Abstract
Aims:
Available literature on the prevalence of Charcot arthropathy (CA) represents mainly Western population. No study has been reported from India so far. Hence we attempted to study the prevalence of CA in patients with type 2 diabetes mellitus and severe peripheral neuropathy (T2DMPN), belonging to Indian population amongst whom type 2 diabetes is on the rise in alarming proportions.
Materials and Methods:
Medical records of 3387 patients who performed an objective vibration perception threshold test during the year 2015 were screened for T2DMPN. Out of these, 1475 T2DMPN patients above 50 years were selected and analyzed in detail for CA. CA was diagnosed based on clinical features and/or radiological investigations. The anatomical localization of the disease distribution of the affected foot was done according to Brodsky's classification.
Results:
The prevalence of CA in T2DMPN patients was found to be 9.8%. The mean age of patients diagnosed with CA was 63 ± 8.36 years, and mean duration of DM for CA to develop was 18.01 ± 8.23 years. About 62.5% of the patients were male and 37.5% female. Bilateral presentation of CA was observed in 20.8% of patients. Multiple sites of the foot were affected in 48.6% of patients and belonged to type 4 classification of Brodsky.
Conclusions:
A high prevalence of CA (9.8%) was observed in the present study conducted on T2DMPN patients who presented to the endocrinology department of a tertiary care South Indian hospital. In the majority of patients, the area of foot affected belonged to type 4 classification of Brodsky.
Keywords: Ankle-brachial index, Brodsky's classification system, peripheral neuropathy, type 2 diabetes mellitus
INTRODUCTION
Charcot arthropathy (CA) of the foot, an infrequent neuromusculoskeletal sequelae of diabetes, progressively ends up in varying degrees of destruction and deformity of the foot and ankle. Morbidity and mortality rates are dangerously high in these patients following ulcerations and/or amputations.[1,2] Besides, CA compromises overall health and quality of life of the affected patient and family.
CA was first described by the French neurologist Jean-Martin Charcot in 1868. Syphilis was believed to be the common cause of CA until Jordan in 1936 established the association between CA and diabetes mellitus (DM). Even though the etiology of the disease is not fully identified, it is well accepted that neuropathy precedes the disease.[2] The prevalence of CA is variably reported in literature ranging from 0.08% to 13%.[3] A general trend for higher frequency of occurrence of CA in patients with DM and severe peripheral neuropathy (DMPN) and those visiting podiatry clinics is reported.[4] The occurrence of racial/ethnic variations in the distribution of this rare disease is also documented.[3]
Despite the voluminous work reported in literature on CA, very few studies mention the prevalence of this disabling disease. Available literature on the prevalence of CA represents mainly Western population.[5,6] The actual incidence of CA may be greater than what is reported, as in many cases, the clinicians fail to diagnose or are late to diagnose. CA is left undiagnosed in about a quarter of cases due to (1) lack of specific markers and/or diagnostic criteria, (2) clinical resemblance to other common bone disorders such as osteomyelitis and cellulitis, (3) lack of specialists in the area, etc.
Diabetes currently affects more than 62 million Indians, which is more than 7.1% of India's adult population. Inaccessibility to optimum health care, lack of knowledge regarding diabetes care, and complications, all contribute to increased rates of secondary complications of diabetes in developing countries like India. Moreover, habit of walking barefoot or using unfit footwear without any support to foot arch can add to increasing incidence of foot complications in patients with diabetes in India.[7] With recent advances in DM medicine which inevitably increase the life expectancy of the patients, podiatrists are likely to come across more number of DM patients with foot complications such as CA. Meticulous screening of those diabetes patients who are at risk to develop CA, for early changes such as widening of Lisfranc joint, flattening of the metatarsal head, cortical erosion of bones of foot, etc., will help bring down the incidence of this life-threatening disease.[8] The prevalence of CA is hardly reported in Indian population. Therefore, an effort has been made to study the prevalence of CA in DM patients who are at risk to develop CA in Indian population.
