Abstract
Background:
While effective for the repair of large skull base defects, the Hadad-Bassagasteguy nasoseptal flap increases operative time and can result in a several-week period of postoperative crusting during re-mucosalization of the denuded nasal septum. Endoscopic transsphenoidal surgery for pituitary adenoma resection is generally not associated with large dural defects and high-flow cerebrospinal fluid (CSF) leaks requiring extensive reconstruction. Here, we present the posterior nasoseptal flap as a novel technique for closure of skull defects following endoscopic resection of pituitary adenomas. This flap is raised in all surgeries during the transnasal exposure using septal mucoperiosteum that would otherwise be discarded during the posterior septectomy performed in binostril approaches.
Methods:
We present a retrospective, consecutive case series of 43 patients undergoing endoscopic transsphenoidal resection of a pituitary adenoma followed by posterior nasoseptal flap placement and closure. Main outcome measures were extent of resection and postoperative CSF leak.
Results:
The mean extent of resection was 97.16 ± 1.03%. Radiographic measurement showed flap length to be adequate. While a defect in the diaphragma sellae and CSF leak were identified in 21 patients during surgery, postoperative CSF leak occurred in only one patient.
Conclusions:
The posterior nasoseptal flap provides adequate coverage of the surgical defect and is nearly always successful in preventing postoperative CSF leak following endoscopic transsphenoidal resection of pituitary adenomas. The flap is raised from mucoperiosteum lining the posterior nasal septum, which is otherwise resected during posterior septectomy. Because the anterior septal cartilage is not denuded, raising such flaps avoids the postoperative morbidity associated with the larger Hadad-Bassagasteguy nasoseptal flap.
Keywords: Cerebrospinal fluid leak, closure, endoscopy, nasoseptal flap, pituitary adenoma
INTRODUCTION
Pituitary adenomas are a common benign intracranial neoplasm comprising 15.5% of all primary central nervous system (CNS) neoplasms.[13,41] The pituitary gland, seated within the sella at the skull base, is difficult to access transcranially, so neurosurgeons have been approaching pituitary adenomas via the sphenoid sinus since Hermann Schloffer first did so in 1907.[46]
Transsphenoidal pituitary surgery may be performed either microscopically or endoscopically. While both techniques are cost-effective compared to medical therapies alone in patients with a life expectancy greater than ten years,[26] studies have shown that endoscopy has become the standard of care due to a higher rate of complete tumor resection, lower incidence of operative complications, and shorter postoperative hospital stay when compared to microsurgical approaches.[1,10,12,16,17,19,29,30,31,33,37,38,44,50,52,56] However, certain risks remain with the endoscopic approach, most commonly cerebrospinal fluid (CSF) leaks and endocrine abnormalities, including SIADH, diabetes insipidus, and pituitary insufficiency.[2,11,25,36,51] While CSF leaks occur intraoperatively in up to 61% of the cases,[15,53] the risk of postoperative CSF leak requiring repair after surgery for resection of a pituitary adenoma has been found to be approximately 4%.[6,15,34]
Repairing CSF leaks is crucial in preventing serious postoperative infectious complications. The creation of a fistula between the subarachnoid space and nasal cavity carries the risks of bacterial spread from the nasal cavity, potentially leading to ascending meningitis.[5] Rates of meningitis from unrepaired CSF leaks have been reported to be up to 19% per year.[8] Concurrent with the development of endoscopic approaches to the pituitary, surgeons have been devising endoscopic techniques to address CSF leaks. Some of the earliest techniques involved the use of tissue grafts from elsewhere in the body, including fascia, muscle, or fat;[40] however, this leads to additional incisions, increased operative time, and further discomfort for the patient. More recently, other methods of closing the sella turcica have involved the use of materials such as cadaveric acellular dermis or silastic.[47] While they improve upon the drawbacks of the previous method, these materials may not support healing as effectively as living autologous tissue and can cause magnetic resonance imaging interference. Finally, vascularized rotational flaps may be used, which have the advantage of promoting healing.
Nasoseptal and middle turbinate rotational flaps have been used since the 1950s, but these early flaps had a random blood supply and unfavorable arcs of rotation. Hadad and Bassagasteguy developed an improved, pedicled vascularized nasoseptal flap in 2006.[21] This relatively large flap significantly reduces the incidence of postoperative CSF leak[15,22] in extended endoscopic approaches and addresses the issues associated with homografts and alloplastic materials. However, it does result in increased operative time and a prolonged healing period with crusting along the denuded caudal nasal septum.[9] While necessary for repair of large skull base and dural defects created during surgical resections that extend beyond the sella, the Hadad-Bassagasteguy nasoseptal flap is typically more than is necessary for reconstruction of the small dural defects and low-flow CSF leaks typically associated with surgery for resection of pituitary adenomas. Given these concerns, here we describe a novel method that employs the use of a posterior nasoseptal flap to prevent postoperative CSF leak following endoscopic transsphenoidal pituitary adenectomy. This flap is raised and positioned in all cases during the transnasal approach regardless of whether an intraoperative CSF leak is either anticipated or present. The flap consists of vascularized mucoperiosteal tissue that would otherwise be removed during posterior nasal septectomy. While not suitable for closure of the larger skull base defects created during extended transsphenoidal or transnasal procedures, this technique is extremely effective in reconstructing sellar dural defects and addressing postoperative CSF leak as a potential risk after aggressive resection of pituitary adenomas. Importantly, raising this flap does not significantly prolong the duration of surgery or generate the postoperative nasal complaints such as crusting of denuded septal cartilage associated with larger flaps.
