Abstract
Background:
Breast milk adiponectin could play a role in the regulation of infants’ growth during lactation.
Aim of Work:
The aim is to evaluate adiponectin concentration in human milk and to investigate its relationship with serum adiponectin concentration in lactating mothers and their breastfed infants and with anthropometric parameters of infants and mothers.
Materials and Methods:
Sixty healthy term infants and their healthy lactating mothers are included at infant age of 1 month then repeated again at the age of 4 months. All subjects included in this study were subjected to history, clinical examination, investigations including serum level of adiponectin of infants and their mothers by RIA test, human milk level of adiponectin by ELISA test.
Results:
There was a significant decrease in serum adiponectin of infant and mothers and maternal breast milk at the age of 4 months when compared to them at the age of 1 month. There was a significant positive correlation between infant serum adiponection, maternal serum adiponectin and breast milk adiponectin at infant's age of 1 month and at infant's age of 4 months. There was a significant negative correlation between maternal serum adiponectin and BMI of mothers. There was a significant negative correlation between infant serum adiponectin and their weight and length of infants at the age of 1 month and at the age of 4 months.
Conclusions:
There's a metabolic link between mothers and their infants through breast milk during the first 6 months of life. A gradual decline in adiponectin level in maternal breast milk is associated with a gradual increase in infant growth up to 6 months of age.
Keywords: Adiponectin, anthropometric parameters, human milk
INTRODUCTION
Human milk contains a wide variety of high biological value proteins that provide adequate nutrition to breastfed infants and simultaneously help in the development of important physiological functions.
Adiponectin, one of the most important hormones related to adipose depots, In addition to its peripheral actions regulating lipids and glucose metabolism,[1] adiponectin has central activity in the regulation of energy homeostasis; stimulate food intake and reducing energy expenditure.[2]
Adiponectin circulates in human blood as three distinct isomeric forms: trimeric low molecular weight, hexameric medium molecular weight, and high molecular weight (HMW) adiponectin, which consist of large multimers of 12–18 subunits.[3] Recent evidence suggest that HMW adiponectin is the most active form exerting metabolic functions and it is the most abundant form present in HM, suggesting that milk adiponectin could play a significant role in the early regulation of infants’ growth during lactation.[3]
Its plasma concentrations range from 0.5 to 30 ug/ml, 1000-fold higher than the concentrations of other hormones, such as leptin.[2] A great evidence suggest that adiponectin serum concentration in neonates and infants is higher than those found in children and adults.[4,5] In previous studies, it has been observed a direct association between cord blood adiponectin concentration and birth weight,[4] whereas other studies have not confirmed this correlation.[6]
These findings suggest that prenatal and early postnatal periods are critical for the development of metabolic homeostasis and that adiponectin could play a role in the programming of energy balance.[7]
The aim of the work is to evaluate adiponectin concentration in human milk and to investigate its relationship with serum adiponectin concentration in lactating mothers and their breastfed infants and with infants’ and mothers, anthropometric parameters (weight and length).
MATERIALS AND METHODS
Design of the study sample size and setting
After research ethical committee approval From Aswan Faculty of Medicine and informed oral or written parental consents from all participants in this research, We performed a follow-up cohort study.
Sample size
The study was conducted on 60 healthy term infants aged 1 month and their healthy lactating mothers at the age of 1 month then had been studied again at the age of 4 months. All the subjects that enrolled in our study were seen in the outpatient clinic of Aswan University hospital for brief observation for mild pathological conditions. The study was carried out in the period between June 2016 and June 2017.
Inclusion criteria
Chronological age <6 months. Their gestational age between 38 and 42 weeks, their birth weight between 2500 and 4000 g, their APGAR score higher than 7 at 5 min and exclusively breastfed infants.
Exclusion criteria
Major neonatal diseases, fever, chronic illness, acute disorder compromising growth e.g., acute gastroenteritis or partial breastfeeding.
All infants and their lactating mothers included in this study were subjected to the following:
Full history taking
Stressing on age and sex of infants’ age of mothers antenatal history (Maternal diseases associated with or complicating pregnancy and antenatal fetal assessment) and duration of exclusive breastfeeding.