MATERIALS AND METHODS
Subjects
A retrospective review of medical records of all patients both inpatient and outpatient who performed an objective vibration perception threshold (VPT) test in the department of endocrinology, part of a tertiary care hospital, was carried out. Only the medical records of those patients who visited the department during the year 2015 were considered. Patients were included in the study on satisfying the following criteria:
Have age more than 50 years
Have type 2 DM (According to the World Health Organization criteria)
Have severe peripheral neuropathy (VPT is more than 25V – measured by a biothesiometer [DSA-India])
Have CA.
Only known cases of diabetic neuropathy were considered. After careful scrutiny of medical records, patients with other causes for neuropathy were excluded from the study.
Thus, out of 3387 medical records of patients evaluated those of 1475, T2DMPN patients over 50 years of age were selected and were thoroughly reviewed for CA. The study was approved by the Institutional Ethics Committee.
Diagnosis of CA
CA was diagnosed on the basis of clinical features and/or radiological investigations (plain radiographs and/or nuclear scan and/or magnetic resonance imaging [MRI]) or a combination of these. Clinical features of CA were the presence of erythema, edema, pain, or soreness, local rise in temperature, strong pedal pulse, loss of sensation in the foot, instability of joints, and foot deformity.[4] Patients with clinical features of CA had performed radiological imaging (X-ray and/or nuclear scan and/or MRI) to rule out CA.
Anteroposterior, lateral, and oblique nonweight-bearing X-rays of the foot and ankle were taken and evaluated for radiological features of CA by an experienced radiologist and podiatric surgeon. The X-rays of patients were analyzed for radiological features of CA such as bone and joint destruction, distension, dislocation, disorganization, debris, and increased bone mineral density.[2,6] Those patients whose X-rays failed to give a clear picture of CA changes were asked to perform either a nuclear scan or an MRI for confirmation of the pathological condition. Nuclear scan was performed as per standard techniques mentioned.[8] Very few patients underwent MRI imaging for diagnosing CA.
Vascularity of both the feet was assessed by measuring ankle-brachial index (ABI). Blood flow was considered normal in those patients whose ABI value was between 0.9 and 1.4. Patients with ABI index values <0.9 were considered as cases with peripheral arterial disease (PAD). Cases with ABI index values >1.4 were considered to have noncompressible vessels.[9] The anatomical location of the disease distribution on the affected foot was done according to Brodsky's classification system.[10]
Statistical analysis
Statistical analysis was performed using IBM SPSS version 20.0 software for windows (SPSS Inc, Chicago, USA). Categorical variables are expressed using frequency and percentage. Numerical variables are presented using mean and standard deviation. The prevalence of CA is calculated as percentage.
RESULTS
A total of 3387 patients performed VPT during the study period. Thorough analysis of medical records of these 3387 patients showed that 1475 patients were above 50 years of age and had type 2 DM along with severe bilateral peripheral neuropathy (VPT score more than 25V). Analysis of medical records of these 1475 patients for clinical and radiological features of CA showed that 144 (9.8%) patients had CA. Data of these 144 patients were reviewed carefully and as detailed below form the basis of this study.
Twenty-six (18.1%) patients were old cases of CA and 118 (81.9%) patients were newly diagnosed cases of CA. Old cases of CA were the ones diagnosed with CA earlier irrespective of whether they did or did not undergo treatment. New cases were newly diagnosed cases of CA irrespective of the stage but with no prior history of being diagnosed or treated for CA. Bilateral presentation was observed in 30 (20.8%) patients and unilateral presentation in 114 (79%) patients. Unilaterality was on the right side in 57 (39.5%) patients and on the left side in 57 (39.5%) patients. Among the patients identified with CA, 90 (62.5%) patients were males and 54 (37.5%) were females. The mean age of these 144 patients at the onset of CA was 63 ± 8.36 years, and mean duration of DM for CA to develop was 18.01 ± 8.23 years.
Vascularity assessment of the foot was carried out based on values of ABI. ABI values were missing in three patients. Among the 141 records assessed, 19 (13.5%) patients had ABI index values <0.9 and were taken positive for PAD. One hundred and two (72.3%) had ABI index values between 0.9 and 1.4 and were considered to have good blood flow in the lower limb. Twenty (14.2%) had values >1.4 and were considered as cases with noncompressible blood vessels.