MATERIALS AND METHODS
This study represents a retrospective analysis of a prospectively constructed database of 43 serial patients who underwent endoscopic transsphenoidal resection of pituitary adenomas with posterior nasoseptal flap placement at our institution between March, 2014 and June, 2015. Patient charts were reviewed for a number of preoperative, intraoperative, and postoperative parameters, including intra and postoperative CSF leaks. This database does not include patients who were deemed to require an extended transsphenoidal approach for resection of their tumor during preoperative surgical planning. Such patients were reconstructed with Hadad-Bassagasteguy nasoseptal flaps.
Surgical technique
After the induction of general endotracheal anesthesia, the patient's nose is decongested with oxymetazoline-soaked cottonoids. The nasal passages are carefully examined, after which the inferior turbinates are first in-fractured and subsequently out-fractured to improve access via the nasal passages. The middle turbinates are injected with 1% lidocaine with epinephrine (1:100,000) for anesthesia and hemostasis, and lateralized using the Freer elevator. The superior turbinates are also visualized and lateralized, allowing for identification of the sphenoid ostium in the sphenoethmoidal recess bilaterally. For larger tumors or cases in which more lateral access is needed, the middle and/or superior turbinates may be partially resected.
The posterior nasoseptal flap is typically raised on the left side unless anatomic or surgical considerations dictate otherwise; however, this is just a matter of surgeon preference. The anterior aspect of the middle turbinate marks the anterior extent of the posterior nasoseptal flap. Directly medial to this point, the mucosa of the midnasal septum is cauterized vertically and incised. From there, the mucoperiosteum is raised posteriorly to the level of the sphenoid rostrum. The sphenoid ostium is identified submucosally as an anatomic landmark, and the septal mucoperiosteum is transected inferiorly along the maxillary crest and dorsally below the expected level of the olfactory epithelium. This creates a posterior septal flap, pedicled on the posterior septal branch of the sphenopalatine artery [Figure 1]. The flap is rotated into the nasopharynx and covered with a cottonoid.
Figure 1.
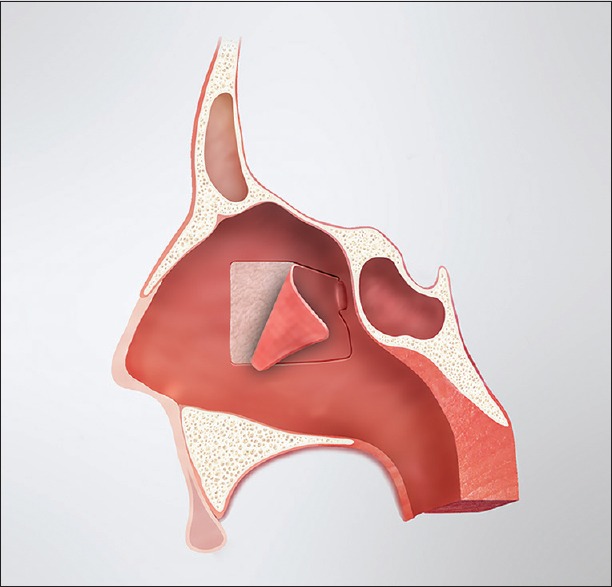
Illustration demonstrating the posterior septal flap in relation to the nasal anatomy
The mucosa of the contralateral septum is then similarly cauterized and incised. The flap is raised from that point posteriorly and the ostium of the sphenoid is identified. The mucosa and bone of the posterior septum on this second side is then resected, thus creating a central surgical corridor between the turbinates with access to both sphenoid sinuses. Because a posterior septectomy is performed to facilitate a binostril approach for tumor resection, the posterior septal flap can be preserved on both sides if it is felt to be necessary for reconstruction.
After elevation of the flap(s) and creation of a central surgical corridor with access to both sphenoid sinuses, the standard tumor resection is performed by the neurosurgeon. If an intraoperative CSF leak occurs, the dural opening is repaired using medium implantable (0.5–1.0 mm) AlloDerm (LifeCell Corporation, New Jersey, USA) placed in an “inlay” fashion in the epidural space just deep to the residual bone of the sella. The graft is then covered with a thin layer of Tisseel (Baxter Inc., Illinois, USA) tissue adhesive to maintain its position.
Whether there has been an intraoperative CSF leak, the posterior septal flap is rotated into position covering the demucosalized bone of the sphenoid rostrum and floor as well as the area of the dural opening. Intersinus septations are removed and the sphenoid rostrum is drilled as needed to allow for direct overlay of the flap along the floor of the sella. The flap is covered with Tisseel tissue adhesive and Gelfoam. A sphenoid pack consisting of 0.5-inch Nu Gauze (Johnson and Johnson, New Jersey, USA) coated in bacitracin ointment is then placed to maintain the position of the flap and underlying grafts. The pack is brought out through the nasal cavity and secured to the membranous septum with a single suture. At the completion of the procedure, the middle turbinates are medialized to their normal anatomic position.
