Abstract
Background:
Diabetes mellitus (DM) is a chronic abnormal metabolic condition, which manifests elevated blood sugar level over a prolonged period. The pancreatic endocrine system generally gets affected during diabetes, but often abnormal exocrine functions are also manifested due to its proximity to the endocrine system. Fecal elastase-1 (FE-1) is found to be an ideal biomarker to reflect the exocrine insufficiency of the pancreas.
Aim:
The aim of this study was conducted to assess exocrine dysfunction of the pancreas in patients with type-2 DM (T2DM) by measuring FE levels and to associate the level of hyperglycemia with exocrine pancreatic dysfunction.
Methodology:
A prospective, cross-sectional comparative study was conducted on both T2DM patients and healthy nondiabetic volunteers. FE-1 levels were measured using a commercial kit (Human Pancreatic Elastase ELISA BS 86-01 from Bioserv Diagnostics). Data analysis was performed based on the important statistical parameters such as mean, standard deviation, standard error, t-test-independent samples, and Chi-square test/cross tabulation using SPSS for Windows version 20.0.
Results:
Statistically nonsignificant (P = 0.5051) relationship between FE-1 deficiency and age was obtained, which implied age as a noncontributing factor toward exocrine pancreatic insufficiency among diabetic patients. Statistically significant correlation (P = 0.003) between glycated hemoglobin and FE-1 levels was also noted. The association between retinopathy (P = 0.001) and peripheral pulses (P = 0.001) with FE-1 levels were found to be statistically significant.
Conclusion:
This study validates the benefit of FE-1 estimation, as a surrogate marker of exocrine pancreatic insufficiency, which remains unmanifest and subclinical.
Keywords: Exocrine pancreatic insufficiency, fecal elastase-1, glycated hemoglobin, type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus (DM) is a chronic, lifelong metabolic disorder characterized by high blood sugar level (hyperglycemia) over a prolonged period. Uncontrolled hyperglycemic condition for an extended period can induce tissue damage, which subsequently causes end-organ damage due to micro and macrovascular complexities.[1,2] The endocrine system is found to be majorly affected in a diabetic patient, but a significant impact of this disease is also expected in exocrine functions due to its adjacency to the endocrine system.[3,4] Therefore, a proper exocrine function is essential for digestion and absorption of food in the gastrointestinal (GI) tract.[1,3,5] During the last two decades of 20th century, indirect pancreatic function tests became accessible; specifically, the fecal elastase-1 (FE-1) concentration measurement. This study was conducted to assess the exocrine dysfunction of the pancreas in type-2 diabetes mellitus (T2DM) patients by measuring FE-1 levels and to correlate the degree of hyperglycemia with exocrine pancreatic dysfunction.[4,6]
METHODOLOGY
The comparative cross-sectional study was conducted on both inpatients and outpatients who attended a multi-specialty hospital based in India, with clinical symptoms of diabetes, without any other comorbid diseases. The study was approved by the Institutional Ethical Committee, and informed consent was obtained from all the patients.
The inclusion criteria of the study were patients with T2DM who were on treatment and within the age range >18 years to <60 years. Nondiabetic healthy individuals served as controls. Patients with alcohol intake > 40 g/day, intake of Orlistat or Acarbose and Gliptins, history of chronic diarrhea and known pancreatic disorders, GI surgery, immunodeficiency, cancer, critical illness, or acute infective disorders were excluded from the study. The patient's demographic data, glycemic parameters, glycated hemoglobin (HbA1c), peripheral neuropathy (PN), retinopathy, peripheral pulses, proteinuria, electrocardiogram (ECG) abnormality, and hemoglobin were used across the level of pancreatic insufficiency (level of FE-1), and statistical significance was estimated. Routine blood investigations such as fasting blood sugar (FBS), postprandial blood sugar (PPBS) by glucose oxidase method using calorimeter, and HbA1c by high-performance liquid chromatography were carried out. FE-1 levels were measured using commercial kit (Human Pancreatic Elastase ELISA BS 86-01 from Bioserv Diagnostics). As per the validated cutoffs, levels of <100 μg/g, 100–200 μg/g and >200 μg/g were considered as severe pancreatic insufficiency, moderate pancreatic insufficiency, and normal pancreatic function, respectively. In addition, possible correlations between FE-1 levels and patients’ characteristics such as gender, the duration of diabetes, and the blood glucose levels were also analyzed. Data analysis was performed based on the important statistical parameters such as mean, standard deviation, standard error, t-test-independent samples, and Chi-square test/cross tabulation using SPSS for windows version 20.0 (IBM statistical software).
