Abstract
Background and Aims
Knowledge of thermal acclimation of physiological processes of boreal tree species is necessary to determine their ability to adapt to predicted global warming and reduce the uncertainty around the anticipated feedbacks of forest ecosystems and global carbon cycle to climate change. The objective of this work was to examine the extent of thermal acclimation of net photosynthesis (An) and dark respiration (Rd) of two distant white spruce (Picea glauca) seed sources (from south and north of the commerial forest zone in Québec) in response to latitudinal and seasonal variations in growing conditions.
Methods
The temperature responses of An, its biochemical and biophysical limitations, and Rd were measured in 1-year-old needles of seedlings from the seed sources growing in eight forest plantations along a regional thermal gradient of 5.5 °C in Québec, Canada.
Key Results
The average optimum temperature (Topt) for An was 19 ± 1.2 °C and was similar among seed sources and plantation sites along the thermal gradient. Net photosynthesis at Topt (Aopt) varied significantly among plantation sites and was quadratically related to the mean July temperature (MJT) of plantation sites. Topt for mesophyll conductance, maximum electron transport rate and maximum rate of carboxylation were 28, 22 and 30 °C, respectively. Basal respiration rate (Rd at 10 °C) was linearly and negatively associated with MJT. Q10 of Rd (the rate of change in Rd with a 10 °C increase in temperature) did not show any significant relationship with MJT and averaged 1.5 ± 0.1. The two seed sources were similar in their thermal responses to latitudinal and seasonal variations in growing conditions.
Conclusions
The results showed moderate thermal acclimation of respiration and no evidence for thermal acclimation of photosynthesis or local genetic adaptation for traits related to thermal acclimation. Therefore, growth of local white spruces may decline in future climates.
Keywords: Picea glauca, thermal acclimation, climate change, photosynthesis, respiration, assisted migration, local adaptation, temperature, maximum rate of carboxylation, maximum electron transport rate, mesophyll conductance
INTRODUCTION
In the large boreal forest of Canada, global warming should be leading to an increase in average daily temperature of at least 2 °C (scenario B1) by 2050 (IPCC, 2014) and to rises in the frequency and duration of summertime episodes of extreme heat waves and drought (Romero-Lankao et al., 2014). It is unclear today whether changes in average climatic factors or extreme events will be the main drivers of species responses to climate change. Downregulation of net CO2 uptake as a result of global warming may reduce species fitness and ecosystem feedback on the global carbon cycle (Sage et al., 2008). It is important to understand how physiological processes involved in photosynthetic and respiration rates will respond to future climatic regimes in order to (1) accurately predict climate change effect on carbon uptake at different scales (Way and Yamori, 2014), (2) reduce the uncertainty around the anticipated feedbacks of forest ecosystems, and global carbon cycle to climate change (Niu et al., 2012; Girardin et al., 2016) and (3) determine the intrinsic acclimation and genetic abilities of tree species to adapt to climate change in the short and longer terms (Bigras, 2000; Way and Sage, 2008b; Gunderson et al., 2010). Thermal acclimation of both dark respiration (Rd) and net photosynthetic rate (An) through biochemical, biophysical and structural adjustments may help plants to maintain a positive carbon balance in warming conditions (Medlyn et al., 2002; Atkin et al., 2005; Sage et al., 2008; Way and Yamori, 2014). However, the extent to which thermal acclimation may help boreal conifer species to cope with global warming remains poorly understood. Currently, few reports show a lack, or very limited thermal acclimation, of An for boreal tree species (Way and Sage, 2008a, b; Dillaway and Kruger, 2010; Ow et al., 2010; Silim et al., 2010; Zhang et al., 2015). Conversely, it has been reported that boreal tree species may show a moderate to strong thermal acclimation of Rd in response to experimental warming (Gunderson et al., 2000; Ow et al., 2008; Way and Sage, 2008b; Silim et al., 2010; Zhang et al., 2015; Reich et al., 2016), or following a thermal latitudinal gradient (Tjoelker et al., 2009; Dillaway and Kruger, 2011).
Thermal acclimation of CO2 exchange has been mostly investigated in controlled conditions using static day–night temperature treatments or from seasonal variation in CO2 exchange in response to the seasonal courses of temperature (Hikosaka et al., 2006; Way and Yamori, 2014; Yamori et al., 2014). Although these studies helped increase our understanding of the processes involved in thermal acclimation, their results cannot realistically be used to infer thermal responses in natural conditions, and consequently to better evaluate the quantitative aspects of tree responses to global warming. The main reason for this is the lack of representation of diurnal and daily temperature variations during the growing season, which are especially important in boreal regions. In addition, it is unclear whether or not thermal acclimation of An to temporal variation in temperature during the growing season and spatial variation in temperature along a climate gradient may result from similar physiological adjustments. In fact, the predominant role of photoperiod in the regulation of the seasonal pattern of photosynthetic rate, also known as the phenology of photosynthesis, is still largely controversial for tree species (Busch et al., 2007; Bauerle et al., 2012; Stinziano and Way, 2017).
Thermal acclimation of both An and Rdvaries widely among tree species depending on their thermal environment (Aitken et al., 2008; Dillaway and Kruger, 2010; Way and Yamori, 2014; Reich et al., 2016). Thermal acclimation of An can improve or at least maintain plant photosynthetic performance when the growth temperature regime shifts from cold to warm through adjustments of one or more photosynthetic components (Way and Yamori, 2014). This may occur via (1) the shift in the thermal optimum of An (Topt) towards the warm growing temperature, (2) the increase or maintenance of the photosynthetic rate at Topt (Aopt) in the new growing temperature conditions, (3) the shift in both Aopt and Topt (Way and Yamori, 2014), or (4) the increase or maintenance of the photosynthetic rate with respect to growth temperature (Agrowth). The mechanisms involved in thermal acclimation of photosynthesis include adjustment in (1) thermal responses of both maximum rate of carboxylation (Vcmax) and maximum electron transport rate (Jmax) (activation and deactivation energy), (2) basal Vcmax measured at a reference temperature of 25 °C (Vcmax25) and Jmax25, (3) the ratio of Jmax25 to Vcmax25, and (4) thermal responses of mesophyll (gm) and stomatal conductance (gs) (Kattge and Knorr, 2007; Warren, 2008; Silim et al., 2010; Way and Yamori, 2014).
Thermal acclimation of Rd in response to the increase in temperature may occur via (i) a downregulation of the basal rate of Rd (so-called type II acclimation), (2) a decrease in Q10 (rise in Rd with a 10 °C increase in temperature) (type I acclimation), or (3) a combination of both types (Atkin and Tjoelker, 2003; Atkin et al., 2005; Way and Yamori, 2014; Reich et al., 2016).
Mineral nutrition is one of several physiological attributes that contribute to the improvement of survival, growth and physiology of tree seedlings after outplanting (Margolis and Brand, 1990). Several studies showed that net photosynthesis, dark respiration and survival were closely related to nitrogen levels in needles (Lamhamedi and Bernier, 1994; Tjoelker et al., 1999; Poorter et al., 2009). Needle nitrogen concentration (Nmass) varies with site climatic conditions, including temperature (Friend et al., 1989). However, little is known about the role of Nmass in thermal acclimation of An and Rd. For instance, Tjoelker et al. (2009) showed that both type I and II acclimation of Rd were unrelated to Nmass in Pinus banksiana.
Clinal variation in growth and other functional traits as a result of local genetic adaptation to climate of origin has been reported for several boreal tree species (Li et al., 1997; Andalo et al., 2005; Aitken et al., 2008; Benomar et al., 2016). However, little evidence exists regarding the genetic differentiation in the thermal acclimation capacity and its involvement in growth clinal variation. Recently, Drake et al. (2017) found similar thermal responses (both An and Rd) in response to an experimental warming (under controlled conditions) of three seed sources of Eucalyptus tereticornis despite a large geographical distance among them and a 13 °C difference in mean annual temperature at seed origin. Similar results were reported for Pinus banksiana (Tjoelker et al., 2009). In contrast, Ishikawa et al. (2007) showed intraspecific variation in thermal acclimation of An among Plantago asiatica populations, which was related to the capacity of adjustement of the Jmax25 to Vcmax25 ratio.
White spruce (Picea glauca) is one of the ecologically and commercially most important conifer species of the boreal forest of Canada (Beaulieu et al., 2009). It is the subject of large reforestation efforts and several intensive breeding programmes in Canada (Mullin et al., 2011). It has been shown not to be optimally adapted to local climate conditions in relation to recent temperature warming (e.g. Andalo et al., 2005). But to our knowledge, no one has investigated the temperature response of both photosynthesis and respiration to determine the thermal acclimation capacity and potential adaptive differences among seed sources from geographically distant regions, which could affect the productivity of forest ecosystems and assisted migration strategies. The objectives of this study were (1) to evaluate the thermal acclimation of photosynthesis and respiration of two geographically distant white spruce seed sources in response to short-term variation in climatic conditions during a growing season and to long-term variation in growing conditions along a regional thermal gradient of 5.5 °C, and (2) to assess the involvement of morphological, biochemical and biophysical processes in the temperature response of photosynthesis.