Postoperative management
Patients are monitored postoperatively for signs of CSF leak, diabetes insipidus, and pituitary dysfunction, and given stool softeners to reduce straining. For patients who did not have intraoperative CSF leaks, the nasal packing is removed before discharge (typically postoperative day 2 or 3). If an intraoperative CSF leak did occur, the packing is left in place until the 1-week postoperative visit.
Calculation of tumor volume
Tumor volumes were calculated based on the (A × B × C)/2 formula, where A, B, and C represent the largest tumor dimension in the anterior-posterior, superior-inferior, and medio-lateral dimensions.
Statistics
All statistical analyses were performed with Prism (Graphpad). Population means were compared with Student's t-test. Population statistics are presented as mean ± standard error. Linear correlations were analyzed with Spearman correlation coefficient. Significance was set at P < 0.05.
RESULTS
Patient information
Patient demographic information and data related to tumors and surgical parameters are shown in Table 1. Among the 43 patients, 35 (81.4%) had nonsecreting pituitary adenomas; 4 (9.3%) had ACTH-producing adenomas; 3 (7.0%) had GH-producing adenomas; and 1 (2.3%) had a prolactin-secreting adenoma. The average preoperative and postoperative tumor volumes were 4.83 ± 0.77 (n = 43) and 0.26 ± 0.11 cm3 (n = 40), respectively (t-test, P < 10-6). The mean extent of resection was 97.16 ± 1.03% (n = 40). Cumulatively, 77.5% of patients had >99% of the tumor removed, and 95% of patients had >80% resection [Figure 2a; Table 1]. The extent of resection inversely correlated with tumor size [Figure 2b], consistent with previous literature showing that tumor size is the single most predictive factor for gross total resection and postoperative pituitary functional status.[14] A representative preoperative and postoperative MRI is shown in Figure 2c. The gross total resection rate was comparable with previously published series of endoscopic transsphenoidal resection of pituitary adenomas.[4,7,16,20,42,48,49,52,55] Among the 8 patients with secreting tumors, 2 were lost to follow-up (1 with acromegaly and 1 with Cushing's disease). Among the remaining patients, 3/3 patients with Cushing's disease are in remission/cured and are maintained on hydrocortisone replacement therapy; 2/2 patients with acromegaly are in remission but not cured, based on IGF-1 levels that have improved but are not normal; and 1 patient with prolactinoma is in remission, with normal prolactin levels.
Table 1.
Patient characteristics and results
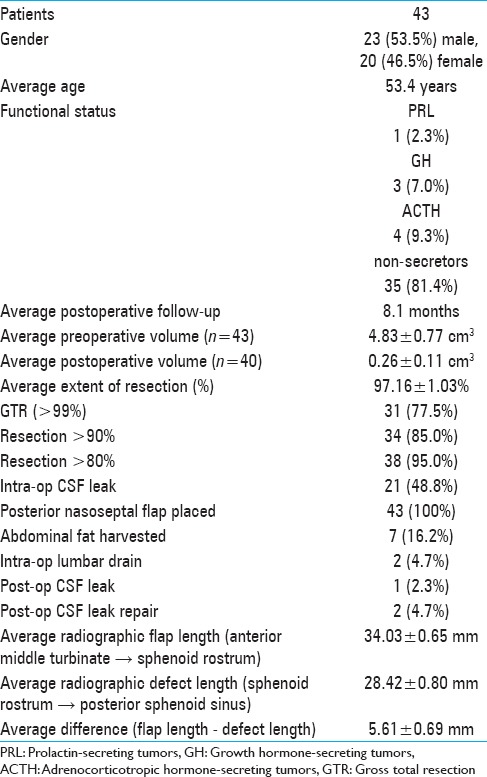
Figure 2.
(a) Distribution of extent of resection (%) among the 43 patients in the study. (b) Linear correlation between the extent of resection and preoperative tumor size. (c) Representative pre- and postoperative coronal gadolinium-enhanced T1-weighted images
Intraoperative CSF leaks were noted in 21 (48.8%) of the patients [Table 2]. All patients, whether a CSF leak was noted intraoperatively or not, had the skull base/sellar defect covered with a posterior nasoseptal flap. Within the group with intraoperative CSF leaks, a multilayered closure was performed in 100% of patients, consisting of Alloderm placed in the epidural space along the floor of the sella, followed by the nasoseptal flap. Furthermore, within this group, intraoperative lumbar drains were placed in 2 patients and abdominal fat was used for closure of the skull defect in 7 patients.
Table 2.
Comparison of patients with and without intraoperative CSF leak
Postoperatively, 2 patients were treated for presumed CSF rhinorrhea, but a CSF leak was confirmed in only one of them. This patient had no documented intraoperative leak, and the skull base defect had been repaired with the posterior septal flap. However, postoperatively, the patient experienced difficulty in breathing and oxygen desaturation, which led us to remove the nasal packing. The leak occurred subsequent to the packing removal while the patient was straining during a bowel movement. She was treated with secondary surgical repair and lumbar drain placement. The second patient had a small intraoperative leak repaired with the multilayered closure. Clear rhinorrhea, suspicious for a CSF leak, was noted on postoperative day 1. A lumbar drain was placed before β2-transferrin results returned negative, indicating that there was, in fact, no CSF leak. Overall, the posterior nasoseptal flap has a 97.7% success rate in preventing postoperative CSF leaks despite a 48.8% rate of observable intraoperative leaks. Furthermore, barring extenuating circumstances, such as Valsalva maneuvers, the success rate is expected to be even closer to 100%.
