Abstract
Background:
Prevalence of Gestational Diabetes Mellitus in India is increasing. In addition to performing physiological role in fetoplacental unit during pregnancy, cytokines also play pathophysiological role if expressed in abnormal amounts or sites.
Objective:
To estimate Proinflammatory Cytokines TNF-α, IL-6, IL-8 and anti-oxidants Glutathione Peroxidase (GTX), Superoxide dismutase (SOD), uric acid and Bilirubin levels in GDM and correlate with pregnancy outcome.
Results:
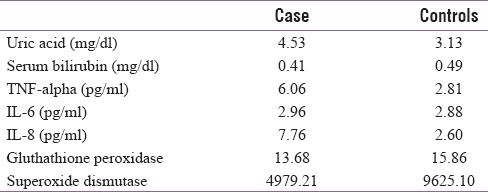
Pregnant women were screened for GDM using Diabetes In Pregnancy Study group, India criteria. The subjects with elevated glucose were grouped into cases (n = 30) and with normal values were taken as controls (n = 30). Mean Uric acid in cases was 4.53 ± 1.2 mg% whereas in controls 3.13 ± 0.58 mg%, mean TNF-α among cases was 6.06 ± 3.6 pg/ml and controls 2.81 ± 1.03 pg/ml. Antioxidants SOD and GTX were markedly decreased with mean value of 4979.21 ± 1006.3 and 13.68 ± 1.5 in cases and 9625.10 ± 1074.1 and 15.86 ± 1.2 in controls. Cytokines IL-6 (2.96 + 1.37 vs 2.88 + 1.21) and IL-8 (7.76 + 3.86 vs 2.60 + 1.45) were increased in subjects with GDM. Those with GDM developed preeclampsia (5%), Preterm labour (2%) and Polyhydromnios (5%). Foetal complications Macrosomia (13.3%) and intra uterine death (3.3%) were observed in GDM mothers. The proinflammatory cytokine levels except IL-6 were significantly increased and antioxidant markers were significantly reduced in GDM group with maternal and foetal complications.
Conclusion:
GDM worsens the oxidative stress and weakens anti-oxidant state. Uric acid, TNF-α and IL-8 were higher with foeto-maternal complications in GDM. Serum bilirubin, GTX and SOD is significantly lower in foeto-maternal complications. TNF-α significantly associated with preeclampsia in GDM mothers.
Keywords: Cytokines, glucose intolerance, intrauterine device, macrosomia, preeclampsia, preterm labor
INTRODUCTION
Gestational diabetes mellitus (GDM) is a common medical disorder complicating pregnancy. It is defined as glucose intolerance of varying severity with onset or first recognition during pregnancy.[1] The prevalence of gestational diabetes in India was l8.9% in 2004.[2] The incidence of GDM, the most common metabolic disorder during pregnancy, is also increasing worldwide owing to advancing maternal age and increasing obesity rates.[3,4] GDM is considered a prediabetic state, offering the opportunity to study the abnormalities that may appear very early in type 2 diabetes mellitus (T2DM).[5] The importance of GDM is that two generations are at risk of developing diabetes in the future. The offspring of these women is prone to adverse effects such as macrosomia, prematurity, birth trauma, and respiratory distress syndrome and more importantly has a higher risk of developing obesity, impaired glucose tolerance, and T2DM in the future. Among Asian Indian women with GDM, over 20% develop dysglycemia within 1-year postpartum and body mass index (BMI) >25 kg/sq. m increases the risk by 4-fold.[6] It also causes maternal morbidity in the form of preeclampsia, polyhydramnios, and operative deliveries.