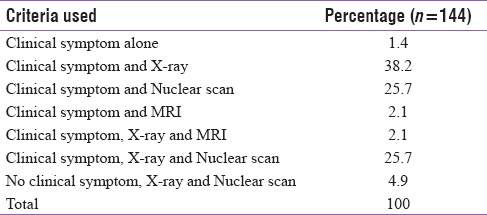
CA was confirmed with clinical symptoms and/or diagnostic imaging techniques (plain radiographs, nuclear scan, and MRI) or a combination of these. A combination of clinical symptoms and X-ray was used in 55 (38.2%) patients, clinical symptoms and nuclear scan in 37 (25.7%) patients, and clinical symptoms and MRI in 3 (2.1%) patients. A combination of clinical symptoms, X-ray and nuclear scan were used in 37 (25.7%) patients and clinical symptoms, X-ray, and MRI in 3 (2.1%) patients. Clinical symptoms alone were the only diagnostic criteria in 2 (1.4%) patients. Seven (4.9%) patients did not have any clinical symptoms of CA, and X-ray and nuclear scan were taken to rule out abnormalities of the foot other than CA and was diagnosed with CA [Table 1].
Table 1.
Diagnostic criteria used to confirm Charcot arthropathy
The anatomical location of foot affected by CA was done according to Brodsky's classification. Sixty-one (43%) had their tarsometatarsal joints affected and belonged to type 1 classification of CA by Brodsky. Four (2.8%) patients had their subtalar, talonavicular, and calcaneocuboid joints involved and belonged to type 2. Four (2.8%) patients had their tibiotalar joints involved and belonged to type 3A. Only 1 (0.7%) patient had his/her calcaneus affected and belonged to type 3B. About 69 (48.6%) patients had more than one area affected and belonged to type 4. Three (2.1%) patients belong to type 5 with forefoot alone affected [Table 2]. Area of disease distribution in two patients could not be located due to nonavailability of any images.
Table 2.
Disease distribution according to Brodskys classification

DISCUSSION
Detailed analysis of available literature on CA shows that only very few studies have been carried out to assess the prevalence of CA in T2DMPN. Among these, limited studies specifically evaluated the prevalence of CA, whereas the remaining merely give information on a sequence of patients with CA, DM, or DMPN. The prevalence of CA as reported in literature diversify from 0.08%–13%.[3] The prevalence of CA is less in general population of patients with diabetes. The pioneers to study the prevalence of CA were Sinha et al. and Amstrong et al. who reported a prevalence of 0.15% and 0.16%, respectively, in patients with DM. A gradual increase in prevalence was observed in studies conducted in the following years. This can be either due to an increased awareness among clinicians or may be because the disease has become more common. In a retrospective survey conducted by Fabrin et al. on 115 patients with DM, a slight increase was noted (0.3%).[11] In another study conducted by Smith et al. on plain radiographs of 456 patients with DM, the prevalence of CA changes was 1.4%, and in all these patients, midfoot region was affected.[12] In a recent study conducted in a specialty clinic for diabetes in Pakistan, the prevalence of CA was found to be 0.4% in patients with diabetes.[13]
Studies have shown that CA is more common in patients with diabetes, neuropathy, and previous history of foot problems. Even though Armstrong et al reported a very low prevalence rate (0.16%) of CA in general population of DM patients, higher rates (13%) were obtained on high-risk DM patients. Evaluation of radiographs of the foot and ankle for changes caused by DM on bones and joints of the foot revealed that while no patients with DM showed CA changes, 14% of patients with diabetes and peripheral neuropathy had CA changes. The rates of CA changes were dangerously high, i.e., 54% in patients with DMPN with foot ulcer.[14] We got a prevalence rate of 9.8% in the current study which may be due to several of the following reasons.
Our population was not a general population of DM. Instead, we selected a subgroup of DM who are at risk to develop CA (T2DMPN with age above 50 years)
Ours is a tertiary care clinic with well-established podiatry department. Fairly good number of patients are referred patients from other primary and secondary health care centers
High index of DM patients in our population
The increased life expectancy of DM patients with advanced medical facility which will definitely add on to a rise in the incidence of secondary complications of diabetes
The availability of advanced medical imaging techniques
The habit of walking barefoot particularly indoors. Continued weight bearing/ambulation on an already weak neuropathic foot in a DM patient without any support to the arches of foot can lead to the development of foot complications in DM patients.