RESULTS
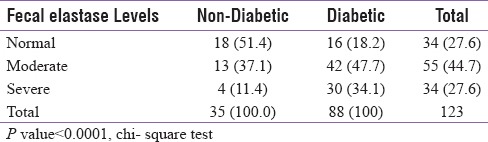
The study was conducted on a total of 123 patients, out of which 88 were T2DM patients, and 35 were nondiabetic healthy volunteers (control group) [Table 1]. Among 88 diabetics, 52 (59.1%) were male, and 36 (40.9%) were female [Table 1]. In control group, 18 (51.4%) subjects had normal FE-1 levels, 13 (37.1%) had moderate, and 4 (11.4%) had severe FE-1 deficiency whereas in the study group (diabetics), only 16 (18.2%) patients had normal FE-1 levels, 42 (47.7%) had moderate, and 30 (34.1%) had severe deficiency of FE-1. Statistically nonsignificant (P = 0.5051) relationship between FE-1 deficiency and age was observed, suggesting that the exocrine pancreatic insufficiency is noncorrelated with age among diabetic patients [Table 2]. The results also implied a statistically nonsignificant relationship of gender with FE-1 levels. There were nondiabetic controls with some degree of exocrine pancreatic dysfunction; however, the FE-1 deficiency was found to be more among diabetics. Statistically nonsignificant relationship (P = 0.641) was observed between the use of antidiabetic medications and FE-1 levels. There was no significant correlation between FE-1 levels and duration of diabetes. A significant linear correlation of FE-1 deficiency among diabetics with the degree of dysglycemia was observed. Statistically significant P values were achieved between glycemic parameters such as FBS (P = 0.001), PPBS (P = 0.012), HbA1c (0.001) [Table 3], and FE-1 levels in diabetic patients whereas the association between glycemic parameters and FE-1 level was found to be nonsignificant in nondiabetic patients. The association between FE-1 levels and PN (P = 0.2) was not found to be statistically significant. The association between retinopathy (P 0.001) and peripheral pulses (P 0.001) with FE-1 levels was found to be statistically significant [Table 2]. As per the results, there was statistically nonsignificant relationship between proteinuria (0.3), ECG abnormality (P = 0.90), and hemoglobin levels with FE-1 level. The mean age of study subjects (diabetics) was 47.53 ± 8.9 years and control group (nondiabetic) was 47.77 ± 9.0 years. The mean FE-1 levels were found to be higher, i.e., 179.85 ± 48.91 μg/g of stool among nondiabetics compared to diabetics (137.38 ± 60.53 μg/g of stool). This difference was found to be statistically significant (P < 0.0001) [Table 4].
Table 1.
Age and Gender distribution of study subjects
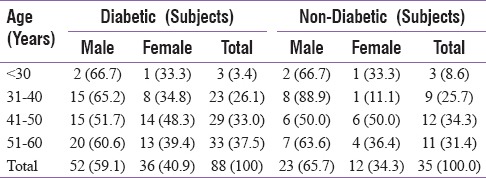
Table 2.
Relationship of various parameters with fecal elastase-1 activity
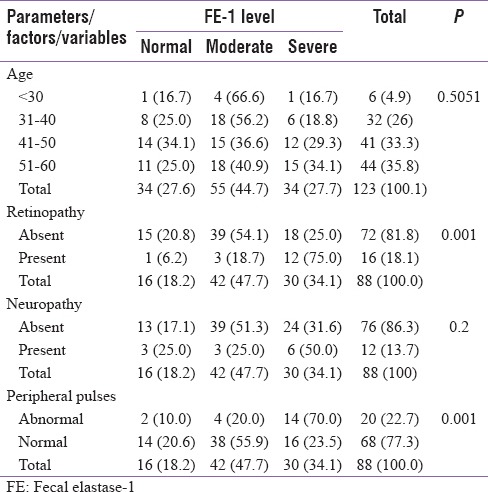
Table 3.
