Abstract
Aims:
This study evaluated the efficacy and safety of teneligliptin in patients with inadequately controlled type 2 diabetes mellitus (T2DM).
Settings and Design:
This was a randomized, doubleblind, placebocontrolled, parallelgroup, multicenter, Phase III study.
Subjects and Methods:
Patients with T2DM and inadequate glycemic control (glycosylated hemoglobin [HbA1c]: >7.0-≤8.5%) were enrolled. Patients were randomly assigned (ratio: 2:1) to receive teneligliptin 20 mg (Glenmark) or placebo. The primary efficacy variable was change from baseline in HbA1c at week 16. Additional analyses included the proportion of patients who achieved target of HbA1c ≤7.0%, changes in fasting plasma glucose (FPG), and postprandial glucose (PPG).
Statistical Analysis:
Mean change in HbA1c was analyzed using an analysis of covariance model, least square (LS) means, 95% confidence intervals (CIs), and P values were calculated.
Results:
Overall, 237 patients were included. Patients of the teneligliptin group showed reduced HbA1c levels (LS mean difference = −0.304% for intent-to-treat [ITT]; −0.291% for per-protocol (PP) populations) after 16 weeks of treatment, and a statistically significant difference was observed between the ITT (LS mean difference = 0.555; 95% CI: 0.176–0.934; P = 0.0043) and PP populations (LS mean difference = 0.642; 95% CI: 0.233–1.052; P = 0.0023). Target HbA1c level was achieved by a greater proportion of teneligliptin group patients (ITT, 43.4%; PP, 43.6%) than placebo group patients (ITT, 27.3%; PP, 26.6%). Reduction in FPG levels was observed in ITT (LS mean difference: 8.829; 95% CI: −4.357–22.016; P = 0.1883) and PP populations (LS mean difference: 11.710 mg/dL; 95% CI: −2.893-26.312; P = 0.1154). Reduction in PPG levels was higher in teneligliptin group than placebo group in both ITT (LS mean difference = 25.849 mg/dL; 95% CI: 7.143–44.556; P = 0.0070) and PP populations (LS mean difference = 25.683 mg/dL; 95% CI: 5.830–45.536; P = 0.0115). Overall, 44 patients (18.6%) experienced at least one adverse event. Three or more hypoglycemic events were experienced by 2.5% patients of teneligliptin group and none in placebo group.
Conclusion:
Treatment with once-daily teneligliptin led to statistically significant and clinically meaningful reductions in HbA1c and PPG, and was well tolerated in Indian patients with T2DM.
Keywords: Glycemic control, teneligliptin, treatment naïve, type 2 diabetes mellitus
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) has risen rapidly over the recent years. The latest diabetes atlas of the International Diabetes Federation (IDF) reports over 415 million individuals with T2DM globally.[1,2] India ranks second in the world after China for the highest number of diabetes cases. The IDF reported 69.2 million diabetic individuals in India, and has anticipated this number to reach 123.5 million by 2040.[1]
Sedentary lifestyle and unhealthy eating habits are the major contributors to the increasing prevalence of T2DM.[3] Adequate glycemic control in T2DM is associated with reduction of mortality and morbidity.[4] Almost 60% patients, who are not able to achieve target glycosylated hemoglobin (HbA1c) level of 6%–7%, are more likely to experience complications.[5] Most therapeutic agents available for the treatment of T2DM reduce the macrovascular complications; however, these may lead to progressive beta-cell damage and deterioration of health due to T2DM.[6] In patients with T2DM, beta-cell function is reduced to 60% as compared with nondiabetic patients. Persistently elevated blood glucose levels result in hypertrophy, hyperplasia, and atrophy of the beta-cell mass.[7]
Beta-cell damage with anti-diabetic medications has prompted researchers to hypothesize that longterm use of these medications may be harmful to the remaining beta-cells.[8] While some drugs such as sulphonylureas are associated with progressive beta-cell loss;[9] gliptins (also known as dipeptidyl peptidase-4 [DPP-4] inhibitors) improve insulin secretion from the beta-cells of the pancreas in response to increased blood glucose levels. The insulin secretion is stimulated by secretion of higher levels of glucagon-like peptide-1 and glucose-dependent insulinotropic peptide that are enzymes released from the intestine and are responsible for regulation of blood glucose levels.[10,11,12] Several studies have demonstrated that gliptins including sitagliptin, vildagliptin, and saxagliptin reduce blood glucose levels.[10] Additionally, the use of gliptins is associated with fewer hypoglycemic events.[13]
Teneligliptin is a relatively new gliptin. It has also produced improvements in fasting plasma glucose (FPG) levels in patients with T2DM.[13,14,15] This placebo-controlled study evaluated the safety and efficacy of teneligliptin 20 mg once daily as monotherapy in Indian patients who had inadequately controlled T2DM with diet and exercise alone.