Proinflammatory cytokines including tumor necrosis factor-alpha (TNF-a), interleukin-6 (IL-6), and IL-8, through their ability to interfere with insulin signaling, have been implicated in insulin resistance in T2DM.[7,8,9] In addition to performing a physiological role in the fetoplacental unit during pregnancy, cytokines may also play a pathophysiological role if expressed in abnormal amounts or sites. TNF-a, IL-6, and IL-8 are released from the placenta at term[10] and have been linked to various states of insulin resistance.[11]
Oxidative stress is a general term used to describe the steady state of oxidative damage in a cell, tissue, or an organ, caused by reactive oxygen species (ROS). Most ROS comes from endogenous sources as by-products of normal and essential reactions such as energy generation from mitochondria. This oxidative stress was found to be greater in women with GDM than in normal pregnant women.[12,13] Many studies have reported increased free radical production and antioxidant depletion in gestational diabetes mother to be a causative factor in increasing the risk of congenital anomalies.[14] The excess amount of glucose in GDM women gets auto-oxidized resulting in the generation of free radicals. The antioxidant defense systems are also found to be depleted in GDM.[15,16]
Need for the study
Studies of T2DM have been critical in elucidating the role of cytokines and oxidative stress in diabetes. However, very limited data are available in relation to GDM. Current evidence has demonstrated that TNF-a is the most significant predictor of insulin resistance during pregnancy.[17] IL-6 and IL-8 may also be involved in the pathogenesis of insulin resistance in GDM.[18,19]
Gestational diabetes is associated with increased oxidative stress leading to generation of free radicals. The antioxidant system in response to this is depleted in GDM. Uric acid possesses antioxidant properties and contributes about 60% free radical scavenging activity in serum. Elevated serum uric acid is a consistent feature of insulin resistance syndrome.[20] Glutathione peroxidase (GTX) and superoxide dismutase (SOD) are also important antioxidants. Serum bilirubin is also a powerful endogenous antioxidant and constitutes one of the biological defense systems in the body.
The incidence of GDM is on the rise and is associated with significant maternal and neonatal morbidity. But relatively, little is known about the importance of these proinflammatory cytokines and antioxidants in GDM and their effect on the pregnancy outcome. Hence, the need to study their role in GDM as preventive and predictive strategies in terms of outcome of the pregnancy can be obtained with these parameters.
Objective of the study
The objective of this study is to estimate proinflammatory cytokines TNF-α, IL-6, IL-8 and antioxidants such as GTX, SOD, uric acid, and bilirubin in GDM and correlate with pregnancy outcome.
MATERIALS AND METHODS
Source of data
Women attending antenatal clinic at JSS hospital, Mysuru, were enrolled in the study.
Study design
This was a prospective observational and diagnostic study.
Inclusion criteria
Women with gestational diabetes diagnosed with 75 g oral glucose challenge test (OGCT) were included in the study.
Exclusion criteria
Women with pregestational diabetes
Women with other systemic illness affecting the levels of proinflammatory cytokines such as bronchial asthma and hypertension were excluded from the study.
The study has been cleared by the Institutional Ethics Committee of JSS Medical College, Mysore.
Method of collection of data
All pregnant women attending antenatal clinic were screened using the diabetes in pregnancy study group, India criteria.[21]
Methodology
Irrespective of the timing of the last meal, as the pregnant woman entered the antenatal clinic, she was given 75 g glucose, and 2 h later, plasma glucose level was estimated. Plasma glucose of 140 mg/dl or more was diagnostic of GDM.
All pregnant women were screened at 24–28 weeks of gestation. The women at high risk for GDM (age >30 years, obesity, family history of diabetes, previous bad obstetric history, and history of GDM in previous pregnancy) were screened earlier at 12–16 weeks of gestation. Those diagnosed with GDM were included in the study (case group). In normally glucose tolerant women, the test was repeated again at 24–28 weeks and 32–34 weeks. Those with blood sugar of <140 mg/dl formed the control group.
At the time of diagnosis, blood was also drawn for estimation of uric acid and bilirubin. For estimation of IL-6, IL-8, TNF-a, GTX, and SOD, 20 ml of blood was drawn and stored at 80o c which was processed in the end after collecting samples from all participants. SOD and GTX were estimated by calorimetric method using Randox kits. TNF-a, IL-6, and IL-8 were evaluated by ELISA using Quantikine R and D.
