Abstract
Background
Ketamine rapidly elicits antidepressive effects in humans and mice in which serotonergic activity is involved. Although α4β2 nicotinic acetylcholine receptor (α4β2 nAChR) in the dorsal raphe nucleus plays a key role in the ketamine-induced prefrontal serotonin release, the source of cholinergic afferents, and its role is unclear.
Methods
Prefrontal serotonin levels after ketamine injection were measured by microdialysis in rats. Electrolytic lesion of pedunculopontine tegmental nucleus and laterodorsal tegmental nucleus was made with constant direct current.
Results
Bilateral lesion of the pedunculopontine tegmental nucleus, but not laterodorsal tegmental nucleus, attenuated prefrontal serotonin release induced by systemic ketamine. Intra-pedunculopontine tegmental nucleus, but not intra-laterodorsal tegmental nucleus ketamine perfusion, increased prefrontal serotonin release. This increase was attenuated by intra-dorsal raphe nucleus injection of dihydro-β-erythroidine, an α4β2 nAChR antagonist, or NBQX, an AMPA receptor antagonist.
Conclusions
These results suggest the ketamine-induced serotonin release in medial prefrontal cortex is mediated by cholinergic neurons projecting from pedunculopontine tegmental nucleus to dorsal raphe nucleus via α4β2 nAChRs.
Keywords: ketamine, serotonin, microdialysis, nicotinic acetylcholine receptors, raphe
Introduction
Major depressive disorder (MDD) is one of the most common mental disorders in the world. Although MDD patients are often treated with antidepressants such as selective serotonin reuptake inhibitors (SSRIs), chronic administration for several weeks is required for therapeutic efficacy. Furthermore, around one-third of MDD patients are resistant to the conventional drug treatment (Rush et al., 2006; Belmaker and Agam 2008). Therefore, rapid-acting antidepressants with higher efficacy are urgently needed. Clinical studies have revealed that an N-methyl-D-aspartate receptor (NMDAR) antagonist, ketamine, at a subanesthetic dose rapidly elicits antidepressive action in the treatment-resistant MDD patients (Berman et al., 2000; Zarate et al., 2006). In rodents, ketamine produces antidepressant-like effects in a variety of behavioral paradigms such as forced swim test and novelty-suppressed feeding test, which are attenuated by depletion of serotonin (Gigliucci et al., 2013; Fukumoto et al., 2014). On the other hand, acute administration of ketamine enhanced extracellular serotonin levels in the medial prefrontal cortex (mPFC), one of the brain regions associated with the pathology of MDD (Amargós-Bosch et al., 2006). These reports indicate that serotonergic neural transmission mediates the effect of ketamine, although the precise mechanism how ketamine activates serotonergic neurons is unclear.
It has been reported that endogenous acetylcholine (ACh) and nicotine activate serotonin neurons by increasing glutamate release through activation of presynaptic α4β2 nicotinic acetylcholine receptors (α4β2 nAChRs) in the dorsal raphe nucleus (DRN) (Garduño et al., 2012). Consistent with these studies, we have reported that systemic ketamine injection increases serotonin levels in the mPFC through indirectly activating α4β2 nAChRs in the DRN (Nishitani et al., 2014). These reports have raised the possibility that the cholinergic neurons projecting to the DRN are involved in ketamine-induced serotonin release. The DRN receives cholinergic innervation from the pedunculopontine tegmental nucleus (PPTg) and laterodorsal tegmental nucleus (LDTg), where large clusters of cholinergic neurons are anatomically defined at the junction of the midbrain and pons (Woolf and Butcher, 1989). However, the role of these cholinergic nuclei in the regulation of serotonergic activity remains to be unveiled.
In this study, we investigated the role of DRN-projecting PPTg and LDTg neurons in the ketamine-induced serotonin release in mPFC by in vivo microdialysis in rats.