SUBJECTS AND METHODS
This randomized, double-blind, comparative, placebo-controlled, parallel-group, multicenter, Phase III study enrolled patients from 24 centers across India. The study was conducted between January 23, 2014, and February 5, 2015.
The study protocol was reviewed and approved by the Drug Controller General of India and the Institutional Review Board. The study was conducted in accordance to the Declaration of Helsinki,[16] Good Clinical Practice, and the International Conference on Harmonization guidelines. Written informed consent was obtained from each patient prior to study participation.
Inclusion and exclusion criteria
Patients of either gender between 18 and 65 years of age with T2DM and inadequate glycemic control (HbA1c: >7.0%–≤8.5%), despite having followed a diet and exercise plan to control blood glucose levels, and who had not taken any other anti-hyperglycemic agent for at least 8 weeks prior to screening, were included in the study.
Patients were excluded if they had T1DM, were pregnant/lactating, had known hypersensitivity to any components of the formulation, had received treatment with insulin within 12 weeks of screening visit, had taken any oral anti-hyperglycemic agent 8 weeks prior to screening, or were treated with systemic corticosteroids, had diabetic complications, had cardiovascular disease, or had abnormal results of creatinine, serum glutamic oxaloacetic transaminase (SGOT), serum glutamate-pyruvate transaminase (SGPT), or serum amylase measurements.
Study design and treatments
The total study duration was between 17 and 18 weeks including 1 week screening and 16 week active treatment period. During the study, patients who did not meet progressively defined glycemic goals were provided with rescue therapy (metformin) until completion of the study (12 weeks). The glycemic rescue criterion was FPG >240 mg/dL at week 12.
All patients randomly received either teneligliptin 20 mg (Glenmark) or placebo in the ratio of 2:1. Randomization was performed using a computer-generated randomization list, with sealed unblinding envelopes. For the first 4 weeks, patients received 1 tablet (teneligliptin 20 mg/placebo) daily before breakfast. On completion of the first 4 weeks, the dose was escalated based on the FPG levels. If FPG levels were ≤180 mg/dL, the same dose was continued for the remaining 12 weeks. However, if the FPG levels were >180 mg/dL, the dose was escalated to two tablets (20 mg teneligliptin/placebo) daily. After the completion of 12 weeks of treatment period, the FPG levels were evaluated using a glucometer and patients with uncontrolled hyperglycemia (>240 mg/dL) were prescribed rescue medication (metformin tablets). The dose of the rescue medication was decided by the investigator.
Study variables
The primary efficacy variable of the study was change in HbA1c levels from baseline at week 16 and the secondary efficacy variables included the proportion of patients who achieved target HbA1c levels (≤7.0%); proportion of patients being prescribed with rescue medication at week 12; and changes in FPG, postprandial glucose (PPG), and body weight. Data of adverse events (AEs), physical examination findings, vital signs measurements, electrocardiogram (ECG) parameters, and body weight were also collected. All AEs were assessed by the investigators for intensity and relationship with the study drug. Laboratory evaluations included complete blood chemistry, hematology, and urinalysis. Laboratory measurements were performed at a central laboratory, and ECG was done at respective sites.
Statistical analysis
Efficacy end points were analyzed using both the intent-to-treat (ITT) and per-protocol (PP) populations. The ITT population comprised all randomized patients who received at least one dose of the study medication and had at least one postbaseline efficacy assessment of HbA1c. The last available observation of the efficacy parameter was carried forward in the ITT and PP analysis sets (last-observation-carried-forward approach), where postbaseline data were missing. To avoid the confounding influence of rescue therapy on efficacy comparisons, efficacy data collected after initiation of rescue therapy were treated as missing. The mean change from baseline (collected at the screening visit) in HbA1c at week 16 was analyzed using an analysis of covariance (ANCOVA) model with baseline HbA1c value as a covariate. The least square (LS) means, 95% confidence interval (CIs), and P values for the difference in mean change of HbA1c levels were calculated. The proportion of patients with HbA1c level ≤7.0% at the end of treatment was summarized by treatment group and compared between groups using logistic regression analysis after adjusting for baseline HbA1c levels. The change from baseline in FPG was analyzed using ANCOVA with baseline value of FPG as a covariate and treatment as a main effect. Similarly, the analysis was performed for PPG and body weight. Safety and tolerability were assessed for patients who received at least 1 dose of study medication by review of the safety data. P < 0.05 was considered to be statistically significant. Software used for statistical analysis was SAS version 9.4 (Cary, NC, USA).