Planning the posterior nasoseptal flap
A review of the preoperative MRI showed the distance between the sphenoid rostrum and the middle turbinate (the length of the flap) to be longer than the distance between the sphenoid rostrum and the posterior sphenoid (the length of the defect) in all but 4 cases; on average, it was 5.61 ± 0.69 mm longer [Figure 3a; Table 1]. When the length of the flap was less than the length of the defect, additional bone was removed from the sphenoidal rostrum and clivus, thus shortening the distance between the rostrum and sella. In all cases, the posterior septal flap was deemed adequate for coverage of the skull defect intraoperatively [Figure 3b]. None of the 4 patients with radiologically “short” flaps experienced a postoperative leak. In all patients, postoperative MRI showed the posterior nasoseptal flap covering the entire skull defect [Figure 3c]. All flaps remained viable upon endoscopic inspection during follow-up appointments. Figure 3d shows an example of a flap visualized endoscopically 2 years after the initial surgery.
Figure 3.
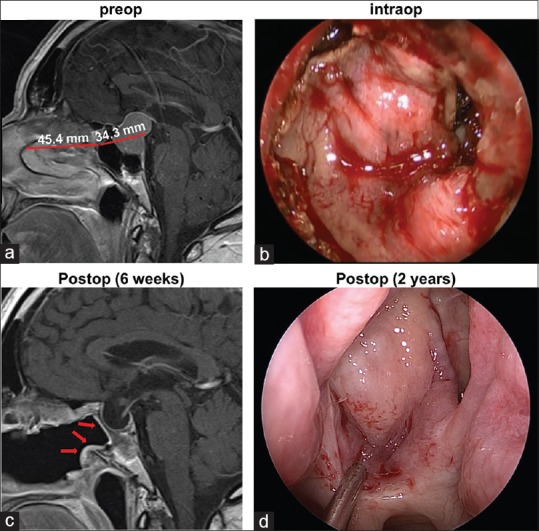
(a) Representative preoperative sagittal gadolinium-enhanced T1-weighted image demonstrates the lengths of the flap and skull defect. (b) Intraoperative photograph through the endoscope demonstrates the posterior nasoseptal flap covering the skull defect. (c) In the postoperative MRI, the vascularized flap (arrows) can be visualized lining the patent sphenoid sinus. (d) Endoscopic photograph of the flap 2 years after the initial surgery
DISCUSSION
Cerebrospinal fluid leak repair
The pituitary gland, seated in the sella turcica, is considered an intradural, extra-arachnoidal part of the brain by virtue of the diaphragma sellae, a reflection of the arachnoid, that separates it from the suprasellar cistern. Because the pituitary is extra-arachnoidal, CSF leaks do not occur as a matter of course during pituitary surgery but rather as a consequence of violation of the diaphragma sellae during tumor resection. The attempt to achieve gross total tumor resection often results in violation of the diaphragma sellae. Our intraoperative CSF leak rate of approximately 49% matches previously published reports,[15,25,39] but reflects our aggressive surgical approach to achieve high resection rates of benign pituitary adenomas. Such arachnoidal defects are generally small and have low CSF flow relative to defects created during extended endoscopic approaches for resection of other skull base tumors. Nevertheless, the rate of postoperative CSF leak following transsphenoidal resection of pituitary adenomas is estimated at 2.7–4.4%.[6,15,24,35,42,45]
Persistent CSF leaks after pituitary adenoma surgery are a concern because they can lead to ascending meningitis. Multiple repair techniques have been described over the years. In 2006, Hadad and Bassagasteguy described a pedicled nasoseptal flap with excellent vascular supply and a wide arc of rotation, and reported success rates in excess of 90%.[21] The Hadad-Bassagasteguy flap, based on the posterior septal branch of the sphenopalatine artery, extends the entire anteroposterior dimension of the nasal septum from the proximal end of the vascular pedicle at the sphenoid rostrum to the caudal incision just posterior to the columella. These vascularized local flaps result in rapid healing at the repair site and eliminate the need for harvesting tissue (fat, fascia) from distant sites.[27] A recent series of 151 patients with various sellar and parasellar pathologies (126 benign, 25 malignant), of whom 144 received Hadad-Bassagestaguy nasoseptal flaps, reported a postrepair postoperative leak rate of 3.3% with no recurrent leaks after 3 months and no incidents of flap death.[54]
The Hadad-Bassagestaguy flap, though extremely effective, was conceived for use in the setting of intraoperative CSF leaks in extended transsphenoidal approaches for resection of skull base tumors other than pituitary adenomas.[21] Because it is only raised when a leak is anticipated and its vascular pedicle is otherwise lost during the posterior septectomy in routine approaches, the flap cannot be used for repair of unexpected leaks after the posterior septectomy has already been performed. In other words, the decision to raise a Hadad-Bassagestaguy nasoseptal flap must be made at the beginning of the operation before the need for it can be determined. Recently, the concept of a “rescue flap” technique has been developed to address this shortcoming.[43] In this technique, as in ours, the incision for elevating the flap occurs at the beginning of all cases; however, only the posterior superior incision is made allowing for inferior reflection of the pedicle and preservation of the vascular supply. It is converted to a full nasoseptal flap in the case of an intraoperative leak; in cases without a leak, the reflected mucosa containing the vascular pedicle is replaced in its normal anatomic position. The shortcoming of this technique lies in its potential to limit the exposure of the sphenoidal rostrum.
