Abstract
Context:
The number of men afflicted with osteoporosis is unknown.
Aims:
This study aims to determine the prevalence of osteoporosis in men.
Settings and Design:
This was a prospective, observational study.
Subjects and Methods:
A total of 200 male attendants of patients attending endocrine outpatient department and who were >55 years were recruited for the study. All the patients with osteopenia and osteoporosis were advised lifestyle interventions, supplementation with calcium carbonate (1000–1500 mg/day) and 25-hydroxyl-Vitamin D (400–600 IU/day) and bisphosphonates if indicated. Vitamin D3 60,000 IU once a week for 8 weeks and once a month thereafter was prescribed to Vitamin D-deficient patients. Androgen-deficient patients were given replacements of either injectable testosterone or oral testosterone undecanoate.
Statistical Analysis Used:
Two sample t-test and paired t-test were used to compare pre- and post-test parameters.
Results:
Overall 80 (40%) subjects had low bone mass, 93 (43.5%) had Vitamin D deficiency/insufficiency, and 39 (19.5%) had androgen deficiency. Osteoporosis was found in 8.5% patients. All patients were above 70 years (Mean age: 73.82 ± 2.79 years). Seventy percentage of these patients had low serum testosterone and 70% of patients had Vitamin D deficiency/insufficiency. About 31.5% of patients had osteopenia (mean age of 67.47 ± 6.35 years). Thirty-five percentage of these patients were androgen deficient and 25% were Vitamin D-deficient/insufficient. Age >70 years, serum testosterone <3 ng/ml, Vitamin D <30 ng/ml were strong risk factors for osteoporosis. Vitamin D supplementation, androgen replacement, and bisphosphonate therapy had beneficial effect on bone mineral density (BMD).
Conclusions:
Low bone mass was common (40%) in males over 55 years of age. Age >70 years, low androgen (<3 ng/ml), steroid use, and low Vitamin D (<20 ng/ml) were independent risk factors of male osteoporosis. Calcium and Vitamin D are effective in improving BMD. Androgen replacement has beneficial effect on BMD in hypogonadism patients.
Keywords: Androgen deficiency, osteopenia, osteoporosis, Vitamin D deficiency
INTRODUCTION
Osteoporosis is a worldwide major public health problem characterized by compromised bone strength predisposing to increased risk of fracture.[1] Most studies on metabolic bone diseases over the past decade have focused on the pathogenesis, diagnosis, and treatment of osteoporosis in women. Nevertheless, recent epidemiological and observational studies have shown that osteoporosis in men is an increasingly important clinical issue. Osteoporosis in men presents a unique array of scientific challenges and opportunities and deserves the same vigorous evaluation as applied to postmenopausal osteoporosis. It has been estimated that the lifetime risk of a man suffering an osteoporotic fracture is actually greater than his likelihood of developing prostate cancer. About one in every four to five hip fractures in people older than 50 occurs in men;[2] however, the number of men afflicted with osteoporosis is not known. Hence, we undertook this cross-sectional, observational prospective study to determine the prevalence of osteoporosis in men.
SUBJECTS AND METHODS
This study was carried out in a tertiary care hospital of North India between July 1st, 2014 and December 31st, 2015 and 223 subjects were screened. A total of 200 male attendants of patients attending endocrine outpatient department (OPD) and who were >55 years were recruited for the study. The Ethics Committee of the hospital approved the protocol. Informed consent was obtained from each individual. All participants underwent a physical examination and routine blood chemistry evaluation. Patients were not included in the present study if they had a history of myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass grafting, proven manifest coronary artery disease, severe or unstable angina pectoris, clinically manifest heart failure (higher than grade II New York Heart Association), or severe cardiac arrhythmia. Patients with acute liver disease or hepatic dysfunction, impaired renal function (plasma creatinine. 1.5 mg/dl), history of partial ileal bypass surgery, any surgical procedure or any systemic inflammatory disease within 3 months before randomization, malignancy, vasculitis, rheumatic arthritis, idiopathic lung fibrosis, ulcerative colitis, or Crohn's disease were excluded from the study. Patients who consumed >20 g of alcoholic drinks per day or who used systemic steroids, androgens, cyclosporine, or other immunosuppressive drugs were also excluded from the study.