RESULTS
Patients
Overall, 237 patients were randomized from 24 study centers across India. The mean age of the patients was comparable between the two treatment groups (teneligliptin: 49.6 years; placebo: 48.9 years) and majority of patients were male (n = 144, 60.8%). Teneligliptin group consisted of 158 patients and placebo group consisted of 79 patients. The majority (n = 213, 89.9%) of the 237 randomized patients completed the study. The detailed demographic parameters are presented in Table 1.
Table 1.
Demographic parameters of the patients
Primary efficacy end point: Mean change in glycosylated hemoglobin
All analysis was performed using both the ITT and the PP populations. The baseline HbA1c was similar in the two treatment groups in both the ITT and PP populations (teneligliptin: 7.75%; placebo: 7.74%). Reduction in HbA1c was statistically significant in the teneligliptin group at week 16 compared with baseline (LS mean difference = −0.31, standard deviation (SD) = 1.246, P = 0.0037) in the ITT population and (LS mean difference = −0.29, SD = 1.263, P = 0.0089 in the PP population) [Figure 1]. Patients treated with teneligliptin showed statistically significant improvement in HbA1c levels compared with the placebo group (LS mean difference = 0.555; 95% CI: 0.176–0.934; P = 0.0043) in the ITT population and (LS mean difference = 0.642; 95% CI: 0.233–1.052; P = 0.0023) in the PP population [Table 2].
Figure 1.
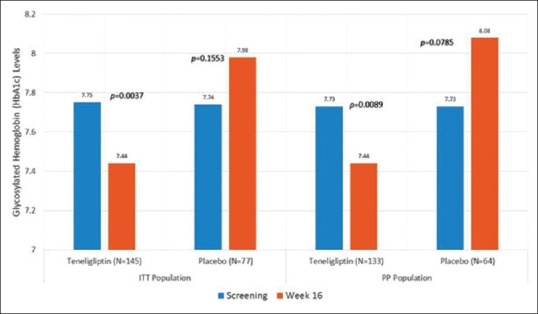
Change in glycosylated hemoglobin levels from screening to week 16 in both intent-to-treat population and per protocol population
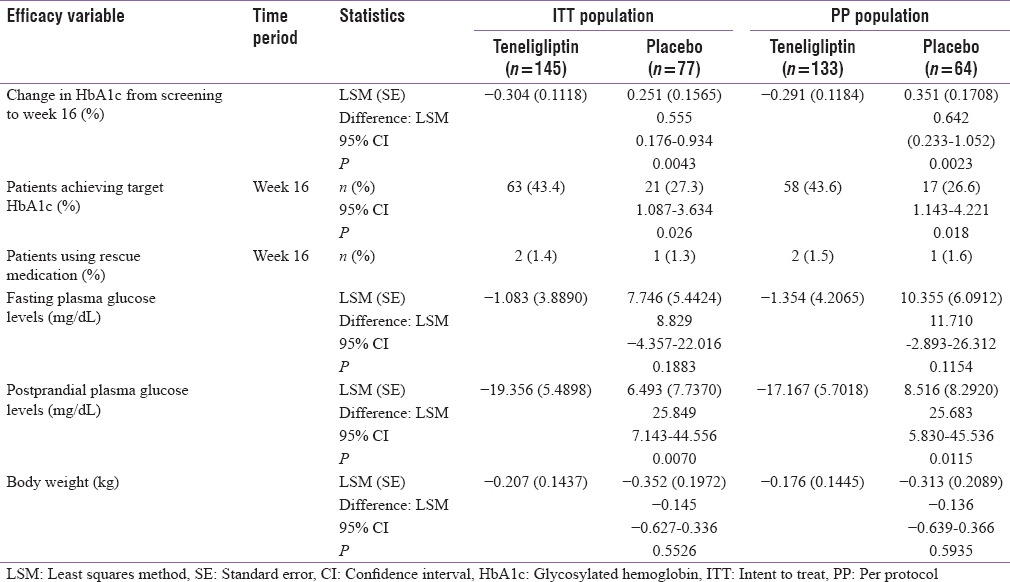
Table 2.