Relationship of glycemic parameters with FE-1 activity
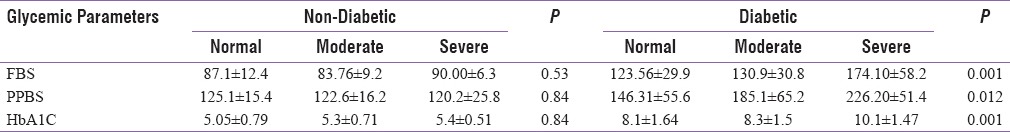
Table 4.
Distribution of FE-1 levels between diabetic and non- diabetic controls
DISCUSSION
The current study validated a significant correlation between pancreatic exocrine dysfunction with endocrinal impairment by involving FE-1 as a consistent biomarker. Elevated FE-1 level was detected in both healthy volunteers and T2DM patients. The study also emphasized a correlation between several parameters such as gender, level of hyperglycemia, and duration of diabetes with exocrine pancreatic dysfunction.
Exocrine dysfunction of pancreas is amongst the newer arena of studies in the field of diabetes, preventive gastroenterology and other fields of medicine. For example, disorders of the pancreas such as acute or chronic pancreatitis, cystic fibrosis, hemochromatosis, and pancreatic carcinomas. primarily affect the functioning of the exocrine system, but in some cases, the endocrine system was also found to be impacted and resulted in diabetes although the reported percentage was minimal (0.5%–1.15%).[7] The previous research validates that the size of the pancreas in diabetic patients is smaller than the healthy individuals.[8] Moreover, exocrine pancreatic insufficiency interrupts the food absorption process, which in turn, causes deregulation of the blood glucose level.[8,9] FE-1 can be utilized as a biomarker for analyzing exocrine pancreatic dysfunction, and a quantitative test can be easily performed using sandwich ELISA to achieve a highly specific report.[10,11,12,13,14] FE-1 is found to possess stability for an extended period and remains unaffected even after medications, gastric surgery, dysmotility, or small intestine diseases.[15,16] FE-1 was reported to be superior to both fecal lipase and fecal chymotrypsin tests in the assessment of exocrine pancreatic function.[17,18,19,20,21,22] However, there is a lack of a single, comparative study focusing only on the intricate relationship between FE-1 measurement and levels of hyperglycemia in T2DM in India. In our study, FE levels were measured and correlated between diabetics and nondiabetics. In 88 diabetics, 30 (34.1%) had severe deficiency, 42 (47.7%) had moderate FE deficiency, and only 16 (18.2%) had normal FE-1 levels. There was a statistically significant association between diabetes and FE-1 levels. The observations, in our current study, were found to be comparable to the results obtained from the study conducted by Andriulli et al. among 556 patients with T2DM where mild/moderate and severe FE-1 were reported in 275 and 281 patients, respectively.[23] After pooling the data, weighted estimates of mild/moderate or severe FE-1 were 49.7% and 50.3%, respectively, (P = 0.023). As per Löser et al., based on FE-1 level measurement, significantly higher proportion of diabetics possessed pancreatic insufficiency.[24] These literature findings substantiate the usefulness of FE-1 levels in assessing pancreatic exocrine insufficiency in T2DM. Various international studies have observed the relationship between T2DM and its relation to exocrine dysfunction of pancreas and various levels of hyperglycemia.[5,16,25,26] In our current study, no correlation between FE-1 levels and duration of diabetes was observed. Study conducted by Hardt and Ewald on 1021 patients (334 females, 687 males; mean age 50 years; mean diabetes duration 11 years; mean age at onset of diabetes 39 years) attained similar outcome.[27] FE-1 was found to be normal (>200 μg/g) in 59.3% and severely reduced (<100 μg/g) in 22.9% which further verified weak associations between FE-1 and diabetes duration. As per Rathmann et al., no significant association was found between diabetes duration and FE-1 levels which was based on a study conducted on 544 T2DM patients, in which FE-1 concentrations were significantly lower in study subjects compared to controls (median: cases 308 μg/g; controls 418 μg/g; P < 0.01).[28] Whereas, contradictory results were obtained from a study conducted by Ewald et al.[27] The study conducted among 307 subjects with 167 diabetics demonstrated a significant (P < 0.004) positive correlation between duration of diabetes and pancreatic exocrine insufficiency. Observations from our study implied no significant correlation between the use of antidiabetic medication and FE-1 level. Similar observation was also evidential from a prospective study conducted by Nunes et al., where 80 patients were enrolled, of which 42 patients with DM, diagnosed for 11.5 ± 8 years, with structural changes of the pancreas detected on ultrasound in three cases and calcifications in one case.