Methods
All animal care and experimental procedures were in accordance with the ethical guidelines of the Kyoto University Animal Research Committee and approved by the Kyoto University Animal Research Committee. Male Wistar/ST rats (8–12 weeks of age, 220–350 g; Nihon SLC) were used. The animals were housed in groups of 3 per cage with free access to food and water and kept under controlled conditions (12-h-light/-dark cycle; 24±1°C). All surgeries were performed on rats anesthetized with sodium pentobarbital (60 mg/kg i.p.). Electrolytic lesions of the PPTg (AP −7.8 mm, ML ±1.9 mm, DV +7.6 mm from the bregma, according to the Brain Atlas; Paxinos and Watson, 2007) or LDTg (AP −8.7 mm, ML ±0.8 mm, DV +7.0 mm) were made bilaterally using stainless-steel pipe (30 gauge) insulated except the tip. Lesions were made with anodal constant direct current at each location (25 V for 25 s). Sham operations were performed in an identical manner, but no current was passed through. After a 7-d recovery period, the animals were implanted with the guide cannula for microdialysis procedures. Each rat was stereotaxically implanted with guide cannula (Eicom) in the mPFC (AP +3.2 mm, ML +0.6 mm or −0.6 mm, DV +4.0 mm) for in vivo microdialysis, in the DRN (AP −7.7 mm, ML +2.4 mm, DV +7.0 mm) for local drug microinjection, and in the PPTg (AP −7.9 mm, ML +1.9 mm or −1.9 mm, DV +7.0 mm) and LDTg (AP −8.7 mm, ML +0.8 mm, DV +6.0) for local drug perfusion. The guide cannulae were secured to the skull with dental cement. After 1 to 2 d of recovery period, microdialysis experiments were performed in unanesthetized and freely moving rats. Microdialysis probes (membrane length 2 mm, o.d. 0.22 mm; Eicom) in the mPFC were inserted through the guide cannula and perfused at a constant flow of 1 μL/min with Ringer buffer (147 mM NaCl, 4 mM KCl, 2.3 mM CaCl2) containing 1 μM citalopram (LKT Laboratories Inc.). Microdialysis probes (membrane length 1 mm, o.d. 0.22 mm; Eicom) for drug perfusion of PPTg or LDTg were also inserted through the guide cannula and perfused at a constant flow of 1 μL/min with Ringer buffer. Dialysates in the mPFC were collected every 10 min and immediately analyzed by high-performance liquid chromatography with electrochemical detector (Eicom). Serotonin was separated on a reverse phase column (PPII-ODS column, 3.0 φ × 150 mm; Eicom) and quantified by reference to a linear calibration curve, as previously described (Nishitani et al., 2014). Ketamine (Ketalar; Daiichi Sankyo Propharma Co., Ltd.) was administered s.c. or locally perfused into the PPTg or LDTg through microdialysis probes. For local perfusion of PPTg or LDTg with ketamine, we used the concentration of 0.1 mM in Ringer buffer. Dihydro-β-erythroidine (DHβE; Santa Cruz Biotechnology) and 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX; Sigma-Aldrich), an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) antagonist, were dissolved in sterile saline (Otsuka Pharmaceutical Co., Ltd.) and microinjected into the DRN through the injection cannula (28 gauge). In microdialysis experiments, all drugs