The adverse maternal and fetal outcomes were studied and were correlated with the cytokines and antioxidant levels.
Statistical analysis
Data collected were entered and stored using Microsoft Excel 2007 and analyzed using IBM SPSS version 22 (United States). Descriptive statistics such as frequencies, percentages, means, and standard deviation were calculated and tabulated for all variables such as age, socioeconomic status and parity, and maternal and fetal complications. Multiple logistic regression analysis was used to assess the influence of various cytokine levels on maternal and fetal complications. The odds ratio and 95% confidence interval for the odds ratio were calculated and significant variables labeled when upper and lower bounds did not include value of “1.”
RESULTS
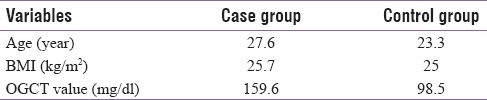
A total of 60 participants were recruited in the study with 30 in each group, i.e., 30 in GDM (case) group and 30 in control group. The mean age in the GDM group was 27.6 years and it was 23.3 years in the control group. The mean BMI in GDM group was 25.7 kg/m2 and the OGCT value was 159.6 mg/dl whereas the OGCT value in control group was 98.5 mg/dl [Table 1].
Table 1.
Mean value of age, BMI and OGCT value between both groups
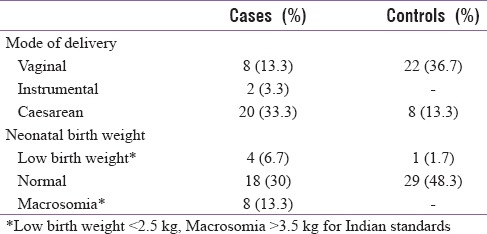
In this study, 56 participants (93.3%) belonged to lower socioeconomic and 4 to middle socioeconomic status with 23 (38.3%) participants from urban and 37 (61.7%) from rural areas. Overall 53.4% were primigravidae, 21.7% in the GDM group and 31.7% in the control group, and rest were multigravidae (46.7%) in the study population. Thirty participants (50%) delivered vaginally in which 8 (13.3%) were from GDM group and 22 (36.7%) from control group and 2 participants in the GDM group underwent instrumental delivery (3.3%). Cesarean was done in 46.7% of participants in which 33.3% (n = 20) were from GDM group and 8 (13.3%) from control group.
Eight babies (13.3%) had macrosomia and were all born to mothers with GDM. Low birth weight was noted in 5 (8.4%) babies out of whom 4 were born to mothers with GDM. Of the 47 babies born with normal weight, 18 (30%) were to GDM mothers and 29 (48.3%) were to normal mothers [Table 2].
Table 2.
Distribution of subjects on the mode of delivery and neonatal birth weight
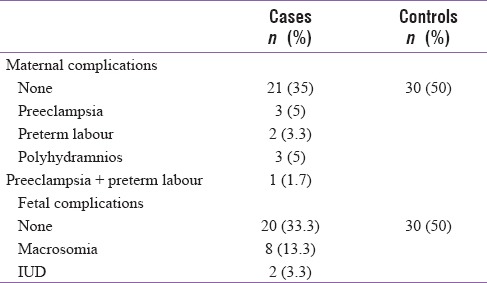
None of the participants in the control group had either maternal or fetal complications while 21 (35%) in the GDM group did not have any maternal complications. Most common maternal complications noted were preeclampsia in 3 (5%), preterm labor in 2 (3.3%), and polyhydramnios in 3 (5%), and 2 intrauterine deaths were reported [Table 3].
Table 3.