Full clinical examination
with special emphasis on anthropometric measurements of infants and their mothers.
Infants were weighed naked before feeding, crown-to-heel length was measured
-
and body mass index (BMI) was calculated by the relation
- BMI = body weight (Kg)/square of length (m2)
We were evaluated anthropometric parameters also in mothers: weight (Kg) height (m) and BMI.
All anthropometric measurement s were taken by a single trained investigator.
LABORATORY INVESTIGATIONS
Which included routine investigations (complete blood count, total serum proteins, serum albumin, and C-reactive protein) and our research laboratory investigations (serum level of adiponectin for mothers and infants measured by radioimmunoassay test (RIA) and level of maternal breast milk adiponectin measured using enzyme-linked immunosorbent assay. (human adiponectin ELISA kit catalog NoE0605 h, Wuhan EIAab Science Co., Ltd, Wuhan, China).
Sampling
Infants’ serum adiponectin levels
Venous blood samples from infants were collected under complete aseptic technique at the same day after fasting of at least 3 h.
Maternal serum adiponectin levels
Venous blood samples from lactating mothers were collected after nocturnal fasting. Serum was separated by centrifugation at 4000 rpm for 10 min and kept at −20° until analysis.
Adiponectin concentration were measured using RIA, with commercial kit adiponectin human RIA-3765, DRG diagnostic (GmbH, Marburg Germany).
Human milk adiponectin levels
Samples were collected in the morning. Milk of lactating mothers who consented to participate in the study.
Milk (5 ml each sample) were collected by manual expression and frozen at −20° until analysis.
Samples were thawed at room temperature and vortex continuously during pipetting to ensure sample uniformity.
The whole mix was centrifuged at 2000 g for 20 min at 4°C, fat layer was removed, and the aqueous phase was used for assays.
Adiponectin was measured in skimmed milk
Adiponectin concentration was determined by enzyme-linked immune sorbet assay kit (human adiponectin ELISA kit catalog NoE0605 h, Wuhan EIAab Science Co., Ltd, Wuhan, China).
Milk samples were collected at the same time as blood samples from infants and mothers for serum adiponectin evaluation.
Test principle
This assay employed the RIA technique in serum and enzyme-linked immunosorbent assay in human milk. A monoclonal antibody specific for the adiponectin globular domain.
The microtitreplate has been precoated with an antibody specific to. Standards or samples are then added to appropriate microtitre plate wells with a biotin-conjugated polyclonal antibody preparation specific for and avid in conjugated to horseradish peroxidase is added to each microplate well and incubated. Then a TMB substrate solution is added to each well. Only those wells that contain biotin-conjugated antibody and enzyme-conjugated avidin will exhibit a change in color.
The enzyme-substrate reaction is terminated by the addition of sulforic acid solution and the color change is measured spectrophotometrically at wavelength of 450 nm ± 2 nm. The concentration of in the samples is then determined by comparing the optical density (OD) of the samples to the standard curve.
Materials and reagents
Reagent, assay plate, standard, sample diluents, assay diluent A, assay diluent B, detection reagent A, detection reagent B, wash buffer (25 × concentrates), substrate, stop solution, plate sealer for 96 wells.
Other supplies were usedas follows: microplate reader, pipettes and pipette tips, EP tube, deionized, or distilled water.
Reagent preparation
All reagents were brought to room temperature before use.
Wash buffer
when crystals had formed in the concentrate, it was warmed to room temperature and mixed gently until the crystals had completely dissolved. A volume of 30 ml of wash buffer concentrate was diluted with deionized or distilled water to prepare 750 ml of wash buffer.
Standard
Was reconstituted with 1 ml of sample diluents. This reconstitution produced a stock solution of 10 ng/ml. The standard was allowed to sit for a minimum of 15 min with gentle agitation before making serial dilution. The undiluted standard was served as the high standard 10 ng/ml). The sample diluent was served as the zero standards (0 ng/ml).
Detection reagent A and B diluted to the working concentration using assay diluent A and B (1:100), respectively.
