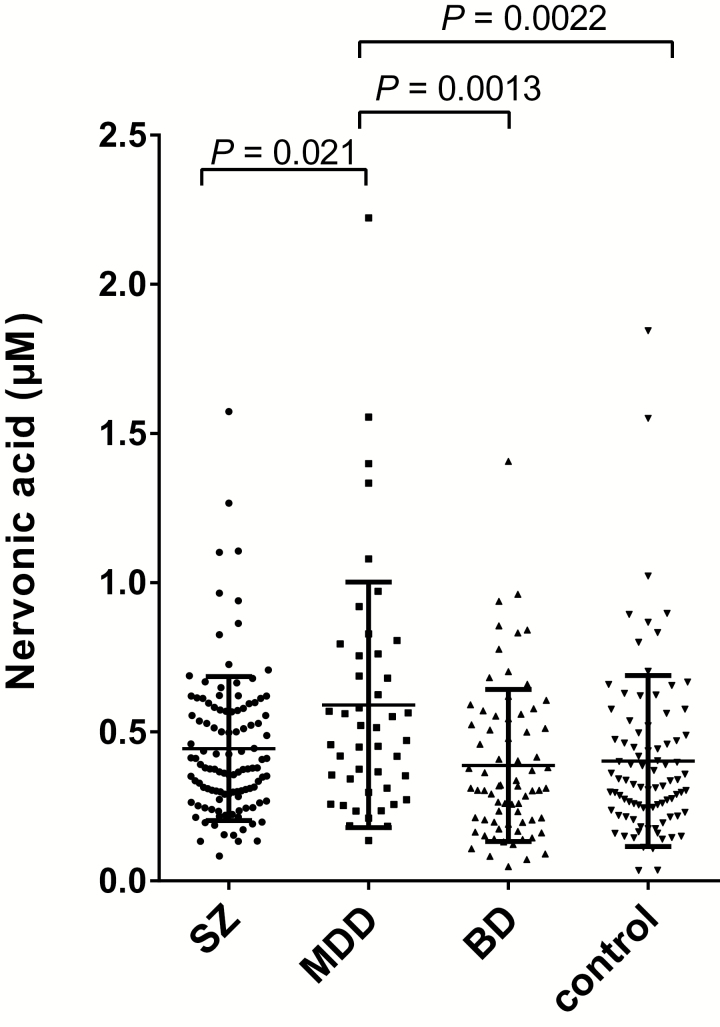
We focused on nervonic acid, because it was the most significantly altered metabolite (P=.005, f=0.78). We confirmed the reproducibility of the results in the medicated condition and the effects of psychotropic medication on nervonic acid by measuring the levels of nervonic acid using GC-TOFMS in an independent sample set. We performed a power analysis to determine the sample size required to replicate the initial nervonic acid finding with reasonable statistical power at an alpha value of 0.05, (1 – β) of 0.80, and an effect size of f=0.2. Because the sample conditions differed between the sets (cohort 1, drug-free; cohort 2, mostly medicated), we estimated that the effect size would be relatively small. The total number of samples required to attain sufficient statistical power to detect a difference was n=280. We measured 321 samples of mostly medicated patients or controls (MDD, n=45; BD, n=71; SZ, n=115; control, n=90) (Table 2). Similar to the results obtained for cohort 1, patients with MDD in cohort 2 had significantly increased plasma nervonic acid levels (0.59±0.41 µM) compared with controls (0.40±0.29 µM; P=.0022), patients with BD (0.39±0.25 µM; P=.0013), and patients with SZ (0.44±0.24 µM; P=.021) (Figure 2). The effect size was f=0.26. The sensitivity and specificity of the plasma nervonic acid levels that discriminated between patients with MDD and controls were 67.1% and 62.2%, respectively. To evaluate the effects of the confounding factors on the plasma levels of nervonic acid, we applied an ANCOVA with a dependent variable of the metabolite levels, an independent variable of diagnosis, and the covariates of age and sex. The effects of diagnosis on the nervonic acid levels remained significant (P=.0016), although the levels of nervonic acid were affected by age (P=.010).
Figure 2.
Absolute plasma levels of nervonic acid in the cohort 2 samples. The bars indicate the mean of each group. The error bars represent the SDs. Comparison of the absolute plasma levels of nervonic acid among the patients with schizophrenia (SZ), major depressive disorder (MDD), bipolar disorder (BD), and healthy controls (1-way ANOVA with posthoc Tukey’s test).