MATERIALS AND METHODS
Genetic material and planting sites
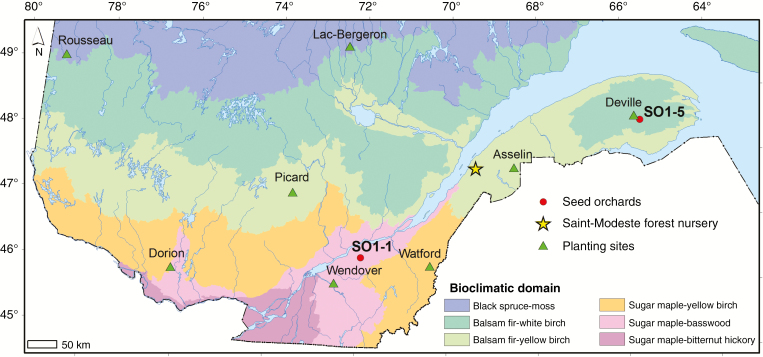
The two white spruce seed sources used in this study were chosen to represent the south and north of the large commercial forest zone in Québec. They came from two first-generation seed orchards (SO1-1 and SO1-5) commonly used for reforestation in Québec, Canada (Fig. 1), distant from each other by 550 km and 2.2° of latitude (Table 1). They are clonal seed orchards made of grafted plus-trees that were selected in local natural stands. They each pertain to one of the two broadly defined white spruce breeding zones in Québec, Canada, which differ mostly in latitude and associated mean annual temperature (Li et al., 1997). Orchard SO1-1 represents the southern seed source and SO1-5 the northern seed source (Fig. 1). Open-pollinated seeds were collected in each seed orchard for two consecutive years (2008 and 2009) and mixed, making up one seedlot per seed orchard. Seedling production was conducted under nursery conditions at the Pépinière forestière of Saint-Modeste (Québec, Canada, 47°50′ N, 69°30′ W) and subjected to standard cultural practices during two consecutive growing seasons (Lamhamedi et al., 2006; Villeneuve et al., 2016).
Fig. 1.
Location of the eight forest plantation sites and the two white spruce seed sources tested in the study. SO1-1, southern seed source; SO1-5, northern seed source.
Table 1.
Location and climatic conditions of the two white spruce seed sources used in this study and the eight forest plantation sites
| Location | Bioclimatic domain* | Latitude | Longitude | Elevation | GDD5 | MAT | MJT | MGST | TGSP | |
|---|---|---|---|---|---|---|---|---|---|---|
| (°N) | (°W) | (m) | (°C) | (°C) | (°C) | (mm) | ||||
| Seed source | ||||||||||
| Northern (SO1-5) | Robidoux | BF-WB | 48.55 | 65.59 | 270 | 1298 | 2.6 | 16.7 | 12.9 | 500 |
| Southern (SO1-1) | St-Cyrille | SM-B | 46.39 | 71.94 | 116 | 1708 | 4.2 | 18.7 | 14.9 | 539 |
| Plantation site† | ||||||||||
| Lac Bergeron | Dolbeau | S-M | 49.65 | 72.41 | 434 | 1347 | 0.3 | 16.3 | 13.8 | 520 |
| Rousseau | Villebois | S-M | 49.15 | 79.20 | 291 | 1516 | 1.4 | 17.3 | 14.9 | 433 |
| Deville | Robidoux | BF-WB | 48.62 | 65.72 | 530 | 1179 | 1.5 | 16.9 | 12.4 | 536 |
| Asselin | Squatec | BF-YB | 47.84 | 68.52 | 370 | 1500 | 2.5 | 18.7 | 14.0 | 564 |
| Picard | La Tuque | BF-YB | 47.34 | 73.57 | 460 | 1397 | 1.5 | 16.2 | 14.4 | 488 |
| Watford | Ste-Rose | SM-YB | 46.30 | 70.40 | 385 | 1644 | 3.8 | 18.6 | 13.9 | 690 |
| Dorion | Maniwaki | SM-B | 46.05 | 76.26 | 201 | 1933 | 4.8 | 19.3 | 16.6 | 465 |
| Wendover | St-Cyrille | SM-B | 45.98 | 72.52 | 68 | 2153 | 5.8 | 20.4 | 15.9 | 650 |
GDD5, number of growing degree-days >5 °C; MAT, mean annual temperature; MJT, mean July temperature; MGST, mean growing season temperature; TGSP, total growing season precipitation.
*SM-B, sugar maple–basswood domain; SM-YB, sugar maple–yellow birch domain; BF-YB, balsam fir–yellow birch domain; BF-WB, balsam fir–white birch domain; S-M, spruce–moss domain.
†Data for plantation sites are means for years corresponding to the first and second growing seasons.
Eight forest sites representative of the white spruce commercial zone in Québec, Canada, were selected for this study (Table 1, Fig. 1). They cover latitudinal and longitudinal transects of 4.6° and 14°, respectively. Plantations were established in the spring of 2013 for the localities of Watford, Asselin and Deville, in the spring of 2014 for the localities of Wendover, Picard and Lac Bergeron, and in the spring of 2015 for the localities of Dorion and Rousseau. The Watford and Wendover sites were formerly occupied by black spruce (Picea mariana) plantations that were harvested in 2012. All other sites, covered by natural forest stands, were also harvested in 2012. Physico-chemical soil properties of plantation sites assessed during the second growing season after plantation are provided in Supplementary Data Table S1.
Two-year-old seedlings were planted at densities of 2000 stems per hectare. Seedling height and root collar diameter (mean ± s.d.) before planting were 38.3 ± 3.6 and 6.9 ± 0.9 mm respectively. Before planting, seedling nutrient concentrations (stem, needles and roots) were assessed using three composite samples (five seedlings/composite sample) per seed orchard (data not shown). The results confirmed that the seedlings met the 28 morphophysiological standards used for white spruce containerized seedling production in Québec (Veilleux et al., 2010).
Seedlings were planted at each site following a randomized complete block design with four blocks. Each seed source was randomly assigned to a plot within a block. The size of each plot was about 730 m2 and contained 144 trees (12 × 12 rows of trees) in which only the 64 interior trees were considered for analyses, leaving 4 × 4 rows of border trees as a buffer zone. The total number of planted seedlings was 9216, corresponding to 144 seedlings × 4 blocks × 2 seed sources × 8 sites.
During the first and second growing seasons after plantation in the field, weed competition was controlled mechanically at all sites, except for Deville and Lac Bergeron, where weedy vegetation was scarce.
Climatic data along the latitudinal gradient
For each plantation site, climatic data (Table 1) were interpolated from data collected from 2013–16 in nearby weather stations using the BioSIM software (Régnière and St-Amant, 2007). Furthermore, permanent weather stations were installed in the Watford, Asselin and Deville sites since 2013, with collected data used to confirm the accuracy of BioSIM data. Climate conditions varied considerably among the sites during the two years of the experiment (Table 1). Mean annual temperature (MAT) was highly influenced by site location. It differed by 5.5 °C between the coolest and the warmest sites. Total growing season precipitation (TGSP) decreased with the longitude of the plantation sites (Table 1).
Natural climatic gradients usually consider geographical and environmental factors such as latitude, longitude, altitude, annual and seasonal mean temperature and precipitation. In the present study, the term ‘climatic gradient’ is used as a simplifier and refers to the annual and seasonal mean temperature gradient, given that the focus of our study was mostly related to change in temperature regime, which was the most variable climatic factor among the plantation sites (Table 1).
Growth
Seedling height (H2) and survival were measured at the end of the second growing season on plantation sites (mid-October) on the 64 central trees in each plot, for a total of 4096 trees (64 plants × 4 blocks × 2 seed source × 8 sites).
Gas exchange measurements
Gas exchange measurements were made with two portable open-path gas-exchange systems (Li-6400, Li-Cor, Lincoln, NE, USA), equipped with a Lighted Conifer Chamber (6400-22L, Li-Cor, Lincoln, NE, USA) and the expanded Temperature Control Kit (6400-88, Li-Cor, Lincoln, NE, USA). Temperature (T) response curves of shoot respiration (Rd–T) and net photosynthesis (An–T) were generated for one randomly selected plant per plot at each plantation site (2 seed sources × 3 blocks × 8 sites = 48). Both An–T and Rd–T curves were assessed using 1-year-old needles of the uppermost lateral shoot. The measurements were carried out in June 2015 in the plantations of Watford, Asselin, Deville, Wendover and Lac Bergeron, and in June 2016 in the plantations of Watford, Dorion, Picard and Rousseau. At the Watford, Asselin, Deville and Picard sites, plants were at their third growing season, whereas those at the remaining sites were at their second growing season. The results obtained in Watford during the third and fourth growing seasons were quite similar for thermal acclimation-related traits, which suggested the absence of an age effect on the observed patterns.
The expanded temperature control kit, which contains two water jackets through which water is circulated using a submersible pump, was used to make measurements at low temperatures (10 and 15 °C). The water channels were connected to a water bath and the temperature was controlled by adding ice water. For temperatures above 25 °C, the seedling and the entire gas-exchange system were covered by a plastic tent (hand-made closed chamber using transparent plastic and wood pickets, and measuring 1 m × 1 m 1.5 m). The temperature within the plastic tent was brought to the desired temperature, i.e. between 30 and 40 °C, using a portable 1500 W ceramic heater (CZ448, Comfort Zone, Pottsville, PA, USA). This made it possible to maintain the difference between the ambient air and that within the conifer chamber below 2 °C, and to prevent water condensation in the exhaust tube or in the cuvette. In fact, because of the large volume of the conifer chambers, there is an important issue of water condensation when the temperature in the chamber is warmer than that of the entering air.