Adiponectin assay procedure
Ten microliter of standard, blank, or sample were added per well, Covered with the plate sealer and Incubated for 2 h at 37°C
The liquid of each well was removed then 100 μl of detection reagent A working solution was added to each well, covered with plate sealer and Incubated for an hour at 37°C
Each well was aspirated and washed. The process was repeated three times for a total of three washes. Wash by filling each well with wash buffer (approximately 400 μl) using a squirt bottle, multichannel pipette, manifold dispenser or auto washer. Complete removal of liquid at each step is essential to good performance. After the last wash, any remaining wash buffer was removed by aspirating or decanting. The plate was inverted and blotted against clean paper towels
One hundred microliter of detection reagent B working solution was added to each well, covered with a new plate sealer and Incubated for 2 h at 37°C
The aspiration/wash were repeated as in step 4
Ninety microliter of substrate solution was added to each well, covered with a new plate sealer, incubated within 15–30 min at 37°C and protected from light
Fifty microliter of stop solution was added to each well. The plate was gently taped when the color change did not appear uniform to ensure thorough mixing
The OD of each well was determined at once, using a microplate reader set to 450 nm.
Detection range: 0.156–10 ng/ml.
Calculation of results
The duplicate readings for each standard control and sample were averaged and the average zero standard OD was subtracted
The mean absorbance for each standard on the X-axis against the concentration on the Y-axis was plotted, and the best fit curve was drawn through the point on the graph. The data were linearzed by plotting the log of the concentration versus the log of the OD, and the best fit line was determined by regression analysis
The samples were diluted with sample diluents when samples generated values higher than the highest standard, and the assay was repeated. The concentration read from the standard curve had been multiplied by the dilution factor.
Statistical analysis
“Data were collected and analyzed using SPSS for windows” (version 12, New Delhi, India). All Data were expressed in terms of mean ± standard deviation comparisons among groups were made using paired t-test. “Two-group comparisons were performed nonparametrically using Mann–Whitney U-test.” “All statistical tests were two-tailed and P < 0.05 was considered to be statistically significant.”[8]
RESULTS
The baseline characteristics of the mother and infants are summarized in Table 1. The study results showed that 24 (40%) of studied infants were males and 36 (60%) were females with male:female ratio 1:1.5. This study showed that there is no significant difference in serum adiponectin of infants as regard to sex (P > 0.05) [Table 2]. Table 2 revealed that there was a significant decrease in serum adiponectin of infant and mothers and maternal breast milk adiponectin at the age of 4 months when compared to them at the age of 1 month. Table 2 and Figures 1 and 2 revealed that there was a significant positive correlation between infant serum adiponection, maternal serum adiponectin, and breast milk adiponectin at infant age of 1 month but there was a significant negative correlation between infant serum adiponectin and their anthropometric parameters (weight and length and BMI) of infants at the age of 1 month and at the age of 4 months.
Table 1.
Baseline characteristics of studied infants and their mothers
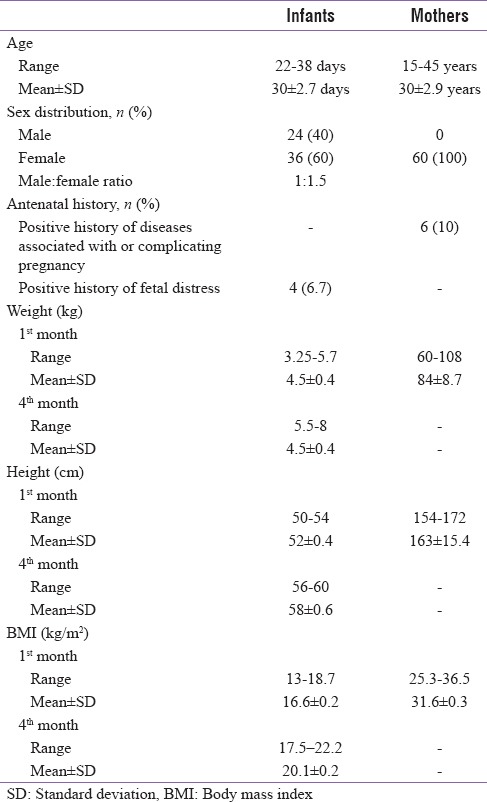
Table 2.