were administered after 3 to 4 h of stabilization period. Intra-DRN drug microinjection (1 μL) was performed at 0.2 μL/min for 5 min by microinfusion pump 10 min before local perfusion with ketamine. After all tests, histological verification of lesion location was performed. Rats were perfused across the heart with PBS followed by 4% paraformaldehyde (Nacalai Tesque) in 0.1 M phosphate buffer. The dissected brains were cryoprotected in 15% sucrose-PBS for overnight and frozen at −80°C for storage. The brains were cryosectioned into 30-μm-thick coronal sections with the cryostat (Leica CM3050S; Leica Biosystems) and stored at −80°C until immunohistochemical processing. For immunohistochemistry, the sections were immersed in PBS with 0.25% Triton-X 100 (Nacalai Tesque) for permeabilization and then incubated overnight at 4°C with goat polyclonal anti-choline acetyltransferase (ChAT) antibody (1:200; AB144P, Merck Millipore), followed by incubation with Alexa Fluor 594-labeled donkey anti-goat IgG (1:200; Thermo Fisher Scientific) for 2 h at room temperature. The sections were then washed in PBS and mounted on glass with VECTASHIELD (Vector Laboratories). Immunofluorescence was visualized using a laser scanning confocal microscopy (Fluoview FV10i, Olympus). Histological analyses were performed to verify the placement of each tip of microdialysis probe (Nissl stain) or injection cannula (Evans Blue injection). The animals, in which the tips were incorrectly located, were excluded from the final analysis. Data are presented as means±SEM of the percentage of the basal values calculated as an average of 4 consecutive dialysate just before drug administration. No significant difference in the basal values was observed throughout the study. Statistical analysis was performed by GraphPad Prism 5 (GraphPad). Microdialysis results were analyzed by 2-way ANOVA for repeated measures, followed by Bonferroni posthoc test. The number of ChAT positive cells after electrolytic lesion was analyzed by Student’s t test. Differences of P < .05 were considered statistically significant.
Results
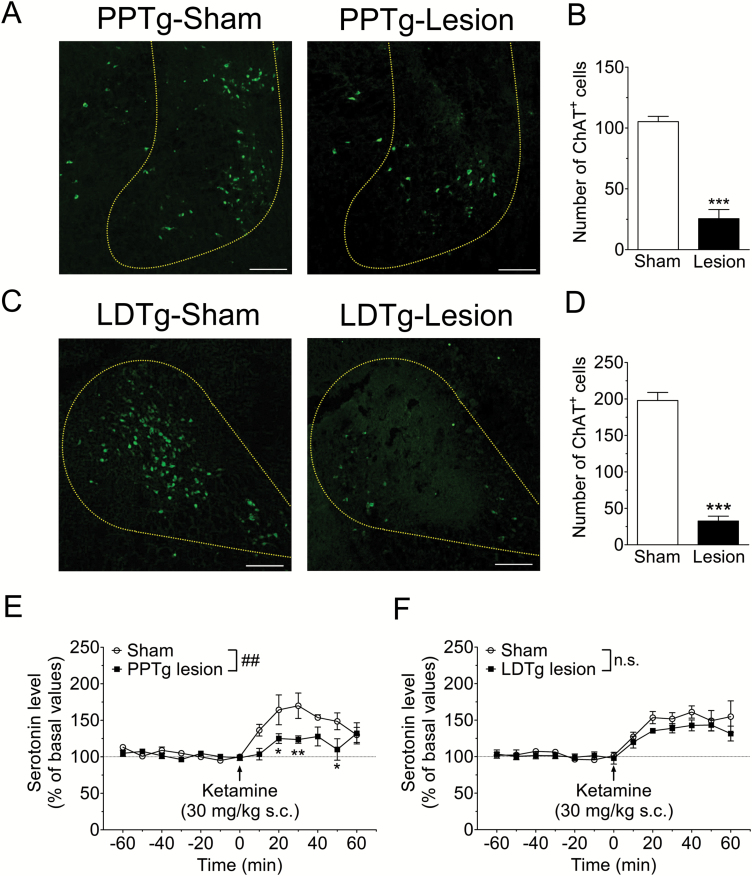
The DRN is mainly innervated by cholinergic neurons from the PPTg and LDTg (Woolf and Butcher 1989). Therefore, we examined the effect of bilateral lesion of PPTg or LDTg on ketamine-induced serotonin release in the mPFC. Histological analyses revealed that PPTg-lesion and LDTg-lesion rats had significantly fewer ChAT positive cells than each sham-operated rat (PPTg sham 105.2±4.3 cells, lesion 25.5±7.5 cells; t7=9.711, P<.001, LDTg sham 198.0±11.0 cells, lesion 32.6±6.7 cells; t8=12.88, P<.001; Figure 1A-D). Microdialysis was performed in these rats. Consistent with previous studies (Amargós-Bosch et al., 2006; Nishitani et al., 2014), acute administration of ketamine (30 mg/kg, s.c.) rapidly increased serotonin release in the mPFC in sham-operated rats. The PPTg lesion significantly attenuated the ketamine-induced serotonin release in the mPFC (F1,84=12.74, P<.01; Figure 1E), whereas the LDTg lesion did not (F1,96=2.703, P=.1388; Figure 1F).