Several authors have asserted the absence of male-female predilections in the presentation of CA.[4] In the study conducted by Younis et al. on DM population of Pakistan, no gender-related difference in presentation of CA was noted.[13] Many studies conclude male gender as a risk factor for developing Charcot foot. Even in the present study, an increased prevalence of CA was observed in males compared to females. Similar results were reported by Sohn et al. and Kensarah et al. with higher frequency 97.1% and 81.2% respectively of presentation of CA in male patients compared to female patients. The higher rates in males may in part be due to the increased physical activity in males compared to females.[1,15]
PAD is less likely in CA patients compared to patients with long history of DM and patients with DM and foot ulcers. Adequate blood supply is a prerequisite for the development of CA, and the increased perfusion observed in CA is due to sympathetic neuropathy. The prevalence rate of PAD in patients with CA reported in literature varies from 4.4% to 35.4%. A low prevalence rate of 4.4% was reported by Carravaggi et al. on CA patients with critical limb ischemia.[16] Chantelau reported a PAD rate of 12.5% in a study assessing early diagnosis of CA.[17] In the present study, 13.5% of patients with CA had ABI index values, less than 0.9. This could probably be due to the presence of any of the possible factors such as chronic smoking and uncontrolled diabetes in these patients, which lead to the development of PAD after the development of CA. Another study conducted by Bem et al. on DM patients with ulcer and CA, 35.4% had been diagnosed with PAD.[18]
Figures representing the age for onset of CA is of great significance and is variably reported in literature. Literature analysis shows that CA is common in younger than elderly patients with diabetes and affects mainly patients in their fifth and sixth decades of life.[6] Cofield et al. reported an average age of 56 years for the development of CA in an observational study done on DMPN patients at Mayo clinic.[19] In another study done by Younis et al., patients within the age group of 60–79 had higher propensity to develop CA, compared to other age groups.[13] In the present study, the average age at the time of diagnosis of CA was 63, and this may be due to the fact that our study population itself was above the age of 50 years.
A history of >10 years of DM is reported for CA to develop.[8,12] In the study conducted by Cofield et al. on 96 patients with DM and peripheral neuropathy, the duration of DM before the diagnosis of CA averaged to 16 years.[19] The mean duration of DM needed for CA to develop is 18 years in the present study. This value is slightly high compared to values reported in general on the duration of DM for CA to develop in literature. This high value may probably be due to the increased awareness on diabetes and its complications among patients and better treatment options available. Results similar to the results of our study were reported in a review by Clouse et al. conducted on DM patients in England.[20] A very low duration of 7.16 ± 6.28 years is reported in a recent study conducted on DM patients of Pakistan.[13]
CA is commonly reported to show unilateral presentation. Bilateral presentation of CA is also reported in numbers varying from 9% to 75%.[21,22] In a study by Clouse on DM patients with CA, 46% of patients had CA on the right foot and 37% had CA on the left foot. In his study, bilateral presentation of CA was observed only in 18% of patients.[20] In a retrospective study conducted on a multiracial society in Malaysia, bilateral presentation was observed in 16.7% of CA cases.[23] Results of our study are in agreement with the figures in above studies in that only 20.80% patients had both the feet affected by CA. With the use of advanced imaging techniques, the values on bilateral presentation are raised up to 75% in CA patients.[24]