The posterior nasoseptal flap represents a modification of the Hadad-Bassagestaguy flap, but is smaller and consists primarily of mucosal tissue that is otherwise discarded during the posterior septectomy for the binostril transsphenoidal approach. As such, the crusting associated with denuded septal cartilage after raising Hadad-Bassagestaguy flaps is minimized with posterior flaps. Another fundamental difference between the two approaches is that we raise the posterior flap in all surgeries, regardless of anticipated or observed intraoperative CSF leak, without much added operative time.
In our experience, the posterior septal flap provides excellent coverage for surgical defects associated with endoscopic transsphenoidal surgery for pituitary adenomas, obviating the need for a full-length nasoseptal flap. The posterior nasoseptal flap has the additional advantage of being placed in all cases, thus preventing postoperative leaks from arachnoidal defects and small CSF leaks that may not have been noted during the procedure, as occurred in one of the patients in the study. Importantly, using this flap in all surgical cases for endoscopic pituitary adenectomy virtually eliminates the risk of postoperative CSF leak. This allows us to pursue aggressive resection of pituitary adenomas, as evidenced by the 97% extent of resection in our series. It should be emphasized that this flap is not conceived for use in cases involving larger skull base defects, as in extended transsphenoidal or transnasal approaches, which require the use of full Hadad-Bassagestaguy flaps.
Multilayered closure in cases with documented intraoperative cerebrospinal fluid leak
In cases where we identify a CSF leak intraoperatively, we employ a multilayered approach for closure of the skull defect. A layer of Alloderm is placed in the epidural space tucked under the edges of the remaining sellar bone and covering the dural opening at the floor of the sella. After a thin layer of Tisseel, we place the posterior nasoseptal flap over the Alloderm. This strategy provides two barriers preventing postoperative leaks. We only rarely have to consider use of abdominal fat graft or lumbar drain using this approach. In fact, none of our patients with documented intraoperative CSF leaks had postoperative CSF rhinorrhea. The only patient in our cohort with postoperative rhinorrhea developed it as a result of straining despite no evidence of an intraoperative leak.
Alloderm has been previously used by itself for closure of skull base defects in endoscopic surgery.[32] In our approach, however, we use Alloderm in combination with the posterior nasoseptal flap as a multilayered closure in patients with intraoperative leak. Although other materials have been used for multilayered reconstructions,[18,23] this is to our knowledge the first description of Alloderm in combination with vascularized flaps.
Crusting
The Hadad-Bassagasteguy nasoseptal flap causes donor site morbidity because of exposure of the anterior septal cartilage. This denuded cartilage takes months to fully re-mucosalize and requires repeated office debridement, as well as the use of topical ointments and saline rinses during the healing period.[28] In contrast, the posterior septal flap makes use of the mucoperiosteum overlying the portion of the septum that is routinely removed and, as such, does not expose any cartilage or result in the attendant donor site morbidity. Furthermore, the flap provides for immediate vascularized mucosal coverage of the exposed portions of the sphenoid bone, eliminating a significant amount of the normal postoperative crusting in the posterior nasal cavity.
Sphenoid sinusitis
In a study of 200 patients who underwent microscopic transsphenoidal hypophysectomy between 1998 and 2001, the incidence of isolated sphenoid sinusitis in the year following surgery was 7.5%.[3] The etiology of the sphenoid sinusitis in this and other retrospective studies was not clear; however, by definition, it had to involve obstruction of the normal sinus drainage pathway. The endoscopic transsphenoidal approach to the sella involves creating a wide, bilateral sphenoidotomy, analogous to the large frontal drainage pathway created when performing a Draf III endoscopic frontal sinus drill-out procedure. In both cases, the expansive but demucosalized opening carries a risk of delayed re-mucosalization, crusting, and potentially scarring.[9] By placing a vascularized mucosal flap, the incidence of postoperative crusting at the level of the sphenoid rostrum, stenosis of the sphenoidotomy, and secondary sinusitis is virtually eliminated. The sphenoid sinus remains surgically patent, allowing for easy postoperative visualization and monitoring of the sphenoid sinus, both endoscopically and radiographically.
CONCLUSIONS
The posterior nasoseptal flap is a novel technique that uses septal mucoperiosteum, which would otherwise be resected during the posterior septectomy, to prevent postoperative CSF leaks following endoscopic transsphenoidal resection of pituitary adenomas. The flap is raised in all cases, regardless of anticipated or observed intraoperative CSF leak. In addition, the flap provides early vascularized coverage of the demucosalized bone of the sphenoid sinus. It is raised at the beginning of the case and provides adequate coverage of the sellar defect without the need for further extension of the flap in the event of an intraoperative CSF leak. In our experience, the flap heals well, maintains a patent sphenoidotomy, and can be elevated and reused if revision surgery is necessary. This approach is uncomplicated, safe, and has advantages relative to alternative flap techniques, such as the rescue flap and the Hadad-Bassagasteguy nasoseptal flap. While not appropriate for closure of larger approaches, the use of the posterior nasoseptal flap virtually eliminates the risk of postoperative CSF leak after pituitary adenectomy, thereby allowing surgeons to pursue complete resection of these benign tumors despite the elevated rate of intraoperative CSF leak associated with aggressive surgery. For these reasons, we propose that the posterior nasoseptal flap is an excellent option for skull defect closure following endoscopic transsphenoidal pituitary adenectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
James Barger, Email: jbarger@mgh.harvard.edu.