Figure 1.
Pedunculopontine tegmental nucleus (PPTg) lesion attenuates ketamine-induced prefrontal 5-HT release. (A, C) Representative photomicrographs of choline acetyltransferase (ChAT) labeled cells in the PPTg (A) and the laterodorsal tegmental nucleus (LDTg) (C). (B, D) The number of ChAT-positive cells was significantly decreased in the PPTg (B) and LDTg (D). ***P<.001 vs sham-operated group (unpaired t test, n=4–5). Scale bar=200 µm (E, F). Effects of bilateral lesion of the PPTg (E) and LDTg (F) on ketamine-induced serotonin release in the mPFC. Extracellular serotonin levels in the mPFC were continuously measured for 120 min by in vivo microdialysis before and after drug administration. Saline or ketamine (30 mg/kg) was administered s.c. at 0 min (arrow). ##P<.01 main effect in 2-way ANOVA, **P<.01, *P<.05 vs sham-operated group by Bonferroni posthoc test. n.s. not significant, n=4–5. Basal values for serotonin concentrations were 0.51±0.12 nM (PPTg-sham), 0.45±0.04 nM (PPTg-lesion), 0.42±0.06 nM (LDTg-sham), and 0.67±0.16 nM (LDTg-lesion).
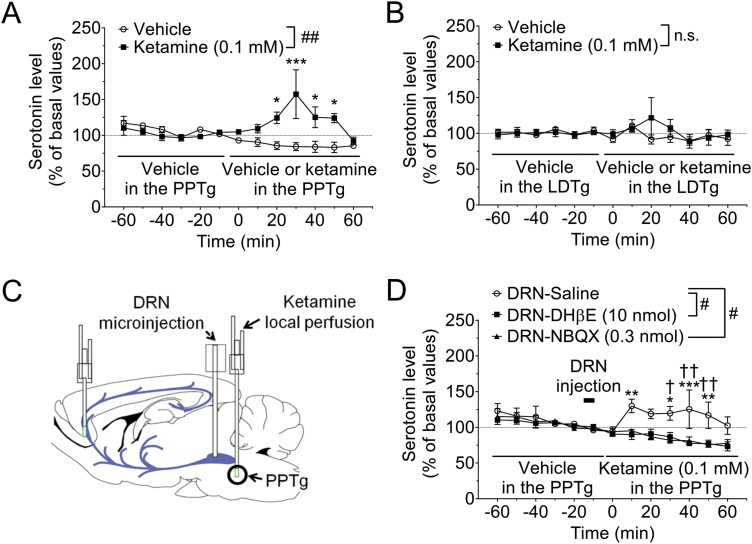
Then we investigated whether ketamine directly affects the PPTg. Unilateral local perfusion of PPTg with ketamine (0.1 mM) significantly increased the serotonin release in the mPFC (F1,72=13.94, P<.01; Figure 2A), indicating that ketamine increases prefrontal serotonin release through action on the PPTg. By contrast, local perfusion of LDTg with ketamine (0.1 mM) did not affect serotonin release (F1,84=0.2563, P=.6282; Figure 2B).
Figure 2.