Clinical and laboratory data
Patients were examined clinically including weight (to the nearest 0.5 kg) and height (to the nearest 0.5 cm) measurements (mean of three values); pulse and blood pressure record; and general physical and systemic examination. At baseline, in the morning after an overnight fast, venous blood was sampled for the measurement of complete hemogram, serum calcium, serum phosphorous, alkaline phosphatase, liver functions, blood urea nitrogen, serum creatinine, level of plasma concentration of glucose, total and high-density lipoprotein (HDL) cholesterol, triglycerides, and hormonal profile (T3, T4, thyroid-stimulating hormone [TSH], cortisol, luteinizing hormone [LH], Follicle-stimulating hormone [FSH], prolactin, testosterone, estradiol, growth hormone, insulin-like growth factor 1 [IGF-1], parathyroid hormone [PTH], and Vitamin D) and serum osteocalcin. Twenty-four hour urinary estimation was done for calcium, phosphorous, creatinine, and N-telopeptide (NTx) of type 1 collagen. Plasma glucose was measured by a glucose-oxidase method. Plasma total cholesterol, HDL cholesterol, and triglycerides were assessed with standard enzymatic spectrophotometric techniques. Plasma low-density lipoprotein cholesterol was calculated with the equation of Friedewald et al. except when triglycerides exceeded 400 mg/d (in that case, data were treated as missing). Estimation of T3, T4, TSH, cortisol, LH, FSH, prolactin, and growth hormone was done by DSL-2100 radioimmunoassay (RIA) kits. The intra- and interassay coefficients of variation (CVs) for all assays were <12%. At baseline, paired serum specimens were collected after an overnight fast and stored at −70°C until assayed. Assays were performed at the Endocrine laboratory of Army Hospital Research and Referral Delhi by single technician. All testosterone and estradiol assays were completed in duplicate, and the average value was used in the analyses. Criteria were implemented to repeat the assay when replicates were highly discrepant within an assay; results from any repeat analyses were averaged with the original sample data. Pooled serum controls were used in every assay run. Serum total testosterone was measured using a solid-phase125 I-RIA (Diagnostic Products Corp., Los Angeles, CA; detectable range 1–16 ng/ml; intraassay CV 5.4%; interassay CV 8.2%). Serum total estradiol was measured with an ultrasensitive RIA (Diagnostic Systems Laboratories, Webster, TX; detectable range 2.5–750 pg/ml; intraassay CV 8.5%; interassay CV 13.3%). Intact-PTH was estimated by DRG ELISA kit (two site enzyme linked immunosorbent assay, DRG international, Inc, Springfield, NJ, USA). The DRG Intact-PTH immunoassay is a two-site ELISA having calculated sensitivity of 1.72 pg/ml. We measured 25-hydroxyl-Vitamin D (25-OH-D) by an equilibrium RIA procedure (DiaSorin Inc., Stillwater, MN, USA). The inter and intraassay CVs were 13% and 10%, respectively. Serum IGF-1 was determined by DRG IGF-1 ELISA kit with sensitivity of 0.15 ng/ml. The NTx of type 1 collagen estimation in urine was done by an enzyme-linked immunosorbent assay-OSTEOMARK by Wampole laboratories. At the baseline participants underwent bone mineral density (BMD) (grams per square centimeter) measurement of total hip, femoral neck, and lumbar spine with dual-energy X-ray absorptiometry (QDR 4500W; Hologic, Inc., Waltham, MA). All hip BMD measurements were made on the right hip unless the participant reported a right hip replacement or metal objects in the right leg, in which case the left hip was measured. Lumbar spine BMD was measured in the anterior-posterior projection and calculated as the mean of the BMD from the first through fourth lumbar vertebrae. A standard phantom was used at all study clinics for cross-calibration. Interclinic CVs were 0.9% for hip and 0.6% for spine. T-score <2.5 was diagnosed to have osteoporosis.
All the patients with osteopenia and osteoporosis were advised lifestyle interventions, supplementation with calcium carbonate (1000–1500 mg/day) and Vitamin D3 60,000 IU once a month. Vitamin D3 60,000 IU once a week for 8 weeks and once a month thereafter was prescribed to Vitamin D-deficient patients. Androgen-deficient patients were given replacements of either injectable testosterone or oral testosterone undecanoate. All patients having osteoporosis were given tablet alendronate 70 mg once a month with usual precautions. Patients were under regular follow-up in our OPD. Patients were advised to undergo repeat testing of biochemical, hormonal, and BMD measurements after 1 year.