[29] There was no relationship between FE-1 determination <200 μg/g and the duration or the type of therapy for DM. Amann et al. validated normal (>200, μg/g) FE-1 concentration in four out of seven patients with mild-to-moderate exocrine pancreatic insufficiency.[30] This low sensitivity might be due to the different subclassification criteria. As per our study, the association between FE-1 levels and PN was not found to be statistically significant function. Moreover, identical outcome was evidential from a study conducted by Rathmann et al. studied where, FE-1 concentrations were significantly lower in experimental group than control (P < 0.01), and therefore, no significant association was found in-between FE-1 level and diabetic PN.[28]
In this current study, statistically significant correlation (P = 0.001) between HbA1c and FE-1 levels was evidential. A similar outcome was also acquired by Ewald et al. where statistically significant (P < 0.031) HbA1c levels were obtained with pancreatic exocrine insufficiency as mild/moderate and severe in 307 subjects with 167 diabetics as study group.[31] Diabetic angiopathy has been proposed to cause arterial lesions and leads to pancreatic fibrosis and exocrine atrophy. The hypothesis that exocrine pancreatic pathology is a diabetes-associated complication mediated by angiopathy implicates that it may be correlated with diabetes duration. In our current study, out of 88 diabetic patients, 20 (22.7%) had abnormality in peripheral pulses (macroangiopathy) among which 2 (10.0%) had normal, 4 (20.0%) had moderate, and 14 (70.0%) had severe elastase insufficiency. Statistically significant relationship was found to be associated between peripheral pulses and FE-1 levels. A compatible outcome was obtained in a study conducted by Larger et al. in 2012 where FE-1 concentration was <200 μg/g in 23% of the patients.[32] In patients with T2DM, association of decreased pancreatic exocrine function with vascular disease suggests a role of pancreatic arteriopathy. Hence, this proves that, diabetic angiopathy is associated with low FE levels and pancreatic exocrine insufficiency. In our study, 73 (82.9%) diabetics had HbA1c ≥7%, of which 30 (41.09%) had severe, 33 (45.20%) had moderate deficiency, and only 10 (13.6%) had normal FE-1 levels with statistically significant correlation (P = 0.003) between HbA1c and FE-1 levels. A similar outcome was analyzed in research conducted by Terzin et al. which included a total of 101 diabetic subjects who were divided into, HbA1c 7% (n = 59) and HbA1c <7% (n = 42) followed by FE-1 level were assessment.[33] It was found that FE-1 levels were significantly higher in group HbA1c <7% (454.6 ± 147.3 μg/g) compared with HbA1c 7% (385.9 ± 171.1 μg/g) with significant P value (0.038).[33] A study conducted by Ewald et al. also exhibited a significant (P < 0.031) positive association between the levels of hyperglycemia by measuring HbA1c levels with pancreatic exocrine insufficiency as mild/moderate and severe in 307 subjects with 167 diabetics as a study group.[31] Research conducted by Yilmaztepe et al. had validated that the exocrine function declined in 28% of T2DM patients while there was no decrease in the control subjects.[34,35] However, there were no significant correlations between pancreatic elastase levels and the duration of diabetes. Hence, this study has revealed that the FE-1 estimated by ELISA can help us to prove exocrine pancreatic function and found to be associated with the levels of hyperglycemia in T2DM concurring with similar studies supporting this finding. A more intense research involving a large sample size and a diverse age group is warranted to substantiate the association.
CONCLUSION
This study demonstrates the benefit of FE-1 estimation as a surrogate marker of exocrine pancreatic insufficiency during T2DM, which remains unmanifest and subclinical. The results obtained indicated significant deficiency of pancreatic FE- 1 level among diabetics as well as some nondiabetic individuals. The severity of exocrine pancreatic insufficiency was found to possess statistically significant association with degree of dysglycemia, retinopathy, and peripheral vascular disease based on the level of FE-1 deficiency. Early identification and treatment with pancreatic enzyme supplementation of may help to improve the quality of life in patients with severe pancreatic insufficiency.