α4β2 Nicotinic acetylcholine receptor (α4β2 nAChR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) in the dorsal raphe nucleus (DRN) mediates prefrontal serotonin release induced by local perfusion of PPTg with ketamine. (A, B) Effects of local perfusion with ketamine of pedunculopontine tegmental nucleus (PPTg) (A) and laterodorsal tegmental nucleus (LDTg) (B) on serotonin release in the medial prefrontal cortex (mPFC). Extracellular serotonin levels in the mPFC were continuously measured for 120 min by in vivo microdialysis before and after local perfusion with ketamine. The horizontal lines indicate the unilateral perfusion with vehicle or ketamine (0.1 mM). ##P<.01 main effect in 2-way ANOVA, ***P<.001, *P<.05 vs vehicle group by Bonferroni posthoc test. n.s., not significant, n=3–6. Basal values for serotonin concentrations were 0.64±0.17 nM (PPTg-vehicle), 0.50±0.05 nM (PPTg-ketamine), 0.55±0.05 nM (LDTg-vehicle), and 0.49±0.05 nM (LDTg-ketamine). (C) Schematic presentation of the experimental procedure. (D) Extracellular serotonin levels in the mPFC were continuously measured for 120 min before and after local perfusion of PPTg with ketamine by in vivo microdialysis. Saline, DHβE (10 nmol), an α4β2 nAChR antagonist, or NBQX (0.3 nmol), an AMPAR antagonist, was microinjected into the DRN from −15 to −10 min (bar). Then, ketamine (0.1 mM) was unilaterally perfused in the PPTg at 0 min (horizontal lines). #P<.05 main effect in 2-way ANOVA, ***P<.001, **P<.01, *P<.05 vs DRN-DHβE group by Bonferroni posthoc test. ††P<.01, †P<.05 vs DRN-NBQX group by Bonferroni posthoc test, n=4–6. Basal values for serotonin concentrations were 0.45±0.09 nM (DRN-saline), 0.44±0.06 nM (DRN-DHβE), and 0.48±0.09 nM (DRN-NBQX).
Since intra-DRN injection of DHβE, an α4β2 nAChR antagonist, attenuates prefrontal serotonin release induced by s.c. administration of ketamine (Nishitani et al., 2014), we investigated whether α4β2 nAChR in the DRN mediates prefrontal serotonin release induced by local perfusion of PPTg with ketamine. A microinjection of DHβE (10 nmol) into the DRN significantly attenuated serotonin release induced by intra-PPTg ketamine perfusion (F1,96=6.240, P<.05; Figure 2D). Previous studies have demonstrated that α4β2 nAChRs are present on serotonergic neurons and synaptic terminals of glutamatergic neurons (Galindo-Charles et al., 2008; Garduño et al., 2012). To investigate whether the effect of intra-PPTg ketamine was mediated by enhancement of glutamate release through stimulation of presynaptic α4β2 nAChR in the DRN, we examined the effect of intra-DRN injection of NBQX, an AMPA receptor antagonist. Similar to DHβE, intra-DRN injection of NBQX significantly attenuated prefrontal serotonin release induced by intra-PPTg ketamine perfusion (F1,96=6.201, P<.05; Figure 2D).
Discussion
In the present study, we showed that bilateral electrolytic lesion of the PPTg attenuated ketamine-induced prefrontal serotonin release and that unilateral perfusion of ketamine in the PPTg increased serotonin levels in the mPFC. Furthermore, pharmacological blockade of α4β2 nAChRs or AMPARs in the DRN suppressed prefrontal serotonin release induced by local perfusion of ketamine in the PPTg. These results suggest that ketamine directly acts on the PPTg and induces serotonin release in the mPFC through activation of the DRN-projecting cholinergic neurons in the PPTg and subsequently presynaptic α4β2 nAChRs in the DRN (supplementary Figure 1).