Cuvette temperature during measurement varied systematically from low (10 °C) to high (40 °C) by 5 °C increments, and measurements were taken systematically in order from low to high temperature. Seedlings were allowed to acclimate for at least 20 min for each new temperature, before recording data. The attachment points of the shoot to the cuvette walls were taped (adhesive putty) to avoid leaks into and out of the cuvette. Photosynthesis (An) was measured under saturating photosynthetically active radiation (PAR = 1000 µmol m−2 s−1) and at 400 µmol mol−1 of CO2. Following An measurement, the light source was turned off and Rd was recorded after at least 15 min of darkness. The vapor pressure deficit (VPD) in the conifer chamber ranged from 0.6 to 3.6 kPa. During measurement, the entering air passed through the drierite column (anhydrous calcium sulphate) to maintain the air humidity (RH) below 75 % at the lower temperature (10 °C). At higher temperatures, a minimum RH of 45 % was maintained by adding water vapour to the air inside the plastic chamber. For each sample, data required to build An–T and Rd–T curves were collected generally within 4–5 h.
Seasonal patterns of Rd–T and An–T were determined at the Watford site during the growing season of 2016. The measurements were taken each month from May to October. The measurements were performed on the same shoot from six seedlings that were different from those used previously for A–Ci–T (see below) and those measured in 2015. For practical reasons, we chose the tallest seedlings (with a long shoot) within each subplot. At the end of measurements, in October, the shoots did not show any visible damage and loss of initial leaf area.
CO2 response of net photosynthesis at different temperatures (A–Ci–T)
During the active growing season, A–Ci response curves at temperature 10, 15, 20, 25, 30, 35 and 40 °C were generated in July 2015 at the Watford and Deville plantation sites (respectively the easiest to access among southern and northern sites) and again in July 2016 at the Watford plantation site. The A–Ci response curve measurements were taken after 20 min of steady-state conditions at the ambient atmospheric CO2 partial pressure (Ca = 400 µmol mol−1) and at saturated photosynthetic active radiation (PAR = 1000 µmol m−2 s−1). Thereafter, for a given temperature, the reference CO2 (Ca) was changed in the following order: 400, 350, 300, 250, 200, 100, 50, 400, 500, 600, 700, 800, 1000, 1200, 1400 and 1500 µmol mol−1. Values were recorded based on the stability of photosynthesis, stomatal conductance, CO2 and water vapour concentrations. For each foliage sample, data collections to build the A–Ci–T curves were completed within two or three consecutive days. Most of the A–Ci curves at 35 and 40 °C measured in 2015 at the Watford and Deville sites failed to converge and estimates could not be obtained. All measured gas exchange were corrected based on the measured projected needles area (see below).
Estimation of gas exchange parameters
The photosynthetic parameters (Vcmax, Jmax and gm) were estimated simultaneously by fitting the A–Ci curves with the non-rectangular hyperbola version of the biochemical model of C3 (Farquhar et al., 1980) following Ethier and Livingston (2004). This method is based on the principle that mesophyll conductance (gm) is not infinite, which reduces the curvature of the A–Ci curve. The net assimilation rate (An) is given by:
| (1) |
with
| (2) |
| (3) |
| (4) |
| (5) |
The combination of eqns (2) and (4) results in a quadratic equation for the Rubisco-limited net photosynthetic rate (Ac), whose solution is the following positive root:
| (6) |
where
The combination of eqns (3) and (4) results in a quadratic equation for the ribulose bisphosphate (RuBP) regeneration-limited net assimilation rate (Aj), whose solution is the following positive root:
| (7) |
where
where Vcmax is the maximum rate of carboxylation (µmol CO2 m−2 s−1), O is the partial atmospheric pressure of O2 (mmol mol−1),Γ* is the CO2 compensation point in the absence of mitochondrial respiration, Rday is mitochondrial respiration in the light (µmol CO2 m−2 s−1), Ci is the intercellular concentration of CO2 (µmol mol−1), Cc is the chloroplastic concentration of CO2 (µmol mol−1), Kc (µmol mol−1) and Ko (mmol mol−1) are the Michaelis–Menten constants of Rubisco for CO2 and O2, respectively, J is the rate of electron transport (µmol CO2 m−2 s−1), Jmax is the maximum rate of electron transport (µmol CO2 m−2 s−1), Q is the incident PAR (µmol m−2 s−1), α is the quantum efficiency, which represents the initial slope of the photosynthetic light response curve, and gm is mesophyll conductance (mol CO2 m−2 s−1).
The model was fitted using non-linear regression techniques (Proc NLIN, SAS). To fit the model, the measured dark respiration (Rd) values were used as proxy for Rday in order to reduce the number of parameters estimated by the model. Respiration occurring in daylight (Rday), which is assumed to be primarily mitochondrial respiration, was assumed to approximate dark respiration (Rd), as observed for black spruce by Way and Sage (2008b). The values at 25 °C used for Kc and Ko were 272 µmol mol−1 and 166 mmol mol−1, respectively (Sharkey et al., 2007), and its temperature dependency is given by:
| (8) |
where c is a scaling constant, Ha (kJ mol−1), is the activation energy and R is the universal gas constant. The scaling constant (c) values are 35.98, 12.37 and 11.18 for Kc, Ko and Γ*, respectively. The values of Ha are 80.99, 23.72 and 24.46 for Kc, Ko and Γ*, respectively (Sharkey et al., 2007).
Characterization of the temperature responses of gas exchange parameters
Individual photosynthesis temperature response curves (An–T) were fitted with a quadratic model (eqn 9) as described by Battaglia et al. (1996):
| (9) |
where An(T) is the photosynthetic rate at temperature T in °C, Aopt is the photosynthetic rate at the temperature optimum (Topt) and the parameter b describes the curvature of the parabola.
Dark respiration temperature response curves (Rd–T) were analysed using eqn (10) to estimate Q10, which is the change in respiration with a 10 °C increase in temperature, following Atkin et al. (2005):
| (10) |
where Rd (T) is the dark respiration rate at temperature T in °C and Rd10 is the measured rate of Rd at the reference temperature of 10 °C.
Both Jmax–T and Vcmax–T data showed a deactivation at high temperatures. Thereafter, the response of Vcmax and Jmax to needle temperature were fitted using a modified Arrhenius function (the peaked model) (Johnson et al., 1942) following Medlyn et al. (2002):
| (11) |
where K(Tk) is the Vcmax or Jmax at temperature Tk which is the leaf temperature in Kelvin, K25 is the value of Vcmax or Jmax at Tref = 25 °C, R is the universal gas constant (8.314 J mol−1 K−1), Ha (kJ mol−1) is the activation energy, Hd (kJ mol−1) is the energy of deactivation and ΔS (J mol−1) is an entropy term.
The Topt for Vcmax and Jmax was calculated as:
| (12) |
The model was fitted using non-linear regression techniques (Proc NLIN, SAS). The value of Hd was fixed to 200 kJmol−1 according to Medlyn et al. (2002) in order to reduce the number of parameters estimated by the model. However, the model underestimates the value of Topt. Consequently, we first estimated Topt using eqn (9) and then used the obtained value to solve for Ha and ΔS using eqns (11) and (12) simultaneously.
Mesophyll conductance was modelled according to the empirical model proposed by Warren and Dreyer (2006):
| (13) |
where gm is the mesophyll conductance, gopt is the value of gm at Topt and b is a scaling factor.
Quantitative limitation analysis
Rubisco-limited (Ac) and RuBP regeneration-limited (Aj) net photosynthetic rates (An) were calculated at temperature ranging from 10 to 40 °C using eqns (2)–(5). Values of Vcmax and J were estimated from fitted parameters in our study. Rday was assumed to approximate Rd (see above).
Needle nitrogen concentrations (Nmass) and specific leaf area
Following the measurements of gas exchange, the shoots were carefully removed from the cuvette, harvested, placed in plastic bags and refrigerated (−20 °C). Projected needle area was measured using WinSeedle (Version 2007 Pro, Regent Instruments, Québec, Canada). Samples were then oven-dried for 72 h at 56 °C and their dry mass was determined. Specific leaf area (SLA) was calculated as the ratio of projected needle area (cm2) to needle dry mass (g). Dried needles were ground to a fine powder in a ball mill. Needle nitrogen concentration (Nmass, mg g−1) was determined using a LECO elemental analyser (LECO Corporation, St Joseph, MI, USA). Nitrogen on a projected area basis (Narea) was calculated as Nmass divided by SLA.
Statistical analyses
All analyses were conducted with SAS/STAT software version 9.4 (SAS Institute, Cary, NC, USA). Response variables were analysed separately using a general linear mixed model with the effects of site and seed source (seed orchard) and their interaction considered as fixed effects, while block was treated as a random effect. Data were transformed whenever required to satisfy normality of residuals and homoscedasticity. Means were compared using the Tukey test; differences were considered significant at P < 0.05. We compared Ha, ΔS and Topt for Vcmax, Jmax and gm between the two seed sources by non-parametric tests using proc NPAR1WAY. Proc REG and proc NLIN were used to examine the relationship between response variables and climate of plantation sites. The lack of A–Ci curves at 35 and 40 °C from measurements at the Watford and Deville sites in 2015 made it difficult to examine the site effect on Ha and Topt of Vcmax and Jmax. To overcome this problem, we used a repeated-measures analysis of variance (ANOVA) with temperature from 10 to 30 °C as a repeated measure factor.