Comparison between levels of adiponectin of infants serum, maternal serum, and breast milk at the age of 1 and 3 months
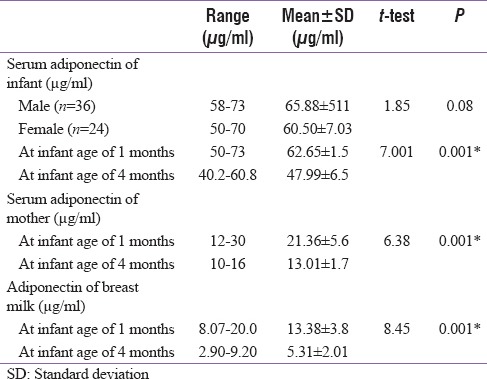
Figure 1.
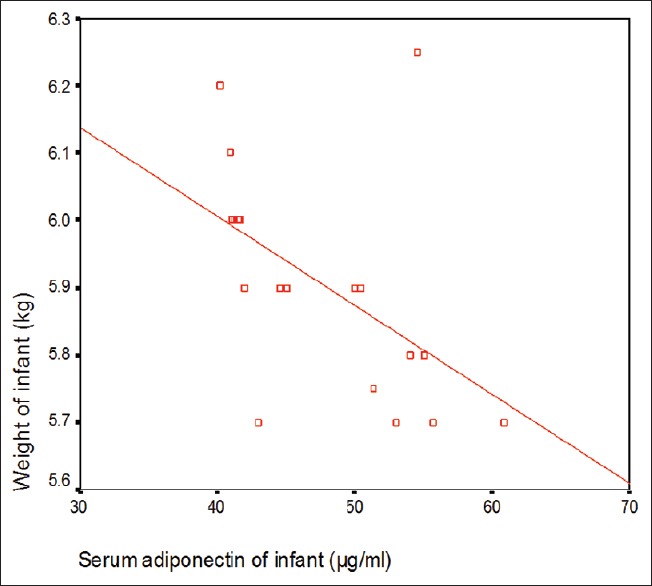
Correlation between infant serum adiponectin and weight of infant at age of 1 months
Figure 2.
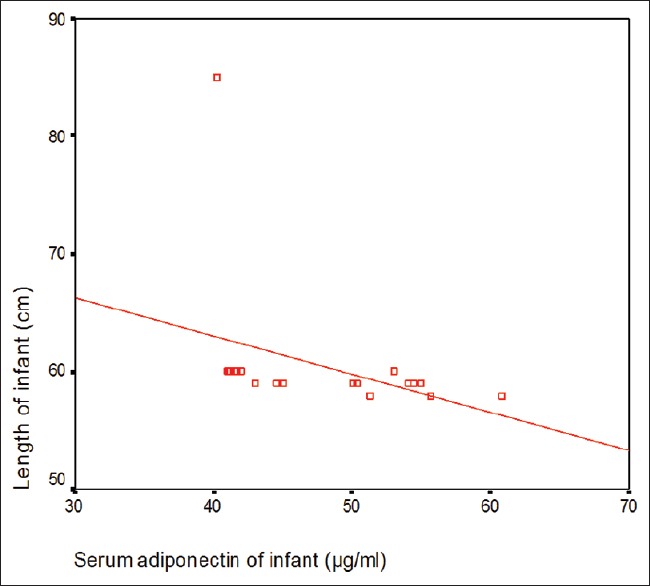
Correlation between infant serum adiponectin and length of infant at age of 4 months
Table 3 and Figure 3 revealed that there was a significant negative correlation between maternal serum adiponectin and BMI of mothers. Figure 4 showed significant positive correlation between maternal serum adiponectin and body mass index (BMI) of mothers.
Table 3.