All the parameters are represented as mean ± SD unless otherwise specified. Database was created in MS Access and statistical program for the Social Sciences (Release 13.0, PC Windows; SPSS Inc., Chicago, IL) was used for analysis. Central tendency and dispersion calculated. Two sample t-test and paired t-test were used to compare pre- and post-test parameters.
RESULTS
A total of 200 male patients were enrolled with the mean age of 63.98 ± 6.51 years. Baseline demographic profile, biochemical profile, markers of bone health, and hormonal parameters are enumerated in Tables 1 and 2, respectively. Three patients taking alternative treatment for generalized weakness were found to have low serum cortisol suggestive of exogenous glucocorticoid exposure. Serum cortisol, thyroid profile, prolactin, and IGF-1 were normal at baseline for rest of the patients.
Table 1.
Demographic profile at baseline and end of follow-up
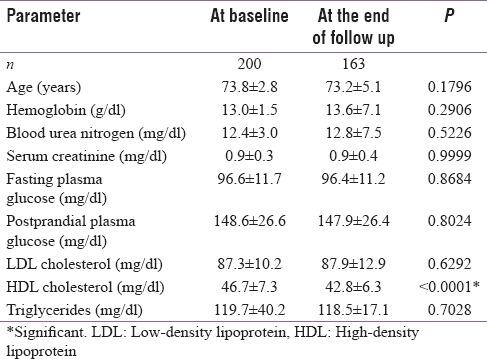
Table 2.
Bone and hormonal parameters at baseline and end of follow-up
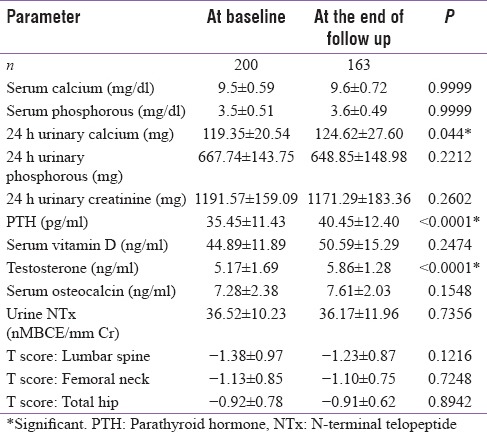
Overall 80 (40%) patients had low bone mass (osteoporosis/osteopenia). Vitamin D deficiency/insufficiency (serum Vitamin D level <30 ng/ml) was noted in 93 (46.5%) of subjects, whereas, androgen deficiency (serum testosterone <300 ng/dl) was found in 39 (19.5%) subjects. There was a positive correlation between serum testosterone and baseline BMD.
Osteoporosis was found in 17 (8.5%) patients, all of whom were above 70 years (mean age 73.82 ± 2.79 years). Twelve (70%) of patients having osteoporosis had low serum testosterone. Vitamin D deficiency (serum Vitamin D level <20 ng/ml) was seen in 3 (17%) patients with osteoporosis, and another 9 (53%) were Vitamin D insufficient (serum Vitamin D level: 20–30 ng/ml). Three patients had inadvertently taken exogenous steroids in the form of indigenous medicines or boosting immunity and stamina, verified by demonstrating low baseline serum cortisol levels while one had subclinical hyperthyroidism. Another 63 (31.5%) of patients with mean age of 67.47 ± 6.35 years had osteopenia of which 22 (35%) were androgen-deficient while 10 (15.9%) had Vitamin D deficiency and 16 (25%) were Vitamin D insufficient.
Age >70 years, serum testosterone <300 ng/dl, Vitamin D <30 ng/ml, and steroid use emerged as independent risk factors for osteoporosis and a negative correlation were observed with serum cortisol with BMD.
At the end of 1 year, 163 (81.5%) patients were available for follow-up having mean age of 64.98 ± 6.88 years. Supplementation of Vitamin D led to significant increase in its level from 23.9 ± 5.62 ng/ml to 28.8 ± 4.86 ng/ml (P < 0.0001) in deficient individuals with concomitant reduction in serum PTH level from 46.82 ± 13.18 pg/ml to 42.2 ± 11.53 pg/ml (P = 0.0196) while serum testosterone levels rose from 2.46 ± 1.04 ng/ml to 4.37 ± 1.12 ng/ml (P < 0.0001) after its replacement in deficient subjects. Vitamin D supplementation, androgen replacement, and bisphosphonate therapy had beneficial effect on BMD. Stabilization, and in fact numerical improvement, was noted in BMD at lumbar spine and total hip (T score: from-3.74 ± 0.57 to-3.10 ± 0.42 and − 2.95 ± 0.43 to-2.54 ± 0.39, respectively) though it did not reach statistical significance.