The most common site on foot affected by CA as depicted in literature is midfoot.[25] Contrary to this in our study, midfoot was affected in only 43% of patients. In a study conducted on a Malaysian population, CA affected midfoot in 45.8% followed by ankle joint in 22.9%. In the same study, multiple sites were affected in 16.7%, hindfoot in 10.4%, and forefoot in only 4.2% of DM patients.[23] In the current study, majority had multiple sites of the foot affected by CA and belonged to type 4 classification by Brodsky. In a study by Sella and Barrette, tarsometatarsal joints were affected in 45% of cases and cuneonavicular, talonavicular, and calcaneocuboid articulations were affected in 35% of cases.[26] In another study by Kensarah et al., forefoot was affected in 65.5% of patients followed by hindfoot and midfoot.[15] According to Armstrong et al., tarsometatarsal (mid foot), talonavicular, and calcaneocuboid (hindfoot) joints are affected in 80% of the patients with CA.[22]
Early detection of the disease before the appearance of clinical symptoms can to a great extent limit the deformities and other long-term outcome of CA including amputation. In our study, majority of patients (95.13%) came to the clinic with clinical symptoms of CA. CA was confirmed in these patients by X-ray and/or nuclear scan and/or MRI or a combination of these. It was interesting to note that 4.9% of DMPN patients without any clinical symptoms of CA were diagnosed with CA, by chance during investigative imaging for other foot abnormalities. This point to the fact the disease develops even before the appearance of clinical symptoms. Since these patients received the appropriate treatment and rest at the correct time, further progression of disease and deformities could be arrested in these patients. Thorough screening of high-risk DMPN patients may help to identify the disease at an early stage and prevent life-threatening complications of the disease. For this, a high degree of vigilance is necessary on the part of the clinicians. Moreover, the patients should be made well aware of the risk factors, symptoms, treatment, and consequences of this devastating condition. Such an education may help reduce the delay in diagnosis of the disease. Development of the disease even before the appearance of clinical symptoms necessitates the development of diagnostic criteria for early detection of this disease.
CONCLUSIONS
In the present study conducted on T2DMPN patients who visited the endocrinology department of a South Indian tertiary care hospital, the prevalence of CA was found to be high (9.8%). This may be due to the fact that we selected a population at high risk to develop CA. Clinicians should be very alert when dealing with such high-risk patients and should screen them for CA changes as part of their routine check-up. In contrary to what is reported in general in literature, multiple areas of the foot were affected in the majority of population followed by midfoot.
Limitations
Our study has some limitations and has to be acknowledged. Since HbA1c levels of all patients were not available, we could not study the correlation between poor glycemic control and prevalence of CA. Few data were missing regarding demographic details, comorbidities of DM, history of foot problems, etc., Another limitation with the study was that we could not stage CA according to Eichenholtz classification system.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sohn MW, Lee TA, Stuck RM, Frykberg RG, Budiman-Mak E. Mortality risk of Charcot arthropathy compared with that of diabetic foot ulcer and diabetes alone. Diabetes Care. 2009;32:816–21. doi: 10.2337/dc08-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, et al. The charcot foot in diabetes. Diabetes Care. 2011;34:2123–9. doi: 10.2337/dc11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg. 2008;25:17–28, v. doi: 10.1016/j.cpm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Rajbhandari SM, Jenkins RC, Davies C, Tesfaye S. Charcot neuroarthropathy in diabetes mellitus. Diabetologia. 2002;45:1085–96. doi: 10.1007/s00125-002-0885-7. [DOI] [PubMed] [Google Scholar]
- 5.Leung HB, Ho YC, Wong WC. Charcot foot in a Hong Kong Chinese diabetic population. Hong Kong Med J. 2009;15:191–5. [PubMed] [Google Scholar]