Summary of efficacy variables in intent-to-treat and per-protocol populations
Secondary efficacy end points
Target glycosylated hemoglobin levels
The greater proportion of patients achieved target HbA1c level (≤7.0%) in the teneligliptin group compared with the placebo group in the ITT population (43.4% vs. 27.3% [P = 0.026]) and the difference between the two groups was statistically significant. A similar trend was observed in the PP population, wherein a greater proportion of patients in the teneligliptin group achieved target HbA1c level compared with the placebo group (43.6% vs. 26.6% [P = 0.018]) [Table 2].
Patients receiving rescue medication after 12 weeks of therapy
The proportion of patients who received rescue medication after 12 weeks of therapy was low and similar in both groups (teneligliptin: 1.4%, ITT and 1.5%, PP; placebo: 1.3%, ITT and 1.6%, PP) [Table 2].
Fasting and postprandial plasma glucose levels
The ITT patients of the teneligliptin group showed a decrease in FPG levels from 144.2 ± 38.57 mg/dL at baseline to 141.9 ± 44.47 mg/dL at week 16. The decrease from baseline to week 16 was 0.9 ± 51.72 mg/dL. In the placebo group, an increase in FPG levels was observed from 145.4 ± 37.94 mg/dL at baseline to 150.8 ± 50.36 mg/dL at week 16. Additionally, the difference between the two groups was not statistically significant (LS mean difference: 8.829; 95% CI: −4.357–22.016; P = 0.1883). In the PP population, the FPG levels decreased from 144 ± 37.55 mg/dL at baseline to 142.3 ± 45.96 mg/dL at week 16 in the teneligliptin group and increased from 145.7 ± 38.88 mg/dL at baseline to 153.9 ± 52.61 mg/dL at Week 16 in the placebo group. The difference between the teneligliptintreated group and the placebo group was not statistically significant (LS mean difference: 11.710 mg/dL; 95% CI:-2.893–26.312; P = 0.1154).
The change in PPG levels showed significantly greater decrease in the teneligliptin group compared with the placebo group in both the ITT (LS mean difference = 25.849 mg/dL; 95% CI: 7.143–44.556; P = 0.0070) and the PP populations (LS mean difference = 25.683 mg/dL; 95% CI: 5.830–45.536; P = 0.0115) [Table 2].
Body weight
The difference in body weight between screening and week 16 was not significant in both the ITT (LS mean difference = −0.145; 95% CI: −0.627–0.336; P = 0.5526) and the PP populations (LS mean difference = −0.136; 95% CI: −0.639–0.366; P = 0.5935) [Table 2].
Safety evaluation
The mean duration of exposure to teneligliptin was 106.7 days and to placebo was 107.7 days. Of the 237 patients included in the study, 44 patients (18.6%) experienced at least one AE. Treatment-emergent AEs (TEAEs) were reported for 19.0% of patients (30/158) in the teneligliptin group and 17.7% of patients (14/79) in the placebo group. Serious AEs (SAEs) were reported in two patients (1.3%), both of whom were in the teneligliptin group. The two SAEs reported were cancer right pyriform fossa and left varicose vein surgery; however, both SAEs were considered not to be related to study drug. A detailed summary of the AEs is presented in Table 3.
Table 3.