RESULTS
Growth
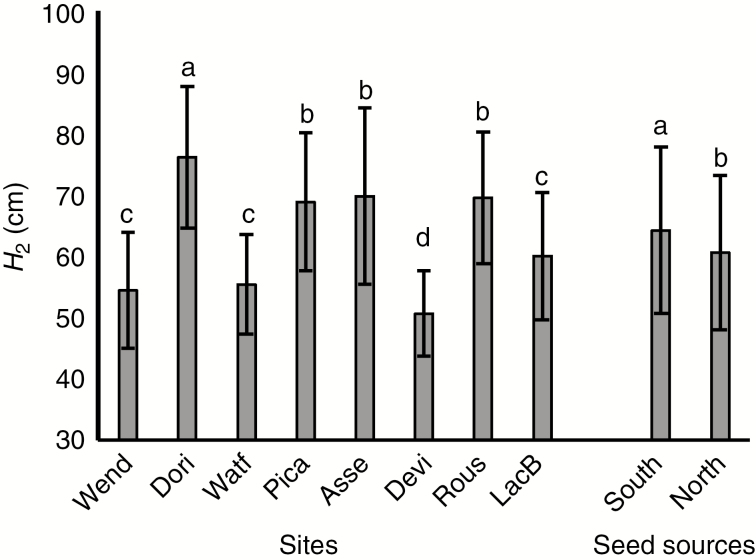
Total height growth after two growing seasons (H2) was affected by both site and seed source effects (Table 2). However, the interaction between the two factors was not significant, suggesting that the two seed sources showed similar patterns of growth in response to changes in growing conditions along the climatic gradient (Table 2). The highest and lowest average of H2 were observed at the Dorion (76 ± 11 cm) and Deville (51 ± 7 cm) sites, respectively (Fig. 2). Seedlings from the southern seed source were significantly taller than those from the northern seed source (Fig. 2). No statistically significant relationships could be found between H2 and prevailing climatic and soil conditions at the plantation sites (Supplementary Data Fig. S1).
Table 2.
Analysis of variance of height growth after two growing seasons (H2) and thermal acclimation-related traits
| Site | Seed source | Site × seed source | |||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P value | d.f. | F | P value | d.f. | F | P value | |
| H 2 | 7 | 84.57 | <0.001 | 1 | 32.76 | <0.001 | 7 | 1.34 | 0.25 |
| T opt (An) | 6 | 1.61 | 0.19 | 1 | 0.22 | 0.64 | 6 | 1.43 | 0.24 |
| A opt (An) | 6 | 6.11 | <.001 | 1 | 3.91 | 0.06 | 6 | 2.46 | 0.06 |
| T opt (Ag) | 6 | 1.03 | 0.43 | 1 | 1.65 | 0.21 | 6 | 1.86 | 0.13 |
| g s_25 | 6 | 6.6 | <0.001 | 1 | 1.19 | 0.29 | 6 | 1.27 | 0.31 |
| Q 10 | 7 | 3.45 | <0.001 | 1 | 0.26 | 0.61 | 6 | 1.21 | 0.32 |
| R d10 | 7 | 6.21 | <0.001 | 1 | 1.09 | 0.31 | 6 | 0.94 | 0.48 |
| N mass | 7 | 2.93 | 0.01 | 1 | 0.2 | 0.65 | 6 | 1.22 | 0.32 |
| SLA | 7 | 2.22 | 0.06 | 1 | 2.85 | 0.10 | 7 | 0.51 | 0.79 |
H 2 (cm), height growth after two growing seasons; Topt (°C), optimal temperature for net (An) and gross (Ag) photosynthetic rate; Aopt (µmol m−2 s−1), photosynthetic rate at Topt. gs_25, stomatal conductance at a reference temperature of 25°C; Q10, rate of change in dark respiration with a 10 °C increase in temperature; Rd10 (µmol m−2 s−1), basal rate of dark respiration (at 10 °C); Nmass, needle nitrogen concentration (mg g−1); SLA, specific leaf area (cm2 g−1).
Significant effects are indicated in bold.
Fig. 2.
Total height (mean ± s.d.) at the end of the second growing season (H2) of white spruce seedlings from two seed sources grown at eight forest plantation sites. Means having the same letters are not significantly different at α = 0.05. Wend, Wendover; Dori, Dorion; Watf, Watford; Pica, Picard; Asse, Asselin; Devi, Deville; Rous, Rousseau; LacB, Lac Bergeron; South, southern seed source (SO1-1); north, northern seed source (SO1-5).
Latitudinal variation in temperature response of photosynthesis and respiration
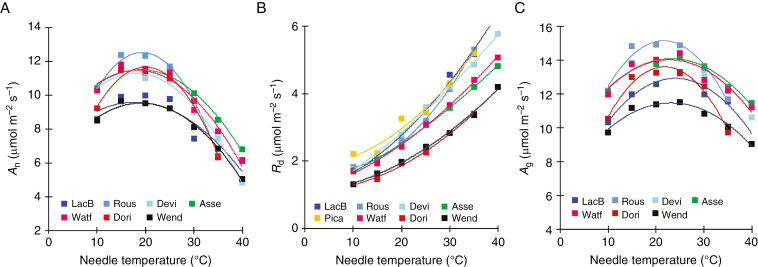
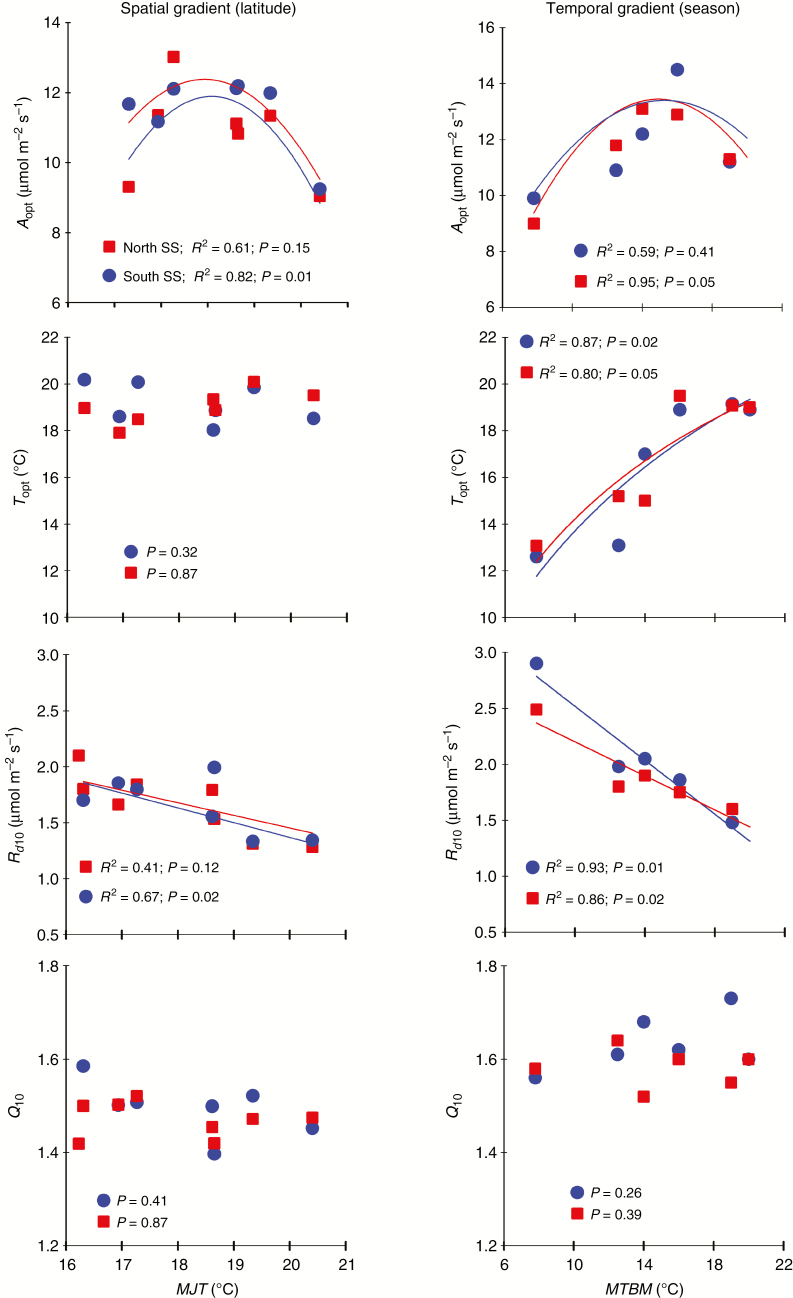
The temperature response curves of both net and gross photosynthesis followed a parabolic shape (Fig. 3). The thermal optimum (Topt) of net photosynthesis (An) averaged 19 ± 1.2 °C and was not variable across plantation sites and seed sources (Tables 2 and 3). Net photosynthetic rate (Aopt) at Topt varied significantly among sites (Table 2), with the lowest value of Aopt occurring at the Wendover and Lac Bergeron sites, which were the warmest and coldest sites, respectively (Table 3). Aopt followed a quadratic relationship with mean July temperature of the plantation site (MJT) for the southern seed source but not for the northern one (Fig. 4). Basal rate of Rd (Rd10) and Q10 showed similar responses among seed sources but differed across plantation sites (Table 2). The lowest mean values of Rd10 were measured at the warmest sites, such as Wendover and Dorion (Table 3), while the highest mean values of Q10 occurred at Lac Bergeron, the coldest plantation site (Table 3). Topt and Q10 of dark respiration were not related to climatic variables at the plantation sites (Fig. 4). Rd10 was negatively and linearly related to MJT for the southern seed source (Fig. 4). The temperature response curve of gross photosynthesis (Ag) showed a shape similar to that of An (Fig. 3). The average Topt of Ag, 22.7 ± 1.3 °C, was not affected by either site or seed source (Table 2).
Fig. 3.