Relationship between Nervonic Acid Levels and the Clinical Assessments
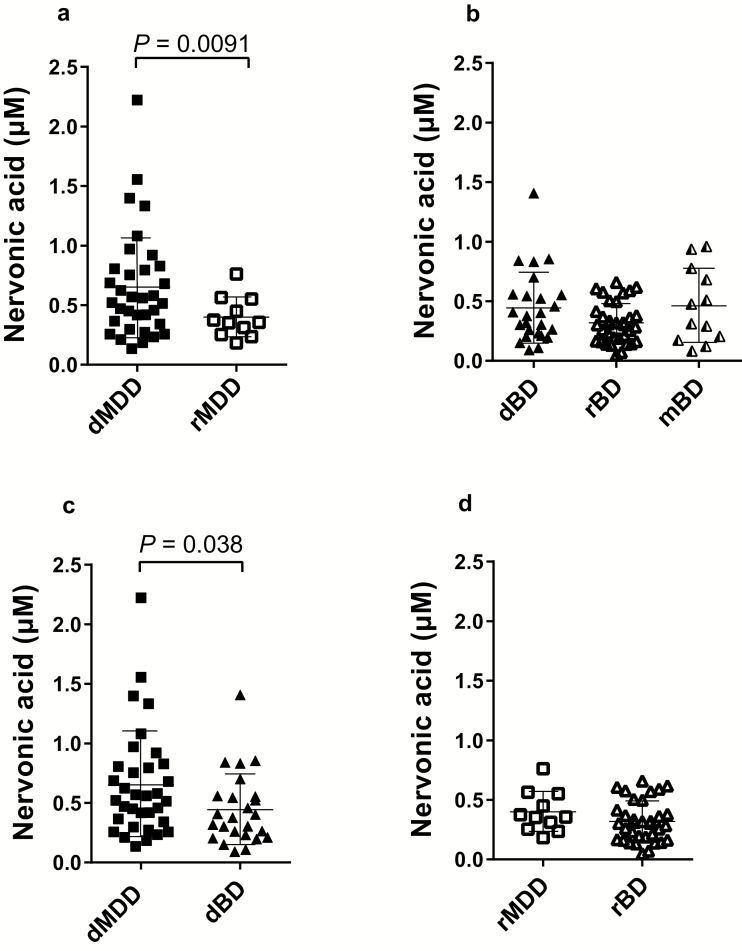
To determine whether the levels of nervonic acid changed in a state-dependent manner in patients with MDD, we divided the patients with MDD into the following 2 groups based on the M.I.N.I. results: depressed (dMDD) patients (n=34) and remitted (rMDD) patients (n=11) (supplementary Table 2). The levels of nervonic acid were higher in the dMDD patients (0.65±0.45 µM) compared with the rMDD patients (0.40±0.17 µM; effect size of Cohen’s d=0.75; P=.0091) (Figure 3a).
Figure 3.
State-dependent changes in plasma levels of nervonic acid in patients with MDD and BD in cohort 2. The bars indicate the mean for each group. The error bars represent the SDs. (a) Comparison between patients in the depressive state of major depressive disorder (dMDD) and the remitted state of major depressive disorder (rMDD) (Welch t test). (b) Comparison among patients in the depressive state of bipolar disorder (dBD), the remitted state of bipolar disorder (rBD), and the manic state of bipolar disorder (mBD) (1-way ANOVA with posthoc Tukey’s test). (c) Comparison between patients with dMDD and dBD (Welch t test). (d) Comparison between patients with rMDD and rBD (Welch t test).
The patients with BD were divided into the following 3 groups based on the M.I.N.I. results: depressed (dBD) patients (n=25), remitted (rBD) patients (n=34), and manic (mBD) patients (n=12) (supplementary Table 3). No significant state-dependent difference were found in the patients with BD (dBD, 0.44±0.30 µM; rBD, 0.32±0.17 µM; mBD, 0.46±0.31 µM; f=0.57, P=.09) (Figure 3b). We compared the plasma levels of nervonic acid in the dMDD and dBD patients (supplementary Table 4) and found that the dMDD patients had increased plasma nervonic acid levels compared with those in the dBD patients (d=0.54, P=.038) (Figure 3c). The plasma nervonic acid levels did not significantly differ between the rMDD and rBD patients (d=0.47, P=.20) (Figure 3d; supplementary Table 5).