Consistent with the present findings, activation of presynaptic α4β2 nAChRs results in the increase of serotonin neuronal firing through enhancement of glutamate release in the DRN (Garduño et al., 2012). Anatomical analyses have demonstrated that the DRN is innervated by cholinergic neurons from PPTg and LDTg (Woolf and Butcher, 1989). Surprisingly, in this study, PPTg but not LDTg was involved in ketamine-induced prefrontal serotonin release. One possible explanation for this difference between PPTg and LDTg is that ketamine preferentially activates PPTg cholinergic neurons. Indeed, local perfusion of ketamine in the PPTg but not in the LDTg mimicked the effect of ketamine, indicating different sensitivity to ketamine between these nuclei. On the other hand, PPTg and LDTg form rostrocaudal continuum of cholinergic neurons and have similar characteristics of structure and neurochemistry (Paxinos and Watson, 2007; Wang and Morales, 2009), suggesting similar sensitivity to pharmacological intervention.
Both PPTg and LDTg innervate basal ganglia and thalamic structures, such as substantia nigra pars compacta, ventral tegmental area, striatum, and thalamus as well as DRN. However, these projections do not overlap; PPTg neurons innervate lateral region, whereas LDTg neurons innervate the medial region in these brain areas (Mena-Segovia, 2016). On the other hand, O’Hearn and Molliver have demonstrated that ventral rather than dorsal neurons in the DRN project to the cortex (O’Hearn and Molliver, 1984). Therefore, it is possible that PPTg and LDTg innervate different sub-nuclei in the DRN and that the PPTg preferentially connects to DRN serotonergic neurons projecting to mPFC, although further anatomical analyses are required.
In this study, prefrontal serotonin release was increased by local perfusion of PPTg with ketamine, strongly indicating that ketamine acts on the PPTg. However, it is unclear how ketamine, an NMDAR antagonist, activates cholinergic neuron in the PPTg. Several reports suggest that blockade of NMDARs increases activity of excitatory neurons by inhibiting GABAergic interneurons, and extracellular glutamate release in the mPFC (Moghaddam et al., 1997; Homayoun and Moghaddam, 2007). Lines of evidence have demonstrated that PPTg contains GABAergic neurons as well as cholinergic neurons (Ford et al., 1995; Wang and Morales, 2009). Thus, it is possible that ketamine activates cholinergic neurons through inhibiting NMDARs on GABA interneurons in the PPTg.
It is reported that social defeat stress suppresses the serotonergic neuronal firing through increasing the activity of GABAergic interneurons in the DRN (Challis et al., 2013), which partly explains why acute SSRI treatment is not effective in depressed animals (Berton et al., 2006). Meanwhile, we have reported that chronic SSRI treatment enhances serotonergic neuronal activity (Asaoka et al., 2017). The present study indicates that ketamine rapidly enhances serotonergic neuronal activity through α4β2 nAChR stimulation, suggesting that ketamine is effective even in the mouse model in which acute SSRI is not effective. Indeed, efficacy of a single dose of ketamine is almost equivalent to that of chronic SSRI in social defeat paradigm wherein acute SSRI is ineffective (Donahue et al., 2014). Considering that antidepressant-like effect of ketamine is attenuated by depletion of serotonin or by inhibiting ketamine-induced prefrontal serotonin release (Gigliucci et al., 2013; Fukumoto et al., 2014), it is possible that increasing the serotonergic neuronal firing is important for achieving an antidepressive effect.
Furthermore, we found that the release of serotonin in the mPFC, induced by perfusion of the PPTg with ketamine, is significantly attenuated by injection of NBQX to the DRN. These results indicate that enhancement of excitatory projection to DRN underlies the effect of ketamine. A previous report demonstrated that optogenetic stimulation of the excitatory afferents from mPFC to DRN leads to the activation of serotonergic neurons of the DRN (Challis et al., 2014). On the other hand, the same stimulation also leads to the activation of GABAergic neurons of the DRN, which, in turn, inhibit serotonergic neurons (Challis et al., 2014). These observations indicate that these excitatory projections exert a bidirectional effect on serotonergic activity. Therefore, it is possible that the cholinergic afferents from PPTg to DRN preferentially enhance the excitatory afferents from mPFC leading to the activation of serotonergic neurons, although further histological and electrophysiological analyses would be required.