Correlations between adiponectin levels and anthropometric parameters of at infant and their mothers
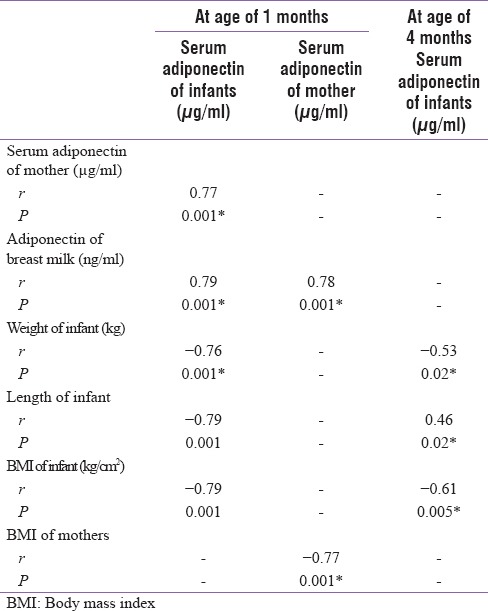
Figure 3.
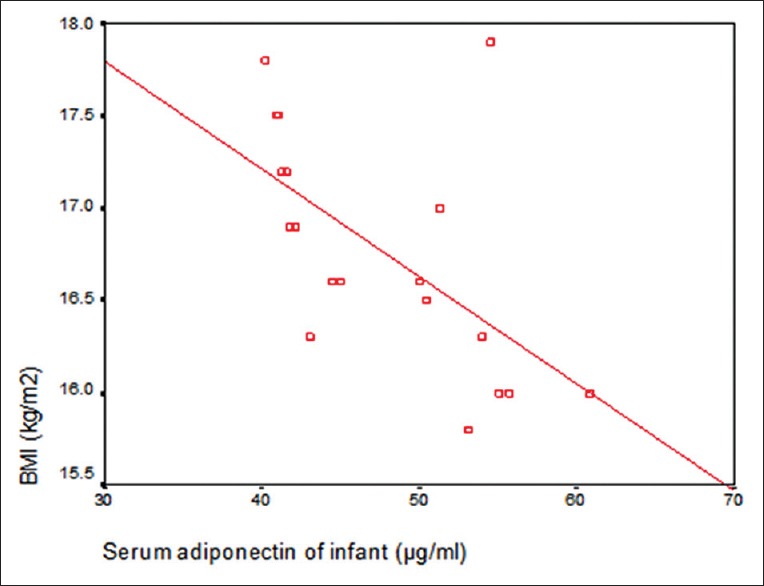
Correlation between infant serum adiponectin and body mass index of infant at age of 4 months
Figure 4.
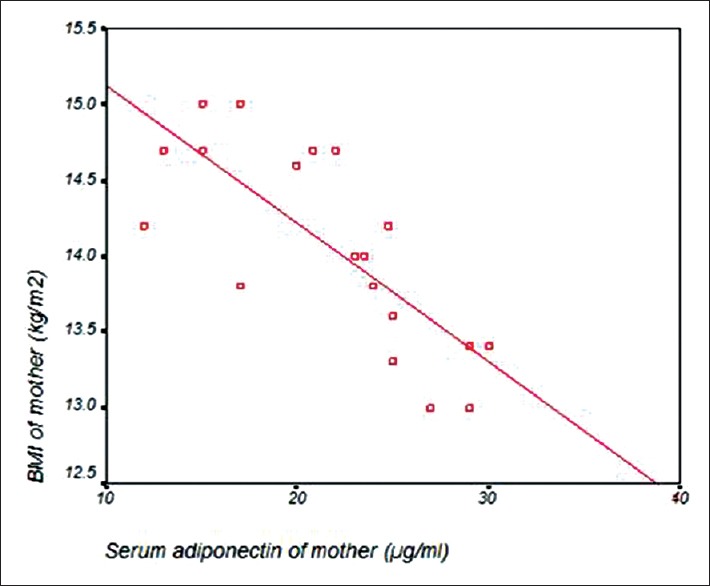
Correlation between maternal serum adiponectin and body mass index (BMI) of mothers
DISCUSSION
Newly identified components of human breast milk, especially regulatory metabolic hormones such as adiponectin, leptin, ghrelin, and adipocyte fatty acid binding protein are considered to influence nutritional status and possibly play a role in the development of components of metabolic syndrome later in adulthood.[9,10,11] In study, 20 forty healthy term infants and their healthy lactating mothers underwent brief observation for mild pathological conditions. They were observed at the age of 1 and 4 months.