Matthew Siow, Email: matthew.siow@nyumc.org.
Michael Kader, Email: michael.kader@nyumc.org.
Katherine Phillips, Email: kgrace.phillips@gmail.com.
Girish Fatterpekar, Email: girish.fatterpekar@nyumc.org.
David Kleinberg, Email: david.kleinberg@nyumc.org.
David Zagzag, Email: david.zagzag@nyumc.org.
Chandranath Sen, Email: chandra.sen@nyumc.org.
John G. Golfinos, Email: john.golfinos@nyumc.org.
Richard Lebowitz, Email: richard.lebowitz@nyumc.org.
Dimitris G. Placantonakis, Email: dimitris.placantonakis@nyumc.org.
REFERENCES
- 1.Aghi MK, Chen CC, Fleseriu M, Newman SA, Lucas JW, Kuo JS, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Management of Patients With Nonfunctioning Pituitary Adenomas: Executive Summary. Neurosurgery. 2016;79:521–3. doi: 10.1227/NEU.0000000000001386. [DOI] [PubMed] [Google Scholar]
- 2.Barkhoudarian G, Cutler AR, Yost S, Lobo B, Eisenberg A, Kelly DF. Impact of selective pituitary gland incision or resection on hormonal function after adenoma or cyst resection. Pituitary. 2015;18:868–75. doi: 10.1007/s11102-015-0664-3. [DOI] [PubMed] [Google Scholar]
- 3.Batra P, Citardi M, Lanza D. Isolated sphenoid sinusitis after transsphenoidal hypophysectomy. Am J Rhinol. 2005;19:185–9. [PubMed] [Google Scholar]
- 4.Beltrame S, Toscano M, Goldschmidt E, Garategui L, Campero A, Yampolsky C, et al. Endoscopic treatment of 140 pituitary tumors, results and complications. Neurocirugia (Astur) 2016 doi: 10.1016/j.neucir.2016.06.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Bernal-Sprekelsen M, Bleda-Vazquez C, Carrau RL. Ascending meningitis secondary to traumatic cerebrospinal fluid leaks. Am J Rhinol. 2000;14:257–59. doi: 10.2500/105065800779954473. [DOI] [PubMed] [Google Scholar]
- 6.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: Results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225. doi: 10.1097/00006123-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Dallapiazza R, Grober Y, Starke R, Laws E, Jr, Jane J., Jr Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery. 2015;76:42–52. doi: 10.1227/NEU.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 8.Daudia A, Biswas D, Jones N. Risk of meningitis with cerebrospinal fluid rhinorrhea. Ann Otol Rhinol Laryngol. 2007;116:902–5. doi: 10.1177/000348940711601206. [DOI] [PubMed] [Google Scholar]
- 9.de Almeida JR, Snyderman CH, Gardner PA, Carrau RL, Vescan AD. Nasal morbidity following endoscopic skull base surgery: A prospective cohort study. Head Neck. 2011;33:547–51. doi: 10.1002/hed.21483. [DOI] [PubMed] [Google Scholar]
- 10.de Divitiis E, Laws ER, Giani U, Iuliano SL, de Divitiis O, Apuzzo ML. The current status of endoscopy in transsphenoidal surgery: An international survey. World Neurosurg. 2015;83:447–54. doi: 10.1016/j.wneu.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 11.de Paiva Neto MA, Vandergrift A, Fatemi N, Gorgulho AA, Desalles AA, Cohan P, et al. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin Endocrinol (Oxf) 2010;72:512–9. doi: 10.1111/j.1365-2265.2009.03665.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH. Cavernous Sinus Invasion in Pituitary Adenomas: Systematic Review and Pooled Data Meta-analysis of Radiological Criteria and Comparison of Endoscopic and Microscopic Surgery. World Neurosurg. 2016;96:36–46. doi: 10.1016/j.wneu.2016.08.088. [DOI] [PubMed] [Google Scholar]
- 13.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas. Cancer. 2004;101:613–9. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 14.Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J, et al. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63:709–19. doi: 10.1227/01.NEU.0000325725.77132.90. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto Y, Balsalobre L, Santos FP, Vellutini E, Stamm AC. Endoscopic combined “transseptal/transnasal” approach for pituitary adenoma: Reconstruction of skull base using pedicled nasoseptal flap in 91 consecutive cases. Arq Neuropsiquiatr. 2015;73:611–5. doi: 10.1590/0004-282X20150070. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Zheng H, Xu S, Zheng Y, Wang Y, Jiang J, et al. Endoscopic Versus Microscopic Approach in Pituitary Surgery. J Craniofac Surg. 2016;27:e157–9. doi: 10.1097/SCS.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C, et al. Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: A meta-analysis. World J Surg Oncol. 2014;12:94. doi: 10.1186/1477-7819-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: Efficacy in a large case series. World Neurosurg. 2013;80:563–8. doi: 10.1016/j.wneu.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: A systematic review and meta-analysis. Clin Otolaryngol. 2011;36:212–20. doi: 10.1111/j.1749-4486.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 20.Guvenc G, Kizmazoglu C, Pinar E, Imre A, Kaya I, Bezircioglu H, et al. Outcomes and Complications of Endoscopic Versus Microscopic Transsphenoidal Surgery in Pituitary Adenoma. J Craniofac Surg. 2016;27:1015–20. doi: 10.1097/SCS.0000000000002684. [DOI] [PubMed] [Google Scholar]