Distribution of cases based on maternal and fetal complications
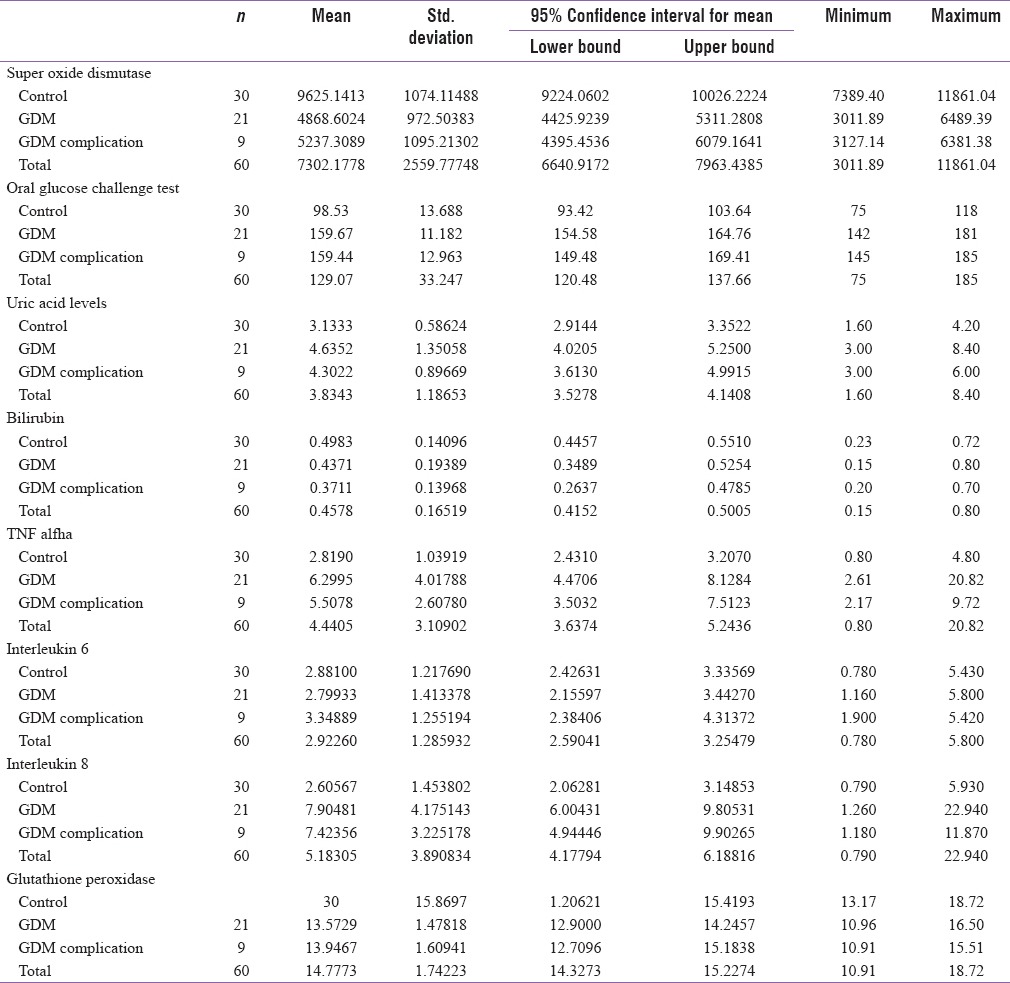
GDM group had a mean uric acid level of 4.53 mg/dl whereas it was 3.13 mg/dl in the control group. The value of TNF-a in GDM group was 6.06 pg/ml and 2.81 pg/ml in control group. Antioxidant enzymes such as SOD and GTX were markedly decreased in the GDM group with a mean value of 4979.21 and 13.68, respectively, in comparison of 9625.10 and 15.86, respectively, in the control group. The proinflammatory cytokines IL-6 and IL-8 were also significantly raised in the GDM group [Table 4].
Table 4.