We then investigated the relationships among the symptoms of the patients (PANSS positive, negative, or general pathology scores; YMRS scores; and HAM-D21 scores), duration of illness, and nervonic acid levels with correlation analyses using Spearman’s rank correlation coefficients. There were no significant correlations between nervonic acid levels and the clinical scores. However, the nervonic acid concentrations positively correlated with the duration of illness in the patients with MDD (ρ=0.55, P=.0011).
Discussion
To the best of our knowledge, this is the first study to examine plasma nervonic acid as a potential biomarker of MDD. Because the profile of plasma metabolites might be potentially affected by antipsychotic (Cai et al., 2012) and antidepressant drugs (Webhofer et al., 2011), we investigated candidate metabolites in a drug-free sample set of patients, who were not currently on medication, to avoid detection of metabolites that are affected by medication. Subsequently, we confirmed that the levels of plasma nervonic acid were altered in an independent sample set of patients who were mostly medicated.
Nervonic acid [(Z)-Tetracos-15-enoic acid or 24:1, n-9 by the International Union of Pure and Applied Chemistry nomenclature] is a long chain of monounsaturated omega-9 fatty acids that are particularly abundant in the white matter of the brain. Nervonic acid is an essential molecule for the growth and maintenance of the brain and peripheral nervous tissue enriched in sphingomyelin (Martínez and Mougan, 2002) and related to psychiatric disorders (Amminger et al., 2012). Because sphingomyelin is a key constituent of myelin, it is abundant in the white matter of the brain. Our results showed that plasma nervonic acid levels were increased in patients with MDD, in whom the integrity of the white matter has been reported as impaired (Nobuhara et al., 2006; Li et al., 2007). Thus, increased levels of plasma nervonic acid might reflect white matter dysfunction in patients with MDD.
White matter dysfunction has also been reported in patients with BD and SZ (Sussmann et al., 2009; Skudlarski et al., 2013). Why were the levels of plasma nervonic acid increased only in the patients with MDD? One possibility is that the cause of white matter dysfunction might be different in the patients with MDD compared with the patients with BD and SZ (Johnston-Wilson et al., 2000). Whereas sphingomyelin is abundant in myelin-forming oligodendrocytes, it also exists in lipid rafts of neuronal cells, which influence the potency and efficacy of neurotransmitter receptors and transporters. The effects of lipid rafts on neurotransmitter signaling have been implicated in neurological and psychiatric diseases (Allen et al., 2007). Sphingomyelin has also been reported to be associated with the regulation of neurogenesis. An association of decreased neurogenesis in the hippocampus and the dysregulation of sphingomyelin metabolic pathway has been suggested to play a role in depression (Gulbins et al., 2015), and antidepressants have been postulated to enhance neurogenesis by inhibiting the acid sphingomyelinase/ceramide system, which is part of the sphingomyelin metabolic pathway (Gulbins et al., 2013). Thus, increased levels of plasma nervonic acid might reflect dysregulation of oligodendrocytes, sphingomyelin-rich lipid rafts, and/or the sphingomyelin metabolic pathway in patients with MDD.
A previous report has shown that the nervonic acid levels that were measured in erythrocyte membranes from patients with recurrent MDD were decreased (Assies et al., 2010). This finding might be due to differences in MDD subtype (recurrent vs not recurrent), methodology (LC-TOFMS vs GC-TOFMS), or the types of samples (plasma vs erythrocyte membrane) (Hayashi-Takagi et al., 2014). In addition, previous studies (Assies et al., 2010; Lin et al., 2010) have shown that omega-3 and omega-6 polyunsaturated fatty acids are altered in patients with MDD compared with controls. We detected omega-3 and omega-6 polyunsaturated fatty acids (supplemental Table 1), but no significant differences were found between patients with MDD and controls. Because nervonic acid does not cross the blood-brain barrier (Coupland et al., 2003), it is not clear whether there is a relationship between peripheral nervonic acid levels and its levels in the brain. More studies, including studies of postmortem brains or cerebrospinal fluid, are necessary to fully elucidate the relationship between MDD and nervonic acid.
A previous study (Kim et al., 2016) has reported a correlation between erythrocyte nervonic acid and positive symptoms on PANSS in ultra-high-risk subjects. However, plasma nervonic acid levels and PANSS scores were not correlated in the results of the present study, and the difference between the previous findings and our findings might have been due to the differences in the subjects (SZ vs ultra-high-risk subjects) or types of samples (plasma vs erythrocyte membrane).