As regard to the demographic data, the results showed that 24 out of 60 infants (40%) were males and 36 (60%) were females with male: female ratio 1:1.5. This study showed that there was no significant difference in serum adiponectin of infants as regard to sex (P > 0.05). These results were in agreement with a study carried by Inami et al. 2007 who revealed that there was no significant difference in serum adiponectin of infants between males and females at the age of 1 month.[12]
As regard to this study, there was a highly significant decrease in serum adiponectin levels of infants at the age of 4 months when compared to them at the age of 1 month (P < 0.01). These results were in agreement with Cesur et al. 2012 who revealed that mean of infant serum adiponectin was 15.09 ± 7.81 μg/ml at the age of 1 month and was 6.60 ± 7.46 μg/ml at the age of 4 months (P < 0.05).[13] The study results were also in agreement with Woo et al., 2012 who revealed that there was a highly significant decrease in serum adiponectin of infants at age of 12 months (mean = 23.5 ± 0.8 μg/ml) when compared to it at age of 6 months (mean = 28.6 ± 0.6 μg/ml) and there was a highly significant decrease in serum adiponectin of infants at age of 6 months when compared to it at age of 3 months (mean = 33.2 ± 0.5 μg/ml) but there is was significant increase in infant serum adiponectin between base line (mean = 31.1 ± 0.6 μg/ml) and at 3 month of age (P < 0.01).[14]
As regards adiponectin in maternal breast milk in our study, there was a highly significant decrease of in its levels at infants’ age of 4 month when compared to it at age of 1 month (P < 0.001). These results were in agreement with a study carried by Burner et al., 2014 who revealed that the median values of both leptin and adiponectin in maternal breast milk were slightly lower at infants’ age of 4 month (median = 0.09 ng/ml and 10.36 ng/ml, respectively) when compared to them at infants’ age of 6 weeks (median = 0.11 ng/ml and 10.93 ng/ml, respectively) (P < 0.001).[15] Another study conducted by Bronsky et al., 2011 revealed that mean value of adiponectin level of breast milk at infant's birth was changeable (22.8 ± 0.8 ng/ml, at the age of 1 month, 20.5 ± 0.6 ng/ml was at age of 3 months and 21.4 ± 0.8 ng/ml was at the age of 6 months).[16] Other studies such as a study done by Cesur et al. 2012 which revealed no significant difference between maternal breast milk adiponectin at infants’ age of 1 month (mean = 23.61 ± 32.95 ng/ml) and at the age of 4 months (mean = 6.66 ± 9.48 ng/ml) (P > 0.05).[13]
In the present study, as regard to maternal serum adiponectin levels, there was a highly significant decrease of maternal serum adiponectin at infants’ age of 4 months when compared to them at infants’ age of 1 month (P < 0.01). These results were in agreement with Burner et al. 2014 who revealed that there was a slight decrease in maternal plasma level of HMW adiponectin at infant age of 4 months when compared to it at infant age of 6 weeks with a significant decrease over time.[15] The results disagreed with a study conducted by Cesur et al. 2012 which revealed that there's no significant difference in maternal serum adiponectin at infants’ age of 4 months (mean = 7.75 ± 3.67 μg/ml) and at infants’ age of 1 month (mean = 8.36 ± 6.46 μg/ml) (P > 0.05).[13]
In our study, there was a significant positive correlation between infant serum adiponectin and their maternal breast milk adiponectin at infants’ age of 1 month (r = 0.79) (P < 0.001) and there was a significant positive correlation between infant serum adiponectin and maternal serum adiponectin at infants’ age of 1 month (r = 0.774) (P < 0.001). These results were in agreement with Woo et al. 2012 who revealed that higher infant serum adiponectin was associated with feeding with breast milk which contains higher level of adiponectin than artificial formula so there was a positive significant correlation between infant and their maternal serum adiponectin (r = 0.29 and P = 0.007) and (r = 0.37 and P = 0.001) at age of birth and 6 months, respectively.[14]