Mean value of antioxidants and cytokines between the 2 groups
Even though maternal and fetal complications occurred only in the GDM group, only preeclampsia was significantly (P < 0.026) associated with TNF-a value, and rest of the markers did not show any statistical significance with any of the complications.
DISCUSSIONS
GDM is identified by an amplification of the low-grade inflammation existing in normal pregnancy. In recent days, the role of the inflammatory system in the pathogenesis of T2DM and GDM has been increasingly examined. Insulin resistance has been related with abnormal secretion of proinflammatory cytokines such as TNF-a and interleukin IL-6 and decreased production of anti-inflammatory mediators such as IL-4 and IL-10. Despite the controversies regarding specific cytokine levels, T2DM is currently regarded as a chronic inflammatory disease. Due to the similarity between T2DM and GDM and the relationship between T2DM and inflammation, it has been hypothesized that inflammation could be also associated in the pathophysiology of GDM.[22]
This theory is supported by increased circulating concentrations of inflammatory molecules such as TNF-α and IL-6 in GDM. TNF-α is one of the aspirant molecules responsible for causing insulin resistance. Comparison of the placental gene expression profile between normal and diabetic pregnant indicates increased leptin synthesis in GDM which is associated with a higher production of proinflammatory cytokines, for example, IL-6 and TNF-a causing a chronic inflammatory environment that enhances leptin production. As a result, compared with normal pregnant women, placental leptin expression in individuals with GDM is increased. Leptin itself increases production of TNF-α and IL-6 by monocytes and stimulates the production of CC chemokine ligands. Raise in leptin concentrations in turn amplifies inflammation.
In this study, women with GDM are characterized by increased antioxidant gene expression. In keeping with this, GDM is characterized by an amplification of the low-grade inflammation which already exists in normal pregnancy. This hypothesis is supported by increased circulating concentrations of inflammatory molecules such as TNF-a and IL-6 in GDM pregnancies. TNF-a is one of the candidate molecules responsible for causing insulin resistance.[23]
Rise in the level of GTX and SOD in GDM is due to the oxidative stress, and various studies have reported the increased levels of these antioxidant enzymes;[24] on the contrary, there are many studies which have reported their decreased levels too.[25]
An elevated serum uric acid concentration may reflect impaired endothelial integrity in which endothelium-dependent vascular relaxation produced by nitric oxide is reduced. High blood glucose levels induce oxidative stress and decrease antioxidant defenses, thus leading to increased free radical formation, and these free radicals may damage not only the organs in which they are formed but also the remote organs.[26]
Rasika et al. showed that in their study, there is an increase in the risk of development of GDM with increased levels of serum uric acid.[26] Studies by Coughla et al., have shown the association of hyperuricemia with GDM.[27]
Zhou et al., in their study, measured lipids and uric acid concentrations in 1000 healthy nulliparous women at 20 weeks of gestation and showed that hyperuricemic women experienced a 1.99-fold risk for preeclampsia and a 2.34-fold risk for GDM.[28] In the present study, the uric acid levels and level of TNF-a and IL-8 were significantly raised in GDM patients compared to controls, and even though level of IL-6 was raised in GDM patients, it was not significant. Serum bilirubin, GTX, and SOD levels were decreased in GDM patient when compared with controls. Even though control group had no complications, maternal complications such as preeclampsia, polyhydramnios, preterm labor, and fetal complications such as macrosomia and intrauterine device were common in GDM group. Only preeclampsia had statistical significance with TNF-a; rest of the markers though elevated in GDM had no statistical significance with any of the complications.
Consistent with literature, the present study also quotes that the incidence of macrosomia was 13.3% (n = 8), all in the study group which can be correlated for the increased operative deliveries in gestational diabetes. This study also showed increased instrumental deliveries and cesarean sections in the GDM group.
Further analysis and comparison of mean levels of oxidative stress markers using ANOVA revealed a significant difference in the mean values of OGCT values, uric acid levels, TNF-a, IL-8, SOD, and GTX levels for control, GDM without complication, and GDM with complications, and post hoc tests revealed a significant mean difference in the level for control and other two categories. However, there is no significant difference between GDM with complications and GDM without complications [Table 5].