We calculated the effect sizes to evaluate if plasma nervonic acid levels would be useful as a diagnostic biomarker. The effect size of the nervonic acid level comparisons between the dMDD and rMDD patients (d=0.75, Figure 3a) and dMDD and dBD patients (d=0.54, Figure 3c) was medium, while the effect size was small in the comparisons among MDD, BD, SZ, and controls (f=0.26, Figure 2). These results suggested that nervonic acid levels can be used as a biomarker to discriminate the depressive state of patients with MDD from the euthymic state of patients with MDD or the depressive state of patients with BD. On the other hand, nervonic acid levels would not be useful to discriminate euthymic patients with MDD from controls or patients with other mental disorders (SZ or BD). Additional longitudinal measurements of plasma nervonic acid levels along with detailed information on clinical symptoms in the same individual are required to determine whether nervonic acid levels show state or trait-dependent changes.
Cortisone is a 21-carbon steroid that is one of the main hormones released by the adrenal gland in response to stress (Tacker et al., 1978). It is converted to the active metabolite hydrocortisone, which is also called cortisol. The results of the current study showed that patients with SZ exhibited increased levels of cortisone. These results may reflect the well-established finding of abnormalities of the hypothalamic-pituitary-adrenal axis in patients with SZ (Walker et al., 2008). A previous report has shown that plasma cortisone levels were increased in patients with MDD compared with controls (Weber et al., 2000). However, no significant differences were found in this study. The discrepancy of these results might have been a result of differences in methodology (LC-TOFMS vs specific radioimmunoassay) (Hayashi-Takagi et al., 2014).
This study had several limitations. First, effects of diet (Cevallos-cevallos et al., 2009; Young et al., 2017), age (Jové et al., 2014), circadian variation (Dallmann et al., 2012), and other confounding factors (Lawton et al., 2008) on the plasma levels of metabolites cannot be excluded. Because we did not strictly control the meal and sample collection time, some of the observed changes might be attributable to these confounding factors. Additional studies involving larger sample size and controlling for all confounding factors are needed to validate the use of plasma nervonic acid levels as a biomarker of MDD. Second, we obtained data only for patients with MDD, BD, and SZ, as well as controls. Additional studies of patients with other neuropsychiatric disorders and demyelinating diseases, such as adrenoleukodystrophy (Moser et al., 1981), are necessary to determine if increased levels of plasma nervonic acid level are specific to patients with MDD. Third, the number of participants examined in this study was too small to draw definitive conclusions. Fourth, a confounding effect of metabolic syndrome on plasma nervonic acid cannot be totally ruled out, because a relationship between nervonic acid and metabolic syndrome has been reported (Yamazaki et al., 2014). In the 30 samples of cohort 1, a weak tendency for correlation between plasma nervonic acid level and cholesterol level was found (ρ=0.32, P=.083). Fifth, there was a relatively consistent effect of age on nervonic acid levels (cohort 1, P=.082; cohort 2, P=.010). Thus, subgroup analyses should be interpreted with caution when the ages between groups are different.
In conclusion, we found that the levels of plasmanervonic acid were increased in patients with MDD. These results suggested that this metabolite would be a good candidate biomarker for the differential diagnosis, depressive state assessment, and treatment optimization of patients with MDD.
Supplementary Material
Acknowledgments
We thank Drs Taku Doi and Mariko Yamada for their help during sample collection. We are grateful to the members of our laboratory for their valuable discussions and technical assistance and to the patients and controls who participated in this study.
Author Contributions
T. Kasahara, T. Kato, and Y. Kageyama conceived and designed the study. Y. Kageyama, T. Nakamura, T. Kasahara, and T. Kato acquired and analyzed the data. K. Hattori, S. Yoshida, Y. Goto, K. Inoue, M. Tani, Y. Deguchi, K. Kuroda, and Y. Kageyama collected the human samples and drafted the manuscript. Y. Kageyama, T. Kasahara, and T. Kato wrote the paper.
Funding
This work was supported by the Japan Agency for Medical Research and Development (T. Kato: 16815678), MEXT/JSPS KAKENHI grants (T. Kato: 24249063 and 17H01573), and a grant-in-aid from the Japanese Ministry of Health and Labor (T. Kato: 201241002A). Y. Kageyama is supported by a RIKEN Junior Research Associate fellowship.