Financial support and sponsorship
Karnataka RSSDI (KRSSDI), Bangalore.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kloppel G, Bommer G, Commandeur G, Heitz P. The endocrine pancreas in chronic pancreatitis. Immunocytochemical and ultrastructural studies. Virchows Arch A Pathol Anat Histol. 1978;377:157–74. doi: 10.1007/BF00427003. [DOI] [PubMed] [Google Scholar]
- 2.Gatling W, Mullee M, Hill R. General characteristics of community based diabetic population. Pract Diabetes. 1988;5:104–7. [Google Scholar]
- 3.Lankisch PG, Manthey G, Otto J, Koop H, Talaulicar M, Willms B, et al. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion. 1982;25:211–6. doi: 10.1159/000198833. [DOI] [PubMed] [Google Scholar]
- 4.Gilbeau JP, Poncelet V, Libon E, Derue G, Heller FR. The density, contour, and thickness of the pancreas in diabetics: CT findings in 57 patients. AJR Am J Roentgenol. 1992;159:527–31. doi: 10.2214/ajr.159.3.1503017. [DOI] [PubMed] [Google Scholar]
- 5.Foulis AK, Frier BM. Pancreatic endocrine-exocrine function in diabetes: An old alliance disturbed. Diabet Med. 1984;1:263–6. doi: 10.1111/j.1464-5491.1984.tb01970.x. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca V, Berger LA, Beckett AG, Dandona P. Size of pancreas in diabetes mellitus: A study based on ultrasound. Br Med J (Clin Res Ed) 1985;291:1240–1. doi: 10.1136/bmj.291.6504.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–5. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- 8.Ammann RW, Akovbiantz A, Häcki W, Largiadèr F, Schmid M. Diagnostic value of the fecal chymotrypsin test in pancreatic insufficiency, particularly chronic pancreatitis: Correlation with the pancreozymin-secretin test, fecal fat excretion and final clinical diagnosis. Digestion. 1981;21:281–9. doi: 10.1159/000198578. [DOI] [PubMed] [Google Scholar]
- 9.Lami F, Callegari C, Miglioli M, Barbara L. A single-specimen fecal chymotrypsin test in the diagnosis of pancreatic insufficiency: Correlation with secretin-cholecystokinin and NBT-PABA tests. Am J Gastroenterol. 1984;79:697–700. [PubMed] [Google Scholar]
- 10.Dürr HK, Otte M, Forell MM, Bode JC. Fecal chymotroypsin: A study on its diagnostic value by comparison with the secretin-cholecystokinin test. Digestion. 1978:404–9. doi: 10.1159/000198143. [DOI] [PubMed] [Google Scholar]
- 11.Sale JK, Goldberg DM, Thjodleifsson B, Wormsley KG. Trypsin and chymotrypsin in duodenal aspirate and faeces in response to secretin and cholecystokinin-pancreozymin. Gut. 1974;15:132–8. doi: 10.1136/gut.15.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sziegoleit A. A novel proteinase from human pancreas. Biochem J. 1984;219:735–42. doi: 10.1042/bj2190735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sziegoleit A, Linder D. Studies on the sterol-binding capacity of human pancreatic elastase 1. Gastroenterology. 1991;100:768–74. doi: 10.1016/0016-5085(91)80024-4. [DOI] [PubMed] [Google Scholar]
- 14.Sziegoleit A, Krause E, Klör HU, Kanacher L, Linder D. Elastase 1 and chymotrypsin B in pancreatic juice and feces. Clin Biochem. 1989;22:85–9. doi: 10.1016/s0009-9120(89)80003-7. [DOI] [PubMed] [Google Scholar]
- 15.Muench R, Ammann R. Fecal immunoreactive lipase: A new tubeless pancreatic function test. Scand J Gastroenterol. 1992;27:289–94. doi: 10.3109/00365529209000077. [DOI] [PubMed] [Google Scholar]
- 16.Domínguez-Muñoz JE, Hieronymus C, Sauerbruch T, Malfertheiner P. Fecal elastase test: Evaluation of a new noninvasive pancreatic function test. Am J Gastroenterol. 1995;90:1834–7. [PubMed] [Google Scholar]
- 17.Stein J, Jung M, Sziegoleit A, Zeuzem S, Caspary WF, Lembcke B. Immunoreactive elastase I: Clinical evaluation of a new noninvasive test of pancreatic function. Clin Chem. 1996;42:222–6. [PubMed] [Google Scholar]