Temperature response curves of (A) net photosynthesis (An), (B) dark respiration (Rd), and (C) gross photosynthesis (Ag) of two geographically distant white spruce seed sources grown in eight plantation sites (n = 6). For site abbreviations see legend of Fig. 2. At Picard (Pica), the measurements were limited to Rd and the northern seed source.
Table 3.
Means (± s.d., n = 6) of thermal acclimation-related traits for photosynthesis and dark respiration of two geographically distant white spruce seed sources established along a latitudinal gradient of eight forest plantation sites
| Plantation site | A opt(µmol m−2 s−1) | T opt(°C) | R d10(µmol m−2 s−1) | Q 10 |
|---|---|---|---|---|
| Lac Bergeron | 10.0 (1.5)b | 19.6 (1.1)a | 1.7 (0.4)a | 1.56 (0.06)a |
| Rousseau | 12.6 (1.5)a | 19.3 (1.1)a | 1.8 (0.5)a | 1.52 (0.10)ab |
| Deville | 11.2 (0.4)a | 18.6 (1.1)a | 1.8 (0.3)a | 1.50 (0.03)b |
| Asselin | 11.5 (1.4)a | 18.9 (0.8)a | 1.7 (0.3)a | 1.42 (0.04)d |
| Picard | – | – | 2.2 (0.6)a* | 1.42 (0.10)d* |
| Dorion | 11.7 (0.9)a | 19.9 (0.5)a | 1.3 (0.3)b | 1.47 (0.10)c |
| Watford | 11.6 (1.4)a | 18.7 (1.3)a | 1.7 (0.4)a | 1.45 (0.05)cd |
| Wendover | 9.6 (1.4)b | 19.1 (1.6)a | 1.3 (0.5)b | 1.48 (0.09)c |
T opt, optimal temperature for net (An) photosynthetic rate; Aopt, photosynthetic rate at Topt; Q10, rate of change in dark respiration with a 10 °C increase in temperature; Rd10, basal rate of dark respiration (at 10 °C).
Within columns, means followed by the same letter do not differ significantly at α = 0.05 based on adjusted Tukey’s tests.
Measurements were carried out during the second growing season in Lac Bergeron, Rousseau, Dorion and Wendover and during the third growing season in other sites.
Plantation sites are ordered from north to south.
*For the Picard site, measurements were limited to the northern seed source.
Fig. 4.
Relationships of thermal acclimation-related traits with climatic conditions along a latitudinal gradient (spatial) and during a growing season (temporal gradient) for two geographically distant white spruce seed sources. MJT, mean July temperature of plantation site during the two growing seasons (1 year before and current year of measurement); MTBM, mean temperature 5 d before measurements; Topt, optimal temperature for net photosynthetic rate; Aopt, photosynthetic rate at Topt; Rd10, basal rate of respiration (T = 10 °C); Q10, rate of change in Rd with a 10 °C increase in temperature. South SS, southern seed source (SO1-1); North SS, northern seed source (SO1-5).
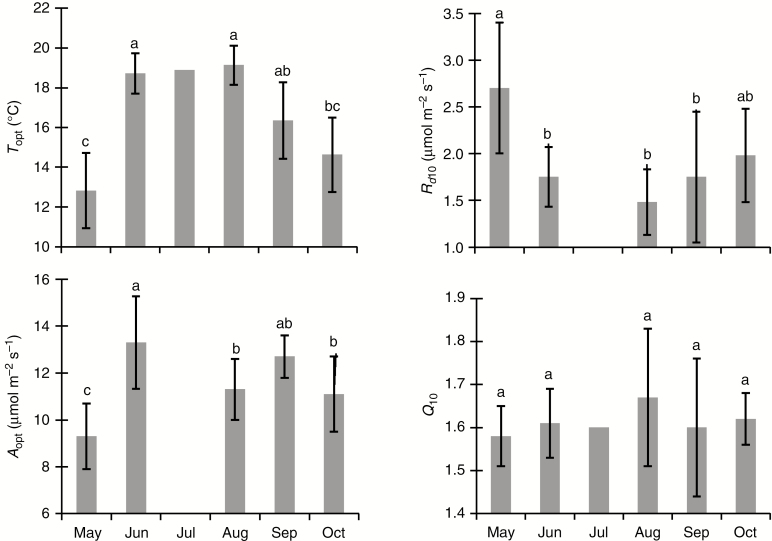
Seasonal variation in temperature response of photosynthesis
Thermal optimum (Topt) and Aopt of An varied greatly during the growing season (from May to October). Topt and Aopt showed no interaction effect of seed source and month during the growing season (Table 4). Mean Topt varied from 12.6 to 19.5 °C and was highest in June and August and lowest in May (Fig. 5). Mean Aopt was higher in June than in May (Fig. 5). Both Aopt for the northern seed source and Topt for both seed sources were correlated with mean temperature 5 d before measurements (Fig. 4). The basal rate of Rd (Rd10) but not Q10 varied greatly during the growing season and neither of them showed any interaction effect of seed source and month (Table 4). Mean Rd10 was higher in May than in the remaining months excluding October (Fig. 5). Rd10 was negatively and linearly related to mean temperature 5 d before measurements (Fig. 4). In contrast, Q10 was not related to mean temperature 5 d before measurements (Fig. 4).
Table 4.
Analysis of variance of thermal acclimation traits during the 2016 growing season in the Watford plantation site
| Month | Seed source | Month × seed source | |||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P value | d.f. | F | P value | d.f. | F | P >value | |
| T opt | 4 | 12.5 | <0.001 | 1 | 0.01 | 0.94 | 4 | 0.62 | 0.65 |
| A opt | 4 | 3.97 | 0.02 | 1 | 0.53 | 0.47 | 4 | 1.47 | 0.25 |
| Q 10 | 4 | 1.02 | 0.42 | 1 | 1.60 | 0.27 | 4 | 1.94 | 0.15 |
| R d10 | 4 | 3.67 | 0.03 | 1 | 0.14 | 0.73 | 4 | 1.19 | 0.35 |
See Table 2 footnote for abbreviations.
Significant effects are indicated in bold.
Fig. 5.
Seasonal patterns of thermal acclimation-related traits of two geographically distant white spruce seed sources during the 2016 growing season in the Watford plantation site. Values are mean ± s.d, n = 6. Values for July are not included in the anlaysis because they are from other seedlings (those used for A–Ci curve measurements). Means having the same letters are not significantly different α = 0.05. For abbreviations see legend of Fig. 4.
Temperature response of Vcmax, Jmax and gm
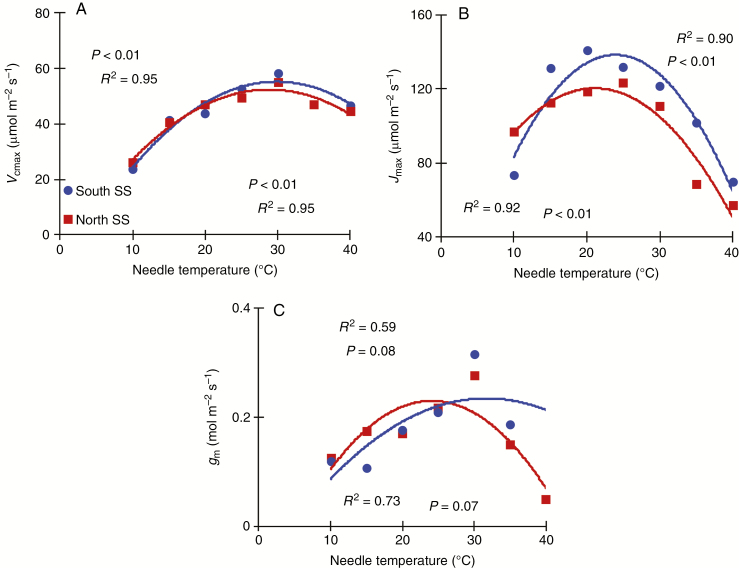
For the 2015 measurements (third growing season), repeated measures ANOVA showed that the temperature response curve of Vcmax and Jmax (for temperatures between 10 and 30 °C) was not different between the southern (Watford) and northern (Deville) plantation sites (Table 5). The Jmax25:Vcmax25 ratio averaged 2.5 ± 0.2 and was also not different between the two plantation sites and not different between the two seed sources. For the measurements conducted only in the Watford plantation site in 2016 (fourth growing season), the temperature response curve of Vcmax, Jmax and gm displayed marked increases with temperature, followed by decreases above Topt (Fig. 6). The optimal temperature (Topt) for Vcmax and Jmax was higher for the southern seed source than for the northern one (Table 6). Topt for gm was similar for the two seed sources and averaged 28 ± 1.1 °C. The activation energy (Ha) for Jmax was greater for the southern seed source than for northern one but this was not the case for Vcmax (Table 6). Also, the entropy term of Vcmax was greater for the northern seed source than for the southern one (Table 6).
Table 5.
Repeated-measures ANOVA of maximal rate of carboxylation (Vcmax) and maximal rate of electron transport (Jmax) in response to needle temperature of two geographically distant white spruce seed sources during the third growing season in Watford and Deville plantation sites (respectively the easiest to access among southern and northern sites)
| V cmax | J max | ||||
|---|---|---|---|---|---|
| Effect | d.f. | F | P value | F | P value |
| Site | 1 | 0 | 0.9858 | 0.77 | 0.4034 |
| Seed source | 1 | 0.16 | 0.7026 | 0.93 | 0.3608 |
| Site × seed source | 1 | 2.28 | 0.1651 | 1.82 | 0.2097 |
| Temperature | 4 | 62.91 | <0.0001 | 16.14 | <0.0001 |
| Site × temperature | 3 | 0.9 | 0.46 | 0.8 | 0.5057 |
| Seed source × temperature | 3 | 0.91 | 0.4787 | 0.22 | 0.9229 |
| Site × seed source × temperature | 3 | 0.67 | 0.5776 | 1.27 | 0.3089 |
Significant effects are indicated in bold.