Statement of Interest
T. Kato has received honoraria for lectures, manuscripts, and/or consultancy from Kyowa Hakko Kirin Co., Ltd.; Eli Lilly Japan K.K.; Otsuka Pharmaceutical Co., Ltd.; GlaxoSmithKline K.K.; Taisho Toyama Pharmaceutical Co., Ltd.; Dainippon Sumitomo Pharma Co., Ltd.; Meiji Seika Pharma Co., Ltd.; Pfizer Japan Inc.; Mochida Pharmaceutical Co., Ltd.; Shionogi & Co., Ltd.; Janssen Pharmaceutical K.K.; Yoshitomiyakuhin; Astellas Pharma Inc.; Wako Pure Chemical Industries, Ltd.; and Takeda Pharmaceutical Co., Ltd. within the last 3 years. T. Kato also received a research grant from Takeda Pharmaceutical Co., Ltd. These companies played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Allen JA, Halverson-Tamboli RA, Rasenick MM(2007)Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8:128–140. [DOI] [PubMed] [Google Scholar]
- Altamura AC, Buoli M, Caldiroli A, Caron L, Cumerlato Melter C, Dobrea C, Cigliobianco M, Zanelli Quarantini F(2015)Misdiagnosis, duration of untreated illness (DUI) and outcome in bipolar patients with psychotic symptoms: a naturalistic study. J Affect Disord 182:70–75. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994)Diagnostic and statistical manual of mental disorders. 4th ed. Arlington, VA. [Google Scholar]
- Amminger GP, Schäfer MR, Klier CM, Slavik JM, Holzer I, Holub M, Goldstone S, Whitford TJ, McGorry PD, Berk M(2012)Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol Psychiatry 17:1150–1152. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schäfer MR, Schlögelhofer M, Klier CM, McGorry PD(2015)Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun 6:7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assies J, Pouwer F, Lok A, Mocking RJT, Bockting CLH, Visser I, Abeling NGGM, Duran M, Schene AH(2010)Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One 5:e10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M, Caldiroli A, Cumerlato Melter C, Serati M, de Nijs J, Altamura AC(2016)Biological aspects and candidate biomarkers for psychotic bipolar disorder: a systematic review. Psychiatry Clin Neurosci 70:227–244. [DOI] [PubMed] [Google Scholar]
- Cai H, Li H, Yan X, Sun B, Zhang Q, Yan M, Zhang W, Jiang P, Zhu R, Liu Y, Fang P, Xu P, Yuan H, Zhang X, Hu L, Yang W, Ye H(2012)Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperdone. J Proteome Res 11:4338–4350. [DOI] [PubMed] [Google Scholar]
- Cevallos-Cevallos JM, Etxeberria E, Danyluk MD, Rodrick GE(2009)Metabolomic analysis in food science: a review. Trends Food Sci Technol 20:557–566. [Google Scholar]
- Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW(2003)Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol 13:99–103. [DOI] [PubMed] [Google Scholar]
- Coupland K, Raoul Y(2003)Nervonic acid derivatives, their preparation and use. U.S. Patent 6,664,406. [Google Scholar]
- Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA(2012)The human circadian metabolome. Proc Natl Acad Sci U S A 109:2625–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt N, Etain B, Lajnef M, Henry C, Raust A, Cochet B, Mathieu F, Gard S, Mbailara K, Zanouy L, Kahn JP, Cohen RF, Wajsbrot-Elgrabli O, Leboyer M, Scott J, Bellivier F(2013)Duration of untreated bipolar disorder: missed opportunities on the long road to optimal treatment. Acta Psychiatr Scand 127:136–144. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A(2007)G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Marangell LB, Richardson AJ, Lake J, Stoll AL(2006)Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 67:1954–1967. [DOI] [PubMed] [Google Scholar]
- Gaebel W, Zielasek J(2015)Schizophrenia in 2020: trends in diagnosis and therapy. Psychiatry Clin Neurosci 69:661–673. [DOI] [PubMed] [Google Scholar]
- Gulbins E, et al. (2013)Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med 19:934–938. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Walter S, Becker KA, Halmer R, Liu Y, Reichel M, Edwards MJ, Müller CP, Fassbender K, Kornhuber J(2015)A central role for the acid sphingomyelinase/ceramide system in neurogenesis and major depression. J Neurochem 134:183–192. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS(1985)Cortisol secretion in endogenous depression. I. Basal plasma levels. Arch Gen Psychiatry 42:904–908. [DOI] [PubMed] [Google Scholar]
- Hamilton M.(1960)A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Vawter MP, Iwamoto K(2014)Peripheral biomarkers revisited: integrative profiling of peripheral samples for psychiatric research. Biol Psychiatry 75:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston-Wilson N, Sims C, Hofmann JP, Anderson L, Shore A, Torrey E, Yolken R(2000)Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. Mol Psychiatry 5:142–149. [DOI] [PubMed] [Google Scholar]