Summary of treatment emergent adverse events and most common adverse events in both intent-to-treat and per protocol population
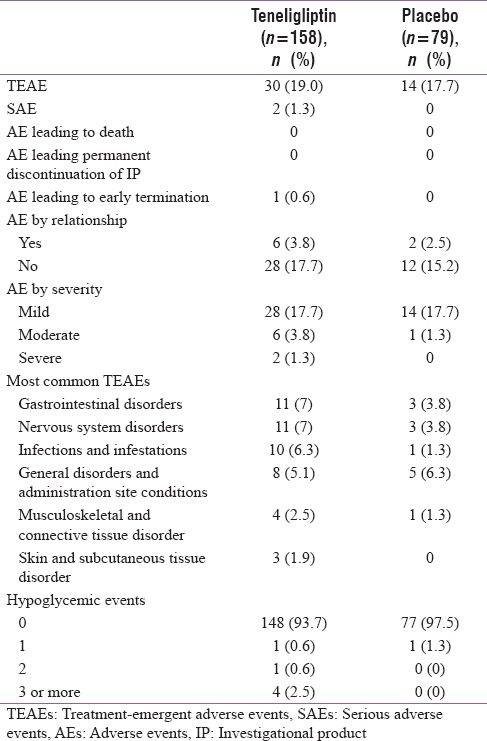
A total of six patients (3.8%) in the teneligliptin group and two patients (2.5%) in the placebo group experienced TEAEs that were related to the study medication. The most frequent TEAEs reported were gastrointestinal disorders and nervous system disorders, both TEAEs occurred with the same incidence in the teneligliptin group and in the placebo group (n = 11/158 patients [7%] and n = 3/79 patients [3.8%], respectively). The other frequent TEAEs were related to general disorders and administration site conditions and were reported by 8/158 (5.1%) patients in the teneligliptin group and 5/79 (6.3%) patients in the placebo group. The majority of TEAEs were mild in severity. TEAEs that were considered to be severe were reported for 2/158 (1.3%) patients in the teneligliptin group. TEAEs that were considered to be moderate in intensity were reported for 6/158 (3.8%) patients in the teneligliptin group and 1/79 (1.3%) patients in the placebo group.
Most patients in both groups did not experience a hypoglycemic event (n = 148, 93.7% and n = 77, 97.5% in the teneligliptin and placebo groups, respectively). At least one hypoglycemic event was experienced 0.6% patients (n = 1) of the teneligliptin group and 1.3% patients (n = 1) of the placebo group. There was a low incidence of patients who experienced three or more hypoglycemic events in the teneligliptin group (n = 4 patients, 2.5%) and none in the placebo group. The mean changes in laboratory values of SGOT, SGPT, alkaline phosphatase (ALP), serum amylase, and serum calcitonin between the baseline values and those observed at the end of week 16 showed no clinically meaningful effect in either the placebo or teneligliptin groups.
DISCUSSION
The present study evaluated the effect of teneligliptin treatment in patients with uncontrolled hyperglycemia who were treatment naïve or had not been treated with hypoglycemic agents for 8 weeks prior to study initiation.
This doubleblind study was performed to provide an assessment of the efficacy and tolerability of teneligliptin at a dose of 20 mg once daily as monotherapy compared with placebo in patients with T2DM with inadequate glycemic control using diet and exercise. Treatment with teneligliptin led to statistically significant and clinically meaningful reductions in HbA1c levels compared with placebo in both the ITT and the PP populations. There was a statistically significant improvement in 2h PPG in patients treated with teneligliptin compared with patients who received placebo. In addition to reducing HbA1c levels, higher proportions of patients treated with teneligliptin achieved the target HbA1c levels (≤7.0%) in the ITT and PP patient populations during the study period.
Although an improvement in FPG was observed in the teneligliptin group, it was not statistically significant compared with the placebo group. Greater improvement was observed in PPG levels in the teneligliptin group than in the placebo group in both the ITT and PP populations. Similar results were observed in another study conducted by Eto et al. wherein 2h PPG levels decreased significantly (P < 0.001) in patients with T2DM.[17]
Hypoglycemic drugs, particularly insulin, insulin secretagogs, and thiazolidinedione, have long been associated with weight gain. This study showed that there was no increase in the body weight after 16 weeks of treatment with teneligliptin. A review by Bohannon reported that the use of gliptins is associated with no increase in weight among patients with T2DM.[10]
A similar proportion of patients experienced AEs in the teneligliptin-treated (19.0%) and placebo (17.7%) groups and most AEs were of mild to moderate in severity. Eto et al. reported lower incidence of AEs with teneligliptin (20 mg: 23.5%; 10 mg: 8.2%) compared with placebo (28.1%).[17] No death was reported in the study. There were two (1.3%) patients who experienced SAEs in the teneligliptin group (cancer right pyriform fossa and left varicose vein surgery) and both SAEs were considered not to be related to study drug. Most patients (93.7%) treated with teneligliptin did not experience a hypoglycemic event. Studies evaluating other anti-diabetic drugs have reported an association with the occurrence of hypoglycemic events.[18] A study conducted by Bodmer et al. reported that the use of sulphonylurea and metformin was significantly (P < 0.0001) associated with the occurrence of hypoglycemic events in patients with T2DM.[19] Similarly, a recently conducted meta-analysis reported that patients who receive basal bolus insulin have a 33.7% risk of a severe hypoglycemic events, while patients treated with sulphonylurea have a 3.5% annual risk of a severe hypoglycemic event.[20] The use of teneligliptin in this study was not associated with any significant changes in laboratory findings (SGPT, ALP, SGOT, serum amylase, and serum calcitonin).