Fig. 6.
Temperature response curve of (A) the maximum carboxylation capacity of rubisco (Vcmax), (B) the maximum electron transport rate (Jmax) and (C) mesophyll conductance to CO2 (gm) of two geographically distant white spruce seed sources measured in the Watford plantation site in 2016 (fourth growing season) (n = 3). South SS, southern seed source (SO1-1); North SS, northern seed source (SO1-5).
Table 6.
Means (± s.d., n = 3) of physiological parameters related to temperature response of needle photosynthetic capacity and mesophyll conductance of two white spruce seed sources measured during the fourth growing season at the Watford plantation site
| Southern seed source | Northern seed source | ||
|---|---|---|---|
| V cmax | T opt (°C) | 31.2 (4.1)a | 28.1 (0.5)b |
| Value at 25 °C (µmol m−2 s−1) | 52.4 (7.5)a | 52.3 (4.5)a | |
| H a (kJ mol−1) | 32.9 (9.2)a | 26.7 (5)a | |
| ∆S (J K−1 mol−1) | 640.1 (7.1)a | 661 (4.5)b | |
| J max | T opt (°C) | 24.2 (3.2)a | 20.9 (1.1)b |
| Value at 25 °C (µmol m−2 s−1) | 131.1 (24.3)a | 122.8 (7.2)a | |
| H a (kJ mol−1) | 18.4 (10.1)a | 14.6 (6.1)b | |
| ∆S (J K−1 mol−1) | 644.9 (8.9)a | 649.2 (2.2)a | |
| g m | T opt (°C) | 28.4 (1.4)a | 28.7 (1.1)a |
| Value at 25 °C (mol m−2 s−1) | 0.22(0.04)a | 0.21(0.07)b |
V cmax, maximal rate of carboxylation; Jmax, maximal rate of electron transport; gm, mesophyll conductance; Ha, activation energy, ∆S, entropy term, Topt, optimal temperature.
Parameters were derived from eqn (11) for Vcmax and Jmax and from eqn (13) for gm. Within rows, means followed by the same letter do not differ significantly at α = 0.05.
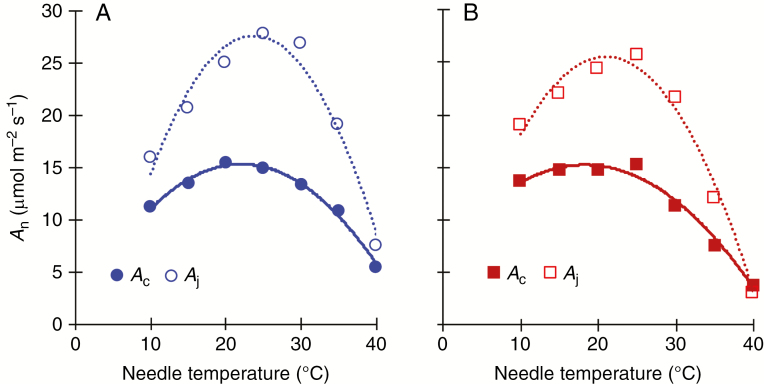
The temperature responses of Rubisco-limited (Ac) and RuBP regeneration-limited (Aj) net photosynthesis (An) were similar among seed sources. Except at 40 °C, Aj was higher than Ac (Fig. 7). Consequently, changes in temperature dependence of photosynthesis were mainly Vcmax-dependent for temperature range between 10 and 40 °C.
Fig. 7.
Biochemical limitations of An in response to needle temperature for (A) the southern seed source and (B) the northern seed source. An was modelled at Ca= 400 ppm by Farquhar modelling using measured gs and estimated gm from A–Ci. The response of An is defined by the minimum value of either Rubisco-limited (Ac) (solid curve) or RuBP regeneration-limited (Aj) net photosynthesis (dashed curve).
Relationships between thermal acclimation-related traits, Nmass and SLA
Specific leaf area was not affected either by site or seed source (Table 2; Supplementary Data Fig. S2). In addition, it was not related to the climate of the plantation sites (MJT) (P ≥ 0.10). Needle nitrogen concentration (Nmass) was similar among seed sources but was affected by sites (Table 2). Similar results were obtained using needle nitrogen content per unit of needle projected area. Mean Nmass was greater in the Asselin, Deville and Picard sites than in the Lac Bergeron, Wendover and Dorion sites (Supplementary Data Fig. S2). Both Rd10 and Aopt were unrelated to leaf nitrogen (Nmass) (Supplementary Data Fig. S3).
DISCUSSION
Under future climate change, temperature will likely remain the most important factor driving boreal forest productivity and species distribution, with potential effects on the composition and structure of natural populations. However, thermal acclimation of physiological processes, particularly photosynthesis, could help mitigate the predicted decrease in net productivity associated with global warming (Way and Yamori, 2014; Girardin et al., 2016). Consequently, a better understanding of acclimation of both photosynthesis (An) and dark respiration (Rd) to warming might provide valuable information to predict the capacity of species to adapt under climate change, and help improve the predictive accuracy of process-based models (Slot et al., 2014; Lombardozzi et al., 2015). Investigations of thermal acclimation of An and Rd in natural conditions are limited compared with those conducted under controlled conditions. Studies conducted along a natural latitudinal gradient should be valuable to investigate growth habit and the thermal acclimation capacity at the intraspecific level, as it takes into account the seasonal fluctuations in temperature (monthly and daily temperature range) as well as the complex interactions with other climatic and non-climatic factors. The present study provides an exhaustive assessment of thermal acclimation of photosynthesis and dark respiration for a conifer under natural conditions, with valuable insights into the temperature responses of photosynthetic capacity attributes (Vcmax and Jmax) and mesophyll conductance under natural conditions. The present results are original in clearly showing a lack of thermal acclimation of net photosynthesis (An) and evidence for type II acclimation of dark respiration (Rd) in response to long-term (2–4 years) variation in temperature along the regional climatic gradient of 5.5 °C for both the southern and northern white spruce seed sources tested.
Boreal tree species, which are well adapted to cold temperature, were often assumed to be limited in their capacity to adapt to warm conditions (Girardin et al., 2016). We showed that the qualitative aspect of thermal acclimation of photosynthesis, such as the ability to shift the thermal optimum (Topt) of An, was lacking. Topt was similar along the climatic gradient and was close to the mean July temperature at southern plantation sites. Our results do not agree with those of previous studies on other species of Picea conducted in controlled conditions, which showed a shift in Topt when the growing temperature was increased by 10 °C (Way and Sage, 2008a; Zhang et al., 2015). Despite the variation in mean temperature (MAT and MJT), a similar range of temperatures (minimum and maximum) along the climatic gradient tested here may explain the unchanging Topt of photosynthesis observed in our study. Increased or constant photosynthetic rate at Topt towards a warmer environment has been widely used as a quantitative proxy of thermal acclimation of photosynthesis (Way and Yamori, 2014). In accordance with results of previous studies on other Picea species (Way and Sage, 2008a; Zhang et al., 2015), we showed the inability of the two seed sources to maintain photosynthetic performance (Aopt) in warmer sites. In addition, the relationship between Aopt and the growing temperature along the gradient followed a quadratic shape, which may suggest adaptation to a narrow climate niche. However, this pattern may also result from complex interaction between soil and climatic conditions (temperature and precipitation) along the climatic gradient tested here rather than temperature per se (Reich and Oleksyn, 2004; Chi et al., 2013; Scafaro et al., 2016). In our study, variation in leaf nitrogen content appeared to play a minor role in photosynthetic adjustments.
In the present study, the temperature response of An was Rubisco-limited (Vcmax) over the entire range of needle temperature (10–40 °C), which follows the common trend observed for cold-adapted species (Kattge and Knorr, 2007; Sage and Kubien, 2007; Sage et al., 2008; Yamori et al., 2010). This result suggests that the observed lack of thermal acclimation potential of white spruce may result from (1) lack of nitrogen reallocation from Aj to Ac and (2) Rubisco activase (RCA) lability. The similar Jmax25:Vcmax25 ratio between the southern and northern plantation sites is in accordance with our previous results during the second growing season at the same sites for six seed sources, including those used here (Benomar et al., 2016), and with other reports (Sage et al., 2008; Dillaway and Kruger, 2010). This lack of adjustment of nitrogen invested in Rubisco (and other soluble proteins involved in the Calvin cycle) may be due to cold adaptation-related constraints on nitrogen allocation. In fact, it has been reported that cold-adapted species allocate more nitrogen to Jmax as a compensatory response to low temperature (Yamori et al., 2010). Vcmax depends not only on Rubisco concentration but also on its activation state (inhibited/activated) (Salvucci and Crafts-Brandner, 2004; Sage et al., 2008). The activation state of Rubisco is regulated by RCA, a heat-labile enzyme using energy via ATP hydrolysis to release inhibitors from the active site of Rubisco (Crafts-Brandner and Salvucci, 2000; Yamori and von Caemmerer, 2009). A decrease in RCA activity has been documented as a primary cause of reduced Rubisco activity and then photosynthetic performance in response to increasing growth temperature (Yamori and von Caemmerer, 2009). Investigations regarding genetic variation in RCA and its activity in response to temperature in white spruce would help refine our understanding of the observed biochemical photosynthetic responses to temperature.