- 6.Sinha S, Munichoodappa CS, Kozak GP. Neuro-arthropathy (Charcot joints) in diabetes mellitus (clinical study of 101 cases) Medicine (Baltimore) 1972;51:191–210. doi: 10.1097/00005792-197205000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Jayasinghe SA, Atukorala I, Gunethilleke B, Siriwardena V, Herath SC, De Abrew K, et al. Is walking barefoot a risk factor for diabetic foot disease in developing countries? Rural Remote Health. 2007;7:692. [PubMed] [Google Scholar]
- 8.Ergen FB, Sanverdi SE, Oznur A. Charcot foot in diabetes and an update on imaging. Diabet Foot Ankle. 2013;4:1–8. doi: 10.3402/dfa.v4i0.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky JW. The diabetic foot. In: Coughlin MJ, Mann RA, Saltzman CL, editors. Surgery of the Foot and Ankle. 8th ed. Louis MO USA: Mosby; 2006. pp. 1281–368. [Google Scholar]
- 11.Fabrin J, Larsen K, Holstein PE. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000;23:796–800. doi: 10.2337/diacare.23.6.796. [DOI] [PubMed] [Google Scholar]
- 12.Smith DG, Barnes BC, Sands AK, Boyko EJ, Ahroni JH. Prevalence of radiographic foot abnormalities in patients with diabetes. Foot Ankle Int. 1997;18:342–6. doi: 10.1177/107110079701800606. [DOI] [PubMed] [Google Scholar]
- 13.Younis BB, Shahid A, Arshad R, Khurshid S, Masood J. Charcot osteoarthropathy in type 2 diabetes persons presenting to specialist diabetes clinic at a tertiary care hospital. BMC Endocr Disord. 2015;15:28. doi: 10.1186/s12902-015-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viswanathan V, Kumpatla S, Rao VN. Radiographic abnormalities in the feet of diabetic patients with neuropathy and foot ulceration. J Assoc Physicians India. 2014;62:30–3. [PubMed] [Google Scholar]
- 15.Kensarah AMA, Zaidi NH, Noorwali A, Aref H, Makki AM, Ghunaim A, et al. Evaluation of Charcot neuroarthropathy in diabetic foot disease patients at tertiary hospital. Surg Sci. 2016;7:250–7. [Google Scholar]
- 16.Caravaggi CM, Sganzaroli AB, Galenda P, Balaudo M, Gherardi P, Simonetti D, et al. Long-term follow-up of tibiocalcaneal arthrodesis in diabetic patients with early chronic Charcot osteoarthropathy. J Foot Ankle Surg. 2012;51:408–11. doi: 10.1053/j.jfas.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Chantelau E. The perils of procrastination: Effects of early vs. delayed detection and treatment of incipient Charcot fracture. Diabet Med. 2005;22:1707–12. doi: 10.1111/j.1464-5491.2005.01677.x. [DOI] [PubMed] [Google Scholar]
- 18.Bem K, Jirkovska A, Dubsky M, Woskova V, Fejfarova V. Charcot Neuropathic Osteoarthropathy and Peripheral Arterial Disease. Presented at the 25th World Congress of the International Union of Angiology. 2015:89–90. [Google Scholar]
- 19.Cofield RH, Morrison MJ, Beabout JW. Diabetic neuroarthropathy in the foot: Patient characteristics and patterns of radiographic change. Foot Ankle. 1983;4:15–22. doi: 10.1177/107110078300400104. [DOI] [PubMed] [Google Scholar]
- 20.Clouse ME, Gramm HF, Legg M, Flood T. Diabetic osteoarthropathy. Clinical and roentgenographic observations in 90 cases. Am J Roentgenol Radium Ther Nucl Med. 1974;121:22–34. doi: 10.2214/ajr.121.1.22. [DOI] [PubMed] [Google Scholar]
- 21.Clohisy DR, Thompson RC., Jr Fractures associated with neuropathic arthropathy in adults who have juvenile-onset diabetes. J Bone Joint Surg Am. 1988;70:1192–200. [PubMed] [Google Scholar]
- 22.Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14:357–63. doi: 10.1002/(SICI)1096-9136(199705)14:5<357::AID-DIA341>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Fauzi AA, Chung TY, Latif LA. Risk factors of diabetic foot Charcot arthropathy: A case-control study at a Malaysian tertiary care centre. Singapore Med J. 2016;57:198–203. doi: 10.11622/smedj.2016074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouveri E, Papanas N. Charcot osteoarthropathy in diabetes: A brief review with an emphasis on clinical practice. World J Diabetes. 2011;2:59–65. doi: 10.4239/wjd.v2.i5.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silvampatti S, Nagaraja HS, Rajasekaran S. Midfoot Charcot arthropathy: Overview and surgical management. J Foot Ankle Surg (Asia pacific) 2016;3:97–106. [Google Scholar]
- 26.Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg. 1999;38:34–40. doi: 10.1016/s1067-2516(99)80086-6. [DOI] [PubMed] [Google Scholar]