Consideration of a narrow target HbA1c window, namely, 7%–8.5% has provided a comparatively lower response than expected for a DPP4 inhibitor. This may be considered as a limitation of the present study. In summary, patients with T2DM who were treated with teneligliptin in this study showed statistically significant and clinically meaningful reductions in HbA1c levels. Teneligliptin treatment was well tolerated. Teneligliptin treatment has been shown to be effective for patients with T2DM that is inadequately controlled using diet and exercise alone.
Financial support and sponsorship
The study was funded by Glenmark Pharmaceuticals Ltd., Mumbai. The authors acknowledge Turacoz Healthcare Solutions for provided writing support for this manuscript.
Conflicts of interest
Dr. Piyush Agarwal, Dr. Chhavi Jindal, and Vinayak Sapakal are employees of Glenmark Pharmaceuticals Ltd, Mumbai. The authors have no other direct or indirect commercial financial incentive associated with publishing the manuscript. The manuscript has been read and approved by all the authors, and the requirements for authorship have been met by all the authors of the manuscript.
REFERENCES
- 1.International Diabetes Federation (IDF) Diabetes Atlas. 7th ed. 2015. [Last accessed on 2015 Dec 28]. Available from: http://www.diabetesatlas.org/component/attachments/?task=download&id=116 .
- 2.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 3.Van Ackerbroeck S, Schepens T, Janssens K, Jorens PG, Verbrugghe W, Collet S, et al. Incidence and predisposing factors for the development of disturbed glucose metabolism and DIabetes mellitus AFter Intensive Care admission: The DIAFIC study. Crit Care. 2015;19:355. doi: 10.1186/s13054-015-1064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redmon B, Caccamo D, Flavin P, Michels R, O’Connor P, Roberts J, et al. Institute for Clinical Systems Improvement. Diagnosis and Management of Type 2 Diabetes Mellitus in Adults. Updated July 2014. Available from: https://www.icsi.org/_asset/3rrm36/Diabetes.pdf .
- 5.Deed G, Barlow J, Kuo I. Early and tight glycaemic control – The key to managing type 2 diabetes. Aust Fam Physician. 2012;41:681–4. [PubMed] [Google Scholar]
- 6.Gupta D, Kono T, Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: Therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:1036–47. doi: 10.1111/j.1463-1326.2010.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108–13. doi: 10.2337/diabetes.54.suppl_2.s108. [DOI] [PubMed] [Google Scholar]
- 9.Rosengren A, Jing X, Eliasson L, Renström E. Why treatment fails in type 2 diabetes. PLoS Med. 2008;5:e215. doi: 10.1371/journal.pmed.0050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med. 2009;121:40–5. doi: 10.3810/pgm.2009.01.1953. [DOI] [PubMed] [Google Scholar]
- 11.Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152:3018–29. doi: 10.1210/en.2011-0286. [DOI] [PubMed] [Google Scholar]
- 12.Omar B, Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Kalra S. Choosing a gliptin. Indian J Endocrinol Metab. 2011;15:298–308. doi: 10.4103/2230-8210.85583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito R, Fukui T, Hayashi T, Osamura A, Ohara M, Hara N, et al. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, improves early-phase insulin secretion in drug-naïve patients with type 2 diabetes. Drugs R D. 2015;15:245–51. doi: 10.1007/s40268-015-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4:576–84. doi: 10.1111/jdi.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–8. [PubMed] [Google Scholar]
- 17.Eto T, Inoue S, Kadowaki T. Effects of once daily teneligliptin on 24h blood glucose control & safety in Japanese patients with Type-2 diabetes mellitus a 4-week randomised double blind placebo controlled trial. J Diabetes Obes Metab. 2012;29:9999. doi: 10.1111/j.1463-1326.2012.01662.x. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto M. Teneligliptin: A DPP-4 inhibitor for the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:187–95. doi: 10.2147/DMSO.S35682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: A nested case-control analysis. Diabetes Care. 2008;31:2086–91. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czech M, Rdzanek E, Pawęska J, Adamowicz-Sidor O, Niewada M, Jakubczyk M, et al. Drug-related risk of severe hypoglycaemia in observational studies: A systematic review and meta-analysis. BMC Endocr Disord. 2015;15:57. doi: 10.1186/s12902-015-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