- 21.Hadad G, Bassagasteguy L, Carrau R, Mataza J, Kassam A, Snyderman C. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 22.Horridge M, Jesurasa A, Olubajo F, Mirza S, Sinha S. The use of the nasoseptal flap to reduce the rate of post-operative cerebrospinal fluid leaks following endoscopic trans-sphenoidal surgery for pituitary disease. Br J Neurosurg. 2013;27:739–41. doi: 10.3109/02688697.2013.795525. [DOI] [PubMed] [Google Scholar]
- 23.Hu F, Gu Y, Zhang X, Xie T, Yu Y, Sun C, et al. Combined use of a gasket seal closure and a vascularized pedicle nasoseptal flap multilayered reconstruction technique for high-flow cerebrospinal fluid leaks after endonasal endoscopic skull base surgery. World Neurosurg. 2015;83:181–7. doi: 10.1016/j.wneu.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Ivan M, Iorgulescu J, El-Sayed I, McDermott M, Parsa A, Pletcher S, et al. Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci. 2015;22:48–54. doi: 10.1016/j.jocn.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Jakimovski D, Bonci G, Attia M, Shao H, Hofstetter C, Tsiouris AJ, et al. Incidence and significance of intraoperative cerebrospinal fluid leak in endoscopic pituitary surgery using intrathecal fluorescein. World Neurosurg. 2014;82:e513–23. doi: 10.1016/j.wneu.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Jethwa P, Patel T, Hajart A, Eloy J, Couldwell W, Liu J. Cost-Effectiveness Analysis of Microscopic and Endoscopic Transsphenoidal Surgery Versus Medical Therapy in the Management of Microprolactinoma in the United States. World Neurosurg. 2016;87:65–76. doi: 10.1016/j.wneu.2015.10.090. [DOI] [PubMed] [Google Scholar]
- 27.Kassam A, Carrau R, Snyderman C, Thomas A, Vescan A, Prevedello D, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63:ONS44–53. doi: 10.1227/01.neu.0000297074.13423.f5. [DOI] [PubMed] [Google Scholar]
- 28.Kimple A, Leight W, Wheless S, Zanation A. Reducing nasal morbidity after skull base reconstruction with the nasoseptal flap: Free middle turbinate mucosal grafts. Laryngoscope. 2012;122:1920–4. doi: 10.1002/lary.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary. 2012;15:150–9. doi: 10.1007/s11102-011-0359-3. [DOI] [PubMed] [Google Scholar]
- 30.Kuo JS, Barkhoudarian G, Farrell CJ, Bodach ME, Tumialan LM, Oyesiku NM, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Surgical Techniques and Technologies for the Management of Patients With Nonfunctioning Pituitary Adenomas. Neurosurgery. 2016;79:E536–8. doi: 10.1227/NEU.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 31.Little AS, Kelly DF, Milligan J, Griffiths C, Prevedello DM, Carrau RL, et al. Comparison of sinonasal quality of life and health status in patients undergoing microscopic and endoscopic transsphenoidal surgery for pituitary lesions: A prospective cohort study. J Neurosurg. 2015;123:799–807. doi: 10.3171/2014.10.JNS14921. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz RR, Dean RL, Hurley DB, Chuang J, Citardi MJ. Endoscopic reconstruction of anterior and middle cranial fossa defects using acellular dermal allograft. Laryngoscope. 2003;113:496–501. doi: 10.1097/00005537-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Lucas JW, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, Litvack Z, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Primary Management of Patients With Nonfunctioning Pituitary Adenomas. Neurosurgery. 2016;79:E533–5. doi: 10.1227/NEU.0000000000001389. [DOI] [PubMed] [Google Scholar]
- 34.Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E, et al. Complications Related to the Endoscopic Endonasal Transsphenoidal Approach for Nonfunctioning Pituitary Macroadenomas in 300 Consecutive Patients. World Neurosurg. 2016;89:442–53. doi: 10.1016/j.wneu.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 35.Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E, et al. Complications Related to the Endoscopic Endonasal Transsphenoidal Approach for Nonfunctioning Pituitary Macroadenomas in 300 Consecutive Patients. World neurosurgery. 2016;89:442–53. doi: 10.1016/j.wneu.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 36.Mascarenhas L, Moshel YA, Bayad F, Szentirmai O, Salek AA, Leng LZ, et al. The transplanum transtuberculum approaches for suprasellar and sellar-suprasellar lesions: Avoidance of cerebrospinal fluid leak and lessons learned. World Neurosurg. 2014;82:186–95. doi: 10.1016/j.wneu.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 37.McCoul ED, Bedrosian JC, Akselrod O, Anand VK, Schwartz TH. Preservation of multidimensional quality of life after endoscopic pituitary adenoma resection. J Neurosurg. 2015;123:813–20. doi: 10.3171/2014.11.JNS14559. [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin N, Eisenberg AA, Cohan P, Chaloner CB, Kelly DF. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J Neurosurg. 2013;118:613–20. doi: 10.3171/2012.11.JNS112020. [DOI] [PubMed] [Google Scholar]