DISCUSSION
Osteoporosis is a common medical problem, with over 1.3 million fractures occurring annually in the United States.[3] Although the majority of these fractures occur in postmenopausal women, the social and economic burden of osteoporosis-related fracture in men is considerable, with 25%–30% of all hip fractures occurring in males. Despite this, there is a lack of understanding of the etiology and epidemiology of male osteoporosis. In many cases, a secondary cause is evident, such as alcohol abuse, glucocorticoid excess (either therapy with glucocorticoids or endogenous Cushing's syndrome), hypogonadism, or hyperparathyroidism. In a large number, however, no cause can be identified (so-called idiopathic osteoporosis).[4] Even though uncertainties remain about whether to use absolute or relative risk in diagnosing male osteoporosis, the International Society for Clinical Densitometry recommends that we use the male database and the T-score of less than-2.5 to diagnose osteoporosis in men. By applying these standards, according to the World Health Organization, it is estimated that 1-2 million men in the United States have osteoporosis (T score-2.5) and another 8–13 million have osteopenia (T-score between-1.0 and-2.5). The respective age-adjusted prevalence figures are impressive: 6% for osteoporosis and 47% for osteopenia. On the other hand, if one applies the female reference standard to men (BMD-2.5 SD below peak bone mass for women), the numbers become much smaller, namely, 0.3–1 million men with osteoporosis (age-adjusted prevalence, 4%) and 4-9 million with osteopenia (age-adjusted prevalence, 33%). These latter figures are not consistent with epidemiological data. Although controversy exists over what database to use, it is clear that men, like women, are at substantial risk for developing osteoporosis throughout the world. In our study of randomly selected patients, osteoporosis was detected in 8.5% and osteopenia in 31.5% patients. Patients having osteoporosis were on an average about 7-year-older than those having osteopenia, possibly giving a glimpse into the natural history of disease. Aging in men, like aging in women, is associated with dramatic increases in fracture risk. The exponential increase in risk occurs approximately one decade later than in women. It has been estimated that the lifetime risk of a man suffering an osteoporotic fracture is actually greater than his likelihood of developing prostate cancer.[5] Age >70 years was a strong risk factor of osteoporosis in our study. The causes of bone loss in men are thought to be related to genetics, environmental, hormonal, and disease-specific factors. As in females, osteoporosis in males can be attributable to specific, underlying etiologies requiring careful clinical evaluation. Approximately, 50% of men with osteoporosis are diagnosed with an underlying “secondary” cause. This leaves a large percentage of men whose osteoporosis is not explained, so-called “primary” or “idiopathic” osteoporosis. Most of the men in this category are <65–70 years of age. Of course, there are men over 70 with osteoporosis in which the cause is not known. The older the patient, however, the more we are likely to relate the osteoporosis to age and not to a specific or unknown cause. Clearly, the younger the patient, the more likely it is that other explanations are needed to account for the condition. The three major causes of secondary osteoporosis in men are alcohol abuse, glucocorticoid excess (either endogenous Cushing's syndrome or, more commonly, chronic glucocorticoid therapy), and hypogonadism.[6,7] We had excluded men with alcohol abuse. Hypogonadism was a strong risk factor for osteoporosis in our study accounting for about 70% cases. Several studies conducted in the second half of the 20th century found an association between osteopenia or osteoporosis and Vitamin D deficiency, which was common in older individuals.[8] The response of the parathyroid glands to a given degree of Vitamin D deficiency increases with age.[9] There is widespread agreement that serum 25-OH-D levels should be kept above 80 nmol/L (32 ng/ml) although several studies fail to support this high target level.[10] Vitamin D was found to be strong risk factor for decreased bone density in our study, which is consistent with literature. Preventive interventions in males are similar to the approach used for women. Dietary calcium intake should be 1200-1500 mg, consistent with the National Institutes of Health and Food and Nutrition Board recommendations for optimal calcium intake.[11] Vitamin D intake must also be adequate and individuals should receive 400–600 IU/day. In men over 70 years of age, many experts make a general recommendation of 600-800 IU/day. Adequate exercise should also be strongly advised. However, drug therapy is almost always indicated in men at high risk for fracture.