Our findings suggest that the DRN-projecting cholinergic neurons in the PPTg and α4β2 nAChR in the DRN play a key role in the effect of ketamine, but the role of cholinergic neurons and α4β2 nAChR in the depressive behavior is controversial. For example, acute stimulation of the α4β2 nAChR produces antidepressant-like responses in the learned helplessness model and the forced swim test (Ferguson et al., 2000), while the α4β2 nAChR antagonists also elicit antidepressant-like effects in forced swim test and tail suspension test (Andreasen et al., 2009). α4β2 nAChRs are widely expressed in many brain regions associated with emotional behaviors, including basal ganglia, striatum, amygdala, ventral tegmental area, locus coeruleus, and DRN (Philip et al., 2010). Because drugs are systemically administered in these previous reports, it is unclear which cholinergic neuronal circuit is important for antidepressant-like effects induced by respective drugs. Although further behavioral analyses are needed, PPTg-DRN cholinergic neurons may play a key role in the antidepressive effect of ketamine.
In conclusion, the present study revealed that ketamine increases serotonin release in the mPFC through directly acting on PPTg, and that α4β2 nAChR in the DRN mediates this effect. The present findings highlight the importance of the DRN-projecting cholinergic neurons in PPTg in the effect of ketamine, which may contribute to rapid antidepressive effect of ketamine.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (to K.N. 16K15125, H.S. 17K19486, T.N. 17H04008, and S.K. 16H05091), and research grants from the Nakatani Foundation for advancement of measuring technologies in biomedical engineering and from Takeda Science Foundation (to K.N.).
Statement of Interest
None.
References
- Amargós-Bosch M, López-Gil X, Artigas F, Adell A(2006)Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int J Neuropsychopharmacol 9:565–573. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP(2009)Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol 23:797–804. [DOI] [PubMed] [Google Scholar]
- Asaoka N, Nishitani N, Kinoshita H, Kawai H, Shibui N, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S(2017)Chronic antidepressant potentiates spontaneous activity of dorsal raphe serotonergic neurons by decreasing GABAB receptor-mediated inhibition of L-type calcium channels. Sci Rep 7:13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH, Agam G(2008)Major depressive disorder. N Engl J Med 358:55–68. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH(2000)Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ(2006)Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. [DOI] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O(2013)Raphe gabaergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci 33:13978–88, 13988a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Beck SG, Berton O(2014)Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front Behav Neurosci 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA Jr(2014)Effects of striatal δfosb overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry 76:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F(2000)Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology (Berl) 152:295–303. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE(1995)Gabaergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol 363:177–196. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S(2014)Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 231:2291–2298. [DOI] [PubMed] [Google Scholar]
- Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduño J, Frías-Dominguez C, Drucker-Colin R, Mihailescu S(2008)Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse 62:601–615. [DOI] [PubMed] [Google Scholar]
- Garduño J, Galindo-Charles L, Jiménez-Rodríguez J, Galarraga E, Tapia D, Mihailescu S, Hernandez-Lopez S(2012)Presynaptic α4β2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. J Neurosci 32:15148–15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A(2013)Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228:157–166. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B(2007)NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J.(2016)Structural and functional considerations of the cholinergic brainstem. J Neural Transm (Vienna) 123:731–736. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D(1997)Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S(2014)Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 17:1321–1326. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME(1984)Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull 13:709–726. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C(2007)The rat brain in stereotaxic coordinates, 6th ed. Waltham, MA: Academic Press. [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Price LH(2010)Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology (Berl) 212:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M(2006)Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M(2009)Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and gabaergic neurons in the rat. Eur J Neurosci 29:340–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL(1989)Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull 23:519–540. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK(2006)A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.