- 39.Mehta G, Oldfield E. Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas: Clinical article. J Neurosurg. 2012;116:1299–303. doi: 10.3171/2012.3.JNS112160. [DOI] [PubMed] [Google Scholar]
- 40.Neligan P, Mulholland S, Irish J, Gullane P, Boyd J, Gentili F, et al. Flap selection in cranial base reconstruction. Plast Reconstr Surg. 1996;98:1159–68. doi: 10.1097/00006534-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Ostrom Q, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro-oncology. 2013;15(suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paluzzi A, Fernandez-Miranda J, Stefko S, Challinor S, Snyderman C, Gardner P. Endoscopic endonasal approach for pituitary adenomas: A series of 555 patients. Pituitary. 2014;17:307–19. doi: 10.1007/s11102-013-0502-4. [DOI] [PubMed] [Google Scholar]
- 43.Rivera-Serrano C, Snyderman C, Gardner P, Prevedello D, Wheless S, Kassam A. Nasoseptal “rescue” flap: A novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope. 2011;121:990–3. doi: 10.1002/lary.21419. [DOI] [PubMed] [Google Scholar]
- 44.Schaberg MR, Anand VK, Schwartz TH, Cobb W. Microscopic versus endoscopic transnasal pituitary surgery. Curr Opin Otolaryngol Head Neck Surg. 2010;18:8–14. doi: 10.1097/MOO.0b013e328334db5b. [DOI] [PubMed] [Google Scholar]
- 45.Schlosser R, Bolger W. Nasal cerebrospinal fluid leaks: Critical review and surgical considerations. Laryngoscope. 2004;114:255–65. doi: 10.1097/00005537-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt RF, Choudhry OJ, Takkellapati R, Eloy JA, Couldwell WT, Liu JK. Hermann Schloffer and the origin of transsphenoidal pituitary surgery. Neurosurg Focus. 2012;33:E5. doi: 10.3171/2012.5.FOCUS12129. [DOI] [PubMed] [Google Scholar]
- 47.Seiler R, Mariani L. Sellar reconstruction with resorbable Vicryl patches, gelatin foam, and fibrin glue in transsphenoidal surgery: A 10-year experience with 376 patients. J Neurosurg. 2000;93:762–5. doi: 10.3171/jns.2000.93.5.0762. [DOI] [PubMed] [Google Scholar]
- 48.Shin S, Tormenti M, Paluzzi A, Rothfus W, Chang Y, Zainah H, et al. Endoscopic endonasal approach for growth hormone secreting pituitary adenomas: Outcomes in 53 patients using 2010 consensus criteria for remission. Pituitary. 2013;16:435–44. doi: 10.1007/s11102-012-0440-6. [DOI] [PubMed] [Google Scholar]
- 49.Singh H, Essayed W, Cohen-Gadol A, Zada G, Schwartz T. Resection of pituitary tumors: Endoscopic versus microscopic. J Neurooncol. 2016 doi: 10.1007/s11060-016-2124-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Singh H, Essayed WI, Cohen-Gadol A, Zada G, Schwartz TH. Resection of pituitary tumors: Endoscopic versus microscopic. J Neurooncol. 2016;130:309–17. doi: 10.1007/s11060-016-2124-y. [DOI] [PubMed] [Google Scholar]
- 51.Strychowsky J, Nayan S, Reddy K, Farrokhyar F, Sommer D. Purely endoscopic transsphenoidal surgery versus traditional microsurgery for resection of pituitary adenomas: Systematic review. J Otolaryngol Head Neck Surg. 2011;40:175. [PubMed] [Google Scholar]
- 52.Tabaee A, Anand V, Barrón Y, Hiltzik D, Brown S, Kacker A, et al. Endoscopic pituitary surgery: A systematic review and meta-analysis. J Neurosurg. 2009;111:545–54. doi: 10.3171/2007.12.17635. [DOI] [PubMed] [Google Scholar]
- 53.Tabaee A, Placantonakis DG, Schwartz TH, Anand VK. Intrathecal fluorescein in endoscopic skull base surgery. Otolaryngol Head Neck Surg. 2007;137:316–20. doi: 10.1016/j.otohns.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Thorp B, Sreenath S, Ebert C, Zanation A. Endoscopic skull base reconstruction: A review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak. Neurosurgery. 2014;37:E4. doi: 10.3171/2014.7.FOCUS14350. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Zhou T, Wei S, Meng X, Zhang, J, Hou Y, et al. Endoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomas. Surg Endosc. 2015;29:1270–80. doi: 10.1007/s00464-014-3815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu T, Peng L, Li H, Wang Y, Liu L, Jiang Y, et al. The safety and efficacy of endoscopic versus microscopic surgery for transsphenoidal pituitary adenoma in China: An updated and cumulative meta-analysis. Zhonghua Yi Xue Za Zhi. 2015;95:3378–81. [PubMed] [Google Scholar]