In our study, there was a significant positive correlation between maternal serum adiponectin and maternal breast milk adiponectin at infants’ age of 1 month (r = 0.784) (P = 0.001). These results were in agreement with Woo et al. 2011 who revealed that maternal serum adiponectin was significantly correlated with their own median breast milk adiponectin concentration at base line, 3 months and 6 months (r = 0.37 and P < 0.001).[14] Furthermore, these results were in agreement with Burner et al. 2014 who revealed that there is a high significant positive correlation between plasma and breast milk levels of adiponectin at infant's age of 6 weeks and 4 months (P < 0.001)[15] while these results disagreed with Cesur et al. 2012 who revealed that there was no significant correlation between maternal barest milk and their serum adiponectin (P > 0.05).[13]
This study showed that there is was a significant negative correlation between maternal serum adiponectin and their (BMI at infant's age of 1 month (r = −0.769 0.77 and P = 0.001). These results were in agreement with Matsubara et al. 2002 who revealed that plasma concentration of adiponectin in mothers with highest percentile of BMI (at least 25 kg/m2) were lower than these in the middle (22–25 kg/m2) or lowest (<22 kg/m2) percentile of BMI (mean = 6.7 ± 0.3 μg/ml versus 8.6 ± 0.4 μg/ml versus 9.2 ± 0.3 μg/ml, respectively) (P < 0.001).[16] This was also in agreement with Nedvidk et al. 2005 who revealed that adiponectin release in mother's serum is positively correlated with fat size and negatively correlated with BMI.[17] The results were are in agreement also with Hirose et al. 2010 which revealed that maternal serum HMW adiponectin was negatively correlated with their BMI (mean = 20.6 ± 2.9) (r = −0.216 and P < 0.001) also change in HMW adiponectin level was most strongly correlated with the change in BMI.[18]
This study showed that there was is a significant negative correlation between infants’ serum adiponectin and their anthropometric measurements (weight and length) at infant's age of 1 month and at infants’ age of 4 months. These results were disagreed by a study carried out performed by Cesur et al. 2012 which revealed that there is was a significant positive correlation between level of infant serum adiponectin at age of 1 month and their weight (r = 0.53 and P = 0.020) and also infant serum adiponectin were positively correlated with weight (r = 0.61 and P = 0.006), and BMI (r = 0.71 and P = 0.001) at age of 4 months.[13]
Limitation of this study was small sample size of studied infants and their mothers. Most of them were from rural community in which there was lack of nutritional health education. Hyperadiponectinemia may be as a possible cause of growth failure in infancy that need further research work on a wider scale.
CONCLUSIONS
Strengths of this study was it concluded that there was correlations between maternal serum, breast milk adiponectin and infants’ serum adiponectin which suggested that there's was a metabolic link between mothers and their infants through breast milk during 1st 6 months of life and that milk adiponectin could play a significant role in early regulation of infants’ growth during lactation. Our results suggest that a gradual decline in adiponectin level in maternal breast milk is associated with greater growth (weight gain and fat mass) in their infants up to 6 months of age.