Although a lack of thermal acclimation of photosynthesis seems to be common to all boreal tree species (Way and Sage, 2008a; Dillaway and Kruger, 2010), thermal acclimation of dark respiration was reported to be moderate to strong for several tree species of the boreal forest, including white spruce (Tjoelker et al., 1999, 2009; Dillaway and Kruger, 2011; Reich et al., 2016; Wei et al., 2017). It seems that the firm acclimation of Rd (predominantly by the downshift in Q10) could reduce the negative impact of rising temperatures on photosynthesis under climate change (Atkin et al., 2005; Reich et al., 2016). In accordance with the recent results of Reich et al. (2016) for white spruce and the results of Tjoelker et al. (1999) for black spruce, we showed evidence for type II acclimation of Rd, as indicated by a downshift of Rd10 with increasing plantation site temperature. However, we could not show consistent evidence for type I acclimation of Rd (Fig. 4) as in Reich et al. (2016), and Tjoelker et al. (1999) for different white spruce and black spruce seed sources. The average Q10 value (1.50 ± 0.15) observed in this study was similar to that obtained with a white spruce population from northern Minnesota, USA (Reich et al., 2016). The small change noted in Q10 was not congruent with the important change in climate observed from site to site. Whether this lack of change in Q10 is related to the regional temperature gradient and seed sources tested in the present study, or to intrinsic species physiological performance needs to be examined (Wei et al., 2017).
Needle nitrogen concentration had little impact on the thermal acclimation of Rd, as indicated by similar results when Rd was expressed in nitrogen units. The observed type II acclimation of Rd might be a consequence of a change in needle mitochondria density or by mitochondrial overexpression of alternative oxidase (AOX) (Atkin et al., 2005).
We observed thermal acclimation of both needle respiration and photosynthetic rate in response to seasonal variation in temperature (i.e. reduction in Rd10 at higher temperatures and increase in Topt and Aopt with increasing temperature). Aopt reached a maximum value in June and September, with a clear linear relationship with temperature as expressed in 5-d mean temperature windows. It is still unclear whether photosynthesis phenology relies on temperature or photoperiod cues (Busch et al., 2007; Bauerle et al., 2012; Stinziano and Way, 2017). Unfortunately, our results cannot help clarify this issue. In fact, the decrease in An from June to August may be related to a decline in photosynthesis following bud set or to the increase in mean temperature. On the other hand, the increase in An from August to September cannot be linked to photoperiod. The similar values of Topt observed from June to August is in accordance to the pattern along the latitudinal gradient. The strong adjustment in Topt to lower temperatures in May and October, which may relate to a change in the activation energy of Vcmax, represents good evidence for higher acclimation of An to cold temperature in white spruce. The latter may explain the higher performance of seed sources from Québec in northern regions of Canada, such as Alberta (Lu et al., 2014).
The temperature at the location of origin of seed sources (climate of origin) in our study had no effect on their thermal acclimation-related traits. This result may be interpreted as a lack of intraspecific genetic variation in the thermal acclimation of photosynthesis, as recently reported for Eucalyptus tereticornis seedlings grown under controlled conditions (Drake et al., 2017). However, the limitations imposed by our experiment do not allow a clear conclusion to be drawn. In fact, the southern seed source experienced only a 1.7 °C warming in mean July temperature at the most southern plantation site of Wendover (Table 1). Consequently, we could formulate two hypotheses. First, despite a difference of 2 °C in mean July temperature of geographical origins between the two white spruce seed sources used in this study, their parents may have experienced historically a similar range of temperatures, which would explain the lack of difference in Topt between them. Thus, it would be interesting to test other seed sources from more meridional origins and warmer local climates, such as from southern Ontario and the USA, to further test the existence of genetic differentiation in thermal acclimation of photosynthesis. Our second hypothesis relates to the warming conditions experienced by seed sources in the southern plantation sites, which are likely insufficient to detect intraspecific variation in the thermal acclimation of photosynthesis that would be related to local genetic adaptation. Thus, using more southern plantation sites or augmented warming conditions in controlled environments would be necessary to test this last hypothesis.
Conclusions
Our results indicate that the photosynthesis of seedlings of two white spruce seed sources from southern and northern origins had a lower thermal optimum of photosynthesis and no ability to acclimate to warmer temperature. In contrast, the seedlings showed a clear acclimation of dark respiration by the downshift of the basal rate of Rd. However, dark respiration acclimation was insufficient to counterbalance the low photosynthetic rate in warmer plantation sites. In addition, the temperature response of photosynthesis was limited by Rubisco capacity, which suggests an effect of Rubisco activase or a lack of adjustment of nitrogen allocation. The results highlight the need for more research on thermal responses of photosynthesis and its biochemical limitations, with particular emphasis on Rubisco activase and on understanding of the main cues of photosynthesis phenology in spruces and other boreal forest trees. Together with monitoring at a more mature stage, this should help evaluate the effect of predicted autumnal warming on the global trend of photosynthesis. Overall, our results on growth and thermal acclimation-related traits for photosynthesis and dark respiration suggest that white spruce populations from southern Québec are already above their thermal thresholds and will remain so under predicted climate warming.
SUPPLEMENTARY DATA
Supplementary data are available online at: www.aob.oxfordjournals.org and consist of the following. Table S1: soil physico-chemical properties during the second growing season in the eight plantation sites; Figure S1: total height growth of two white spruce seed sources at the end of the second growing season (H2) plotted against site mean July temperature (MJT), soil total nitrogen and soil C:N ratio. Figure S2: Nmass and SLA of plants of two white spruce seed sources growing at eight plantation sites; Figure S3: Aopt and Rd10 plotted against Nmass for the two white spruce seed sources; raw data on An response to Ci and temperature.
ACKNOWLEDGEMENTS
We thank Guildo Gagnon, Mario Renaud, Jean Noël and Pascal Desjardins (Ministère des Forêts, de la Faune et des Parcs of Québec), Bakry Mustapha and Marie Coyea (Université Laval) for their technical assistance throughout the project. We also thank Raed Elferjani (Agriculture and Agri-Food Canada) for his valuable comments. This study was conducted in collaboration with the Direction de la Recherche Forestière of the ministère des Forêts, de la Faune et des Parcs of Québec. This research was funded by grants to H.A.M., M.S.L., A.R., J.Bo. and J.Be. from the programme Partenariat sur l’aménagement et l’environnement forestiers of the Fonds de la Recherche du Québec sur la Nature et les Technologies (FRQ-NT, 2015-FV-185886) and the Discovery Grant programme of the Natural Sciences and Engineering Research Council of Canada. Major additional support was also provided to M.S.L. (142332093) and A.R. (142332078) by the Direction de la Recherche Forestière of the ministère des Forêts, de la Faune et des Parcs of Québec.