Bisphosphonate therapy is becoming a mainstay in the treatment of male Osteoporosis.[12] All of our patients with osteoporosis were on calcium, Vitamin D and weekly 70 mg of alendronate. This form of therapy was supplemented by androgen replacement in hypogonadal patients. Repeat BMD after 1 year did not show further deterioration. Weekly alendronate was found to be safe and efficacious. Similar results have been shown by Orwoll et al.,[13] who conducted a large, randomized, placebo-controlled trial reporting an increase in BMD in a subgroup of men with osteoporosis treated with alendronate. In multivariate analysis, androgen replacement was effective in improving BMD at lumbar spine. A meta-analysis of placebo-controlled trials of testosterone treatment in men with any degree of androgen deficiency (most of them showing low normal or normal testosterone levels at baseline) suggested a beneficial effect on lumbar spine BMD, but equivocal findings at the femoral neck.[14]
Interesting findings noted in our study were increase in HDL that can be ascribed to improved lifestyle measures, frequent counseling, and possibly Vitamin D supplementation.[15] Similarly, 24 h urinary calcium showed minimal increase in serum calcium levels (within normal limits) that can be explained by in calcium and Vitamin D supplementation. Increase in serum iPTH despite improvement in serum Vitamin D levels can be attributed to bisphosphonate therapy and aging[16] of the study population.
Limitation of our study was about 20% dropouts in follow-up. In our study, we were unable to find out association of bone markers with BMD as has been reported from various studies. This could be attributed to standardization issues.
CONCLUSIONS
In our study, osteoporosis was found in 8.5% patients while another 31.5% of patients had osteopenia. Age >70 years, low androgen level (<3 ng/ml), steroid use, and low Vitamin D (<20 ng/ml) were independent risk factors of male osteoporosis. Calcium, Vitamin D, and alendronate are effective in improving BMD. Androgen replacement has beneficial effect on BMD in hypogonad patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: A world-wide projection. Osteoporos Int. 1992;2:285–9. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ., 3rd The prevalence of osteoporosis. J Bone Miner Res. 1997;12:1769–71. doi: 10.1359/jbmr.1997.12.11.1769. [DOI] [PubMed] [Google Scholar]
- 4.Orwoll ES, Klein RF. Osteoporosis in men. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ. Epidemiology of fractures. In: Riggs BL, Melton LJ, editors. Osteoporosis: Etiology, Diagnosis and Management. Philadelphia: Lippincott-Raven Publishers; 1995. pp. 225–47. [Google Scholar]
- 6.Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab. 1999;84:3431–4. doi: 10.1210/jcem.84.10.6060. [DOI] [PubMed] [Google Scholar]
- 7.Seeman E. Osteoporosis in men: Epidemiology, pathophysiology, and treatment possibilities. Am J Med. 1993;95:22S–8S. doi: 10.1016/0002-9343(93)90377-2. [DOI] [PubMed] [Google Scholar]
- 8.Lips P. Suboptimal vitamin D status: A risk factor for osteoporosis? Adv Nutr Res. 1994;9:151–66. doi: 10.1007/978-1-4757-9092-4_9. [DOI] [PubMed] [Google Scholar]
- 9.Reginster JY, Frederick I, Deroisy R, Dewe W, Taquet AN, Albert A, et al. Parathyroid hormone plasma concentrations in response to low 25-OH vitamin D circulating levels increases with age in elderly women. Osteoporos Int. 1998;8:390–2. doi: 10.1007/s001980050080. [DOI] [PubMed] [Google Scholar]
- 10.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 11.Bilezikian JP. Panel members. Optimal calcium intake: Statement of the consensus development panel on optimal calcium intake. JAMA. 1994;272:1942–8. [PubMed] [Google Scholar]
- 12.Orwoll ES. Treatment of osteoporosis in men. Calcif Tissue Int. 2004;75:114–9. doi: 10.1007/s00223-004-0288-5. [DOI] [PubMed] [Google Scholar]
- 13.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–10. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 14.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 15.Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50:303–12. doi: 10.1016/j.plipres.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Zjacić-Rotkvić V, Kavur L, Cigrovski-Berković M. Hormones and aging. Acta Clin Croat. 2010;49:549–54. [PubMed] [Google Scholar]