Recommendations
Exclusive breast feeding is essential for 6 months because serum adiponectin in infants and mothers as well as in breast milk reach its maximal levels collectively during the first 6 months of life ( first half of 1st year of life) when compared with their levels during the second half of 1st year of life. Measurement of maternal serum and breast milk adiponectin and infant’ s serum adiponectin in the first 6 months of life are important for assessing adequate growth of these infants. In infants with retarded growth (weight and/or height), it is recommended to measure their serum adiponectin level as well as their mothers serum and breast milk adiponectin to exclude hyper adiponectinemia as a possible cause of growth failure and that need further research work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Savino F, Petrucci E, Nanni G. Adiponectin: An intriguing hormone for paediatricians. Acta Paediatr. 2008;97:701–5. doi: 10.1111/j.1651-2227.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 2.Newburg DS, Woo JG, Morrow AL. Characteristics and potential functions of human milk adiponectin. J Pediatr. 2010;156(2 Suppl):S41–6. doi: 10.1016/j.jpeds.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schöndorf T, Maiworm A, Emmison N, Forst T, Pfützner A. Biological background and role of adiponectin as marker for insulin resistance and cardiovascular risk. Clin Lab. 2005;51:489–94. [PubMed] [Google Scholar]
- 4.Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, Kuroda Y. Plasma adiponectin levels in new born are higher than those in adult and positively correlated with birth weight. Clin Endocrinol. 2004;61:418–23. doi: 10.1111/j.1365-2265.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- 5.Iniguez G, Soto N, Avila A, Salazar T, Ong K, Dunger D, et al. Adiponectin levels in first Two years of life in a prospective cohort: Relations with weight gain, leptin levels and insulin sensitivity. J Clin Endocrinol Metab. 2004;11:5500–3. doi: 10.1210/jc.2004-0792. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay RS, Walker JD, Havel PJ, Hamilton BA, Calder AA, Johnstone FD. Scottish Multicentre Study of Diabetes Pregnancy. Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care. 2003;26:2244–9. doi: 10.2337/diacare.26.8.2244. [DOI] [PubMed] [Google Scholar]
- 7.Savino F, Liguori SA, Lupica MM. Adipokines in breast milk and preterm infants. Early Hum Dev. 2010;86(Suppl 1):77–80. doi: 10.1016/j.earlhumdev.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Khothari CR, editor. Research Methodology, Methods and Techniques. 2nd ed. New Delhi: New Age International; 2012. pp. 95–7. [Google Scholar]
- 9.Savino F, Liguori SA, Petrucci E, Lupica MM, Fissore MF, Oggero R, et al. Evaluation of leptin in breast milk, lactating mothers and their infants. Eur J Clin Nutr. 2010;64:972–7. doi: 10.1038/ejcn.2010.105. [DOI] [PubMed] [Google Scholar]
- 10.Martin LJ, Woo JG, Geraghty SR, Altaye M, Davidson BS, Banach W, et al. Adiponectin is present in human milk and is associated with maternal factors. Am J Clin Nutr. 2006;83:1106–11. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- 11.Bronsky J, Mitrova K, Karpisek M, Mazoch J, Durilova M, Fisarkova B, et al. Adiponectin, AFABP, and leptin in human breast milk during 12 months of lactation. J Pediatr Gastroenterol Nutr. 2011;52:474–7. doi: 10.1097/MPG.0b013e3182062fcc. [DOI] [PubMed] [Google Scholar]
- 12.Inami I, Okada T, Fujita H, Makimoto M, Hosono S, Minato M, et al. Impact of serum adiponectin concentration on birth size and early post natal growth. Pediatr Res. 2007;61:604–6. doi: 10.1203/pdr.0b013e3180459f8a. [DOI] [PubMed] [Google Scholar]
- 13.Cesur G, Ozguner F, Yilmaz N, Dundar B. The relationship between ghrelin and adiponectin levels in breast milk and infant serum and growth of infants during early postnatal life. J Physiol Sci. 2012;62:185–90. doi: 10.1007/s12576-012-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo JG, Guerrero ML, Guo F, Martin LJ, Davidson BS, Ortega H, et al. Human milk adiponectin affects infant weight trajectory during the second year of life. J Pediatr Gastroenterol Nutr. 2012;54:532–9. doi: 10.1097/MPG.0b013e31823fde04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner S, Schmid D, Zang K, Much D, Knoeferl B, Kratzsch J, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. 2015;10:67–73. doi: 10.1111/j.2047-6310.2014.222.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173–80. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 17.Nedvídková J, Smitka K, Kopský V, Hainer V. Adiponectin, an adipocyte-derived protein. Physiol Res. 2005;54:133–40. [PubMed] [Google Scholar]
- 18.Hirose H, Yamamoto Y, Seino-Yoshihara Y, Kawabe H, Saito I. Serum high-molecular-weight adiponectin as a marker for the evaluation and care of subjects with metabolic syndrome and related disorders. J Atheroscler Thromb. 2010;17:1201–11. doi: 10.5551/jat.6106. [DOI] [PubMed] [Google Scholar]