- 18.Borowitz D, Baker SS, Duffy L, Baker RD, Fitzpatrick L, Gyamfi J, et al. Use of fecal elastase-1 to classify pancreatic status in patients with cystic fibrosis. J Pediatr. 2004;145:322–6. doi: 10.1016/j.jpeds.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 19.Hardt PD, Marzeion AM, Schnell-Kretschmer H, Wüsten O, Nalop J, Zekorn T, et al. Fecal elastase 1 measurement compared with endoscopic retrograde cholangiopancreatography for the diagnosis of chronic pancreatitis. Pancreas. 2002;25:e6–9. doi: 10.1097/00006676-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Walkowiak J, Lisowska A, Przyslawski J, Grzymislawski M, Krawczynski M, Herzig KH. Faecal elastase-1 test is superior to faecal lipase test in the assessment of exocrine pancreatic function in cystic fibrosis. Acta Paediatr. 2004;93:1042–5. doi: 10.1111/j.1651-2227.2004.tb02715.x. [DOI] [PubMed] [Google Scholar]
- 21.Walkowiak J, Herzig KH, Strzykala K, Przyslawski J, Krawczynski M. Fecal elastase-1 is superior to fecal chymotrypsin in the assessment of pancreatic involvement in cystic fibrosis. Pediatrics. 2002;110(1 Pt 1):e7. doi: 10.1542/peds.110.1.e7. [DOI] [PubMed] [Google Scholar]
- 22.Major RH. A History of Medicine. Springfield, IL: Charles C Thomas; 1954. [Google Scholar]
- 23.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–48. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gröger G, Layer P. Exocrine pancreatic function in diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:740–6. [PubMed] [Google Scholar]
- 25.Cavalot F, Bonomo K, Perna P, Bacillo E, Salacone P, Gallo M, et al. Pancreatic elastase-1 in stools, a marker of exocrine pancreas function, correlates with both residual beta-cell secretion and metabolic control in type 1 diabetic subjects. Diabetes Care. 2004;27:2052–4. doi: 10.2337/diacare.27.8.2052. [DOI] [PubMed] [Google Scholar]
- 26.Hardt PD, Ewald N. Exocrine pancreatic insufficiency in diabetes mellitus: A complication of diabetic neuropathy or a different type of diabetes? Exp Diabetes Res. 2011;2011:761950. doi: 10.1155/2011/761950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andriulli A, Ippolito AM, Festa V, Valvano MR, Merla A, Bossa F, et al. Exocrine Pancreatic Insufficiency, as Assessed by Fecal Elastase-1 Levels, in Diabetic Patients: An Estimate of Prevalence in Prospective Studies. Journal of Diabetes & Metabolism. 2014;5:1–6. [Google Scholar]
- 28.Rathmann W, Haastert B, Icks A, Giani G, Hennings S, Mitchell J, et al. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroenterol. 2001;36:1056–61. doi: 10.1080/003655201750422657. [DOI] [PubMed] [Google Scholar]
- 29.Terzin V, Várkonyi T, Szabolcs A, Lengyel C, Takács T, Zsóri G, et al. Prevalence of exocrine pancreatic insufficiency in type 2 diabetes mellitus with poor glycemic control. Pancreatology. 2014;14:356–60. doi: 10.1016/j.pan.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Amann ST, Bishop M, Toskes PP. Fecal pancreatic elastase 1: Is it the test we have been looking for? Gastroenterology. 1995;108:A341. [Google Scholar]
- 31.Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: A novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39:580–6. doi: 10.1136/gut.39.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunes AC, Pontes JM, Rosa A, Gomes L, Carvalheiro M, Freitas D. Screening for pancreatic exocrine insufficiency in patients with diabetes mellitus. Am J Gastroenterol. 2003;98:2672–5. doi: 10.1111/j.1572-0241.2003.08730.x. [DOI] [PubMed] [Google Scholar]
- 33.Keller J, Layer P. The pathophysiology of malabsorption. Viszeralmedizin. 2014;30:150–4. doi: 10.1159/000364794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larger E, Philippe MF, Barbot-Trystram L, Radu A, Rotariu M, Nobécourt E, et al. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29:1047–54. doi: 10.1111/j.1464-5491.2012.03597.x. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaztepe A, Ulukaya E, Ersoy C, Yilmaz M, Tokullugil HA. Investigation of fecal pancreatic elastase-1 levels in type 2 diabetic patients. Turk J Gastroenterol. 2005;16:75–80. [PubMed] [Google Scholar]