- Jové M, Portero-Otín M, Naudí A, Ferrer I, Pamplona R(2014)Metabolomics of human brain aging and age-related neurodegenerative diseases. J Neuropathol Exp Neurol 73:640–657. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein AOL(1987)The positive and negative syndrome scale for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kim SW, Jhon M, Kim JM, Smesny S, Rice S, Berk M, Klier CM, McGorry PD, Schäfer MR, Amminger GP(2016)Relationship between erythrocyte fatty acid composition and psychopathology in the Vienna omega-3 study. PLoS One 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi H, Hori H, Ogawa S(2015)Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci 69:597–608. [DOI] [PubMed] [Google Scholar]
- Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV(2008)Analysis of the adult human plasma metabolome. Pharmacogenomics 9:383–397. [DOI] [PubMed] [Google Scholar]
- Li L, Ma N, Li Z, Tan L, Liu J, Gong G, Shu N, He Z, Jiang T, Xu L(2007)Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res 1168:124–128. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP(2010)A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 68:140–147. [DOI] [PubMed] [Google Scholar]
- Martínez M, Mougan I(2002)Fatty acid composition of human brain phospholipids during normal development. J Neurochem 71:2528–2533. [DOI] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrowa L, Lieberman J(2006)Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology 31:1249–1263. [DOI] [PubMed] [Google Scholar]
- Moser HW, Moser AB, Frayer KK, Chen W, Schulman JD, O’Neill BP, Kishimoto Y(1981)Adrenoleukodystrophy: increased plasma content of saturated very long chain fatty acids. Neurology 51:334. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T(2006)Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 77:120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet M, Shah S, Selvam K, Ramchand CN(2004)Polyunsaturated fatty acid levels in red cell membranes of unmedicated schizophrenic patients. World J Biol Psychiatry 5:92–99. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS, Kupka RW, Keck PE, McElroy SL, Altshuler LL, Frye MA, Luckenbaugh DA, Rowe M, Grunze H, Suppes T, Nolen WA(2010)Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry 71:864–872. [DOI] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Dennis EA(2011)High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim Biophys Acta 1811:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK(2004)Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull 30:901–911. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC(1998)The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–33, quiz 34–57. [PubMed] [Google Scholar]
- Singh I, Rose N(2009)Biomarkers in psychiatry. Nature 460:202–207. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney J, Tamminga C, Clementz B, O’Neil K, Pearlson GD(2013)Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry 170:886–898. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Bosetti F, DeMar JC, Ma K, Bell JM, Fagin-Jones S, Russ MJ, Rapoport SI(2007)Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord 9:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, Hall J, Bastin ME, Johnstone EC, Lawrie SM, McIntosh AM(2009)White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord 11:11–18. [DOI] [PubMed] [Google Scholar]
- Tacker MM, Leach CS, Owen CA, Rummel JA(1978)Levels of cortisol, corticosterone, cortisone and 11-deoxycoritsol in the plasma of stressed and unstressed subjects. J Endocrinol 76:165–166. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K(2008)Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 4:189–216. [DOI] [PubMed] [Google Scholar]
- Weber B, Lewicka S, Deuschle M, Colla M, Vecsei P, Heuser I(2000)Increased diurnal plasma concentrations of cortisone in depressed patients. J Clin Endocrinol Metab 85:1133–1136. [DOI] [PubMed] [Google Scholar]
- Webhofer C, Gormanns P, Tolstikov V, Zieglgänsberger W, Sillaber I, Holsboer F, Turck CW(2011)Metabolite profiling of antidepressant drug action reveals novel drug targets beyond monoamine elevation. Transl Psychiatry 1:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Kondo K, Maeba R, Nishimukai M, Nezu T, Hara H(2014)Proportion of nervonic acid in serum lipids is associated with serum plasmalogen levels and metabolic syndrome. J Oleo Sci 63:527–537. [DOI] [PubMed] [Google Scholar]
- Young AJ, Marriott BP, Champagne CM, Hawes MR, Montain SJ, Johannsen NM, Berry K, Hibbeln JR(2017)Blood fatty acid changes in healthy young Americans in response to a 10-week diet that increased n-3 and reduced n-6 fatty acid consumption: a randomised controlled trial. Br J Nutr 117:1257–1269. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA(1978)A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.