LITERATURE CITED
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. 2008. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications 1: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalo C, Beaulieu J, Bousquet J. 2005. The impact of climate change on growth of local white spruce populations in Quebec, Canada. Forest Ecology and Management 205: 169–182. [Google Scholar]
- Atkin OK, Tjoelker MG. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8: 343–351. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Bruhn D, Tjoelker MG. 2005. Response of plant respiration to changes in temperature: mechanisms and consequences of variations in Q10 values and acclimation. In: Lambers H, Ribas-Carbo M eds. Plant respiration: from cell to ecosystem. Dordrecht: Springer Netherlands. [Google Scholar]
- Battaglia M, Beadle C, Loughhead S. 1996. Photosynthetic temperature responses of Eucalyptus globulus and Eucalyptus nitens. Tree Physiology 16: 81–89. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Oren R, Way DA et al. 2012. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proceedings of the National Academy of Sciences of the USA 109: 8612–8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J, Daoust G, Deshaies A, Lamhamedi MS, Rainville A, Tourigny M. 2009. Amélioration génétique des arbres, gestion des vergers à graines et de semences, et production de plants forestiers. In: Ordre des ingénieurs forestiers du Québec, ed. Manuel de foresterie, 2nd edn Québec: Presses de l’Université Laval, Québec. [Google Scholar]
- Benomar L, Lamhamedi MS, Rainville A, Beaulieu J, Bousquet J, Margolis HA. 2016. Genetic adaptation vs. ecophysiological plasticity of photosynthetic-related traits in young Picea glauca trees along a regional climatic gradient. Frontiers in Plant Science 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigras FJ. 2000. Selection of white spruce families in the context of climate change: heat tolerance. Tree Physiology 20: 1227–1234. [DOI] [PubMed] [Google Scholar]
- Busch F, Hüner NPA, Ensminger I. 2007. Increased air temperature during simulated autumn conditions does not increase photosynthetic carbon gain but affects the dissipation of excess energy in seedlings of the evergreen conifer jack pine. Plant Physiology 143: 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Xu M, Shen R, Yang Q, Huang B, Wan S. 2013. Acclimation of foliar respiration and photosynthesis in response to experimental warming in a temperate steppe in Northern China. PLoS ONE 8: e56482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2000. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proceedings of the National Academy of Sciences of the USA 97: 13430–13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillaway DN, Kruger EL. 2010. Thermal acclimation of photosynthesis: a comparison of boreal and temperate tree species along a latitudinal transect. Plant, Cell & Environment 33: 888–899. [DOI] [PubMed] [Google Scholar]
- Dillaway DN, Kruger EL. 2011. Leaf respiratory acclimation to climate: comparisons among boreal and temperate tree species along a latitudinal transect. Tree Physiology 31: 1114–1127. [DOI] [PubMed] [Google Scholar]
- Drake JE, Vårhammar A, Kumarathunge D et al. 2017. A common thermal niche among geographically diverse populations of the widely distributed tree species Eucalyptus tereticornis: no evidence for adaptation to climate of origin. Global Change Biology 23: 5069–5082. [DOI] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell & Environment 27: 137–153. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Friend AD, Woodward FI, Switsur VR. 1989. Field measurements of photosynthesis, stomatal conductance, leaf nitrogen and δ13C along altitudinal gradients in Scotland. Functional Ecology 3: 117–122. [Google Scholar]
- Girardin MP, Bouriaud O, Hogg EH et al. 2016. No growth stimulation of Canada’s boreal forest under half-century of combined warming and CO2 fertilization. Proceedings of the National Academy of Sciences of the USA 113: E8406–E8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson CA, Norby RJ, Wullschleger SD. 2000. Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations of Acer saccharum: laboratory and field evidence. Tree Physiology 20: 87–96. [DOI] [PubMed] [Google Scholar]
- Gunderson CA, O’Hara KH, Campion CM, Walker AV, Edwards NT. 2010. Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Global Change Biology 16: 2272–2286. [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y. 2006. Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. Journal of Experimental Botany 57: 291–302. [DOI] [PubMed] [Google Scholar]
- IPCC 2014. Fifth assessment report: climate change (AR5). Geneva: Intergovernmental Panel on Climate Change; https://www.ipcc.ch/report/ar5/. [Google Scholar]
- Ishikawa K, Onoda Y, Hikosaka K. 2007. Intraspecific variation in temperature dependence of gas exchange characteristics among Plantago asiatica ecotypes from different temperature regimes. New Phytologist 176: 356–364. [DOI] [PubMed] [Google Scholar]
- Johnson FH, Eyring H, Williams RW. 1942. The nature of enzyme inhibitions in bacterial luminescence: sulfanilamide, urethane, temperature and pressure. Journal of Cellular and Comparative Physiology 20: 247–268. [Google Scholar]
- Kattge J, Knorr W. 2007. Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant, Cell & Environment 30: 1176–1190. [DOI] [PubMed] [Google Scholar]
- Lamhamedi M, Bernier P. 1994. Ecophysiology and field performance of black spruce (Picea mariana): a review. Annals of Forest Science 51: 529–551. [Google Scholar]
- Lamhamedi MS, Labbé L, Margolis HA, Stowe DC, Blais L, Renaud M. 2006. Spatial variability of substrate water content and growth of white spruce seedlings. Soil Science Society of America Journal 70: 108–120. [Google Scholar]
- Li P, Beaulieu J, Bousquet J. 1997. Genetic structure and patterns of genetic variation among populations in eastern white spruce (Picea glauca). Canadian Journal of Forest Research 27: 189–198. [Google Scholar]
- Lombardozzi DL, Bonan GB, Smith NG, Dukes JS, Fisher RA. 2015. Temperature acclimation of photosynthesis and respiration: a key uncertainty in the carbon cycle-climate feedback. Geophysical Research Letters 42: 8624–8631. [Google Scholar]
- Lu P, Parker WH, Cherry M et al. 2014. Survival and growth patterns of white spruce (Picea glauca [Moench] Voss) rangewide provenances and their implications for climate change adaptation. Ecology and Evolution 4: 2360–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HA, Brand DG. 1990. An ecophysiological basis for understanding plantation establishment. Canadian Journal of Forest Research 20: 375–390. [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D et al. 2002. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell & Environment 25: 1167–1179. [Google Scholar]
- Mullin TJ, Andersson B, Bastien JC. et al. eds 2011. Genetics, Genomics and Breeding of Conifers. Enfield, NH, USA: Science Publishers. [Google Scholar]
- Niu S, Luo Y, Fei S et al. 2012. Thermal optimality of net ecosystem exchange of carbon dioxide and underlying mechanisms. New Phytologist 194: 775–783. [DOI] [PubMed] [Google Scholar]
- Ow LF, Griffin KL, Whitehead D, Walcroft AS, Turnbull MH. 2008. Thermal acclimation of leaf respiration but not photosynthesis in Populus deltoides x nigra. New Phytologist 178: 123–134. [DOI] [PubMed] [Google Scholar]
- Ow LF, Whitehead D, Walcroft AS, Turnbull MH. 2010. Seasonal variation in foliar carbon exchange in Pinus radiata and Populus deltoides: respiration acclimates fully to changes in temperature but photosynthesis does not. Global Change Biology 16: 288–302. [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. [DOI] [PubMed] [Google Scholar]
- Régnière J, St-Amant R. 2007. Stochastic simulation of daily air temperature and precipitation from monthly normals in North America north of Mexico. International Journal of Biometeorology 51: 415–430. [DOI] [PubMed] [Google Scholar]
- Reich PB, Oleksyn J. 2004. Global patterns of plant leaf N and P in relation to temperature and latitude. Proceedings of the National Academy of Sciences of the USA 101: 11001–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Sendall KM, Stefanski A, Wei XR, Rich RL, Montgomery RA. 2016. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531: 633. [DOI] [PubMed] [Google Scholar]
- Romero-Lankao P, Smith JB, Davidson DJ. et al. eds. 2014. Climate Change 2014: impacts, adaptation, and vulnerability. Part B: Regional aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Sage RF, Kubien DS. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 30: 1086–1106. [DOI] [PubMed] [Google Scholar]
- Sage RF, Way DA, Kubien DS. 2008. Rubisco, Rubisco activase, and global climate change. Journal of Experimental Botany 59: 1581–1595. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ. 2004. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiology 134: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro AP, Xiang S, Long BM et al. 2016. Strong thermal acclimation of photosynthesis in tropical and temperate wet-forest tree species: the importance of altered Rubisco content. Global Change Biology 23: 2783–2800. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. 2007. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment 30: 1035–1040. [DOI] [PubMed] [Google Scholar]
- Silim SN, Ryan N, Kubien DS. 2010. Temperature responses of photosynthesis and respiration in Populus balsamifera L.: acclimation versus adaptation. Photosynthesis Research 104: 19–30. [DOI] [PubMed] [Google Scholar]
- Slot M, Rey-Sánchez C, Gerber S, Lichstein JW, Winter K, Kitajima K. 2014. Thermal acclimation of leaf respiration of tropical trees and lianas: response to experimental canopy warming, and consequences for tropical forest carbon balance. Global Change Biology 20: 2915–2926. [DOI] [PubMed] [Google Scholar]
- Stinziano JR, Way DA. 2017. Autumn photosynthetic decline and growth cessation in seedlings of white spruce are decoupled under warming and photoperiod manipulations. Plant, Cell & Environment 40: 1296–1316. [DOI] [PubMed] [Google Scholar]
- Tjoelker MG, Reich PB, Oleksyn J. 1999. Changes in leaf nitrogen and carbohydrates underlie temperature and CO2 acclimation of dark respiration in five boreal tree species. Plant, Cell & Environment 22: 767–778. [Google Scholar]
- Tjoelker MG, Oleksyn J, Lorenc-Plucinska G, Reich PB. 2009. Acclimation of respiratory temperature responses in northern and southern populations of Pinus banksiana. New Phytologist 181: 218–229. [DOI] [PubMed] [Google Scholar]
- Veilleux P, Bonneau A, Girard JP et al. 2010. Guide terrain. Inventaire de qualification des plants résineux cultivés en récipient. Québec: Direction Générale des Pépinières et des Stations Piscicoles, Ministère des Ressources Naturelles et de la Faune. [Google Scholar]
- Villeneuve I, Lamhamedi MS, Benomar L et al. 2016. Morpho-physiological variation of white spruce seedlings from various seed sources and implications for deployment under climate change. Frontiers in Plant Science 7: 1450. doi:10.3389/fpls.2016.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CR. 2008. Does growth temperature affect the temperature responses of photosynthesis and internal conductance to CO2? A test with Eucalyptus regnans. Tree Physiology 28: 11–19. [DOI] [PubMed] [Google Scholar]
- Warren CR, Dreyer E. 2006. Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. Journal of Experimental Botany 57: 3057–3067. [DOI] [PubMed] [Google Scholar]
- Way DA, Sage RF. 2008a. Elevated growth temperatures reduce the carbon gain of black spruce Picea mariana (Mill.) BSP. Global Change Biology 14: 624–636. [Google Scholar]
- Way DA, Sage RF. 2008b. Thermal acclimation of photosynthesis in black spruce Picea mariana (Mill.) BSP. Plant, Cell & Environment 31: 1250–1262. [DOI] [PubMed] [Google Scholar]
- Way DA, Yamori W. 2014. Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynthesis Research 119: 89–100. [DOI] [PubMed] [Google Scholar]
- Wei X, Sendall KM, Stefanski A et al. 2017. Consistent leaf respiratory response to experimental warming of three North American deciduous trees: a comparison across seasons, years, habitats and sites. Tree Physiology 37: 285–300. [DOI] [PubMed] [Google Scholar]
- Yamori W, von Caemmerer S. 2009. Effect of rubisco activase deficiency on the temperature response of CO2 assimilation rate and rubisco activation state: insights from transgenic tobacco with reduced amounts of rubisco activase. Plant Physiology 151: 2073–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hikosaka K, Terashima I. 2010. Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances. Plant Physiology 152: 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Hikosaka K, Way DA. 2014. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research 119: 101–117. [DOI] [PubMed] [Google Scholar]
- Zhang XW, Wang JR, Ji MF et al. 2015. Higher thermal acclimation potential of respiration but not photosynthesis in two alpine Picea taxa in contrast to two lowland congeners. PLOS ONE 10: e0123248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.