Table 5.
Mean values of cytokines among controls, GDM with complications and GDM without complications
Early intervention and right treatment in individuals with GDM or at increased risk for developing of GDM will be helpful in preventing the adverse maternal and fetal outcome and also save them from long-term complications.
CONCLUSION
Serum uric acid and TNF-a levels were significantly increased in GDM patients, and levels of GTX and SOD were decreased in GDM. Oxidative stress is elevated in GDM and TNF-a had a statistical significance with preeclampsia.
Financial support and sponsorship
The study was funded by RSSDI, Karnataka chapter.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We sincerely thank Dr. Madhu Srinath for statistical programming assistance. We thank Research Society for Study of Diabetes in India (RSSDI), Karnataka chapter, for their financial grant to the study.
REFERENCES
- 1.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. [PubMed] [Google Scholar]
- 2.Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A. Gestational diabetes mellitus in India. J Assoc Physicians India. 2004;52:707–11. [PubMed] [Google Scholar]
- 3.Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. 2007;34:173–99, vii. doi: 10.1016/j.ogc.2007.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: A population-based study. Lancet. 2007;369:750–6. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 5.Pendegrass M, Fazioni E, De Fronzo R. NIDDM and GDM: Same disease, another name? Diabetes Rev. 1995;3:556–83. [Google Scholar]
- 6.Balaji A, Mohan R, Mohanraj M, Bhavadharani, Mahalakshmi Diabetes Res Clin Pract. 2016;117:22–7. doi: 10.1016/j.diabres.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–7. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 8.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–56. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: Potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32(Suppl 3):24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 10.Rice GE. Cytokines and the initiation of parturition. Front Horm Res. 2001;27:113–46. doi: 10.1159/000061023. [DOI] [PubMed] [Google Scholar]
- 11.Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5:551–9. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Lappas M, Permezel M, Rice GE. Release of proinflammatory cytokines and 8-isoprostane from placenta, adipose tissue, and skeletal muscle from normal pregnant women and women with gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:5627–33. doi: 10.1210/jc.2003-032097. [DOI] [PubMed] [Google Scholar]
- 13.Lappas M, Hiden U, Hauguel JF, de M. Alicia J, Desoye G. Antioxidants and redox signally. 2011;15:3061–87. doi: 10.1089/ars.2010.3765. [DOI] [PubMed] [Google Scholar]
- 14.Reece EA, Homko CJ, Wu YK, Wiznitzer A. The role of free radicals and membrane lipids in diabetes-induced congenital malformations. J Soc Gynecol Investig. 1998;5:178–87. doi: 10.1016/s1071-5576(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson UJ, Borg LA. Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformations in vitro. Diabetologia. 1991;34:325–31. doi: 10.1007/BF00405004. [DOI] [PubMed] [Google Scholar]
- 16.Tho LL, Candlish JK, Thai AC. Correlation of diabetes mellitus with erythrocvte enzyme decomposing reactive oxygen species. Annu Chem Biochem. 1988;25:426–31. doi: 10.1177/000456328802500420. [DOI] [PubMed] [Google Scholar]
- 17.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 18.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–51. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 19.Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S, et al. Cytokine levels in gestational diabetes mellitus: A systematic review of the literature. Am J Reprod Immunol. 2013;69:545–57. doi: 10.1111/aji.12088. [DOI] [PubMed] [Google Scholar]
- 20.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B, et al. Reduced adiponectin concentration in women with gestational diabetes: A potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 21.Seshiah V, Das AK, Joshi SR, Parikh MN, Guptha S. Available from: https://www.japi.org / august 2006/ DIPSI-662 . [PubMed]
- 22.Nobata Y, Urakaze M, Temaru R, Sato A, Nakamura N, Yamazaki K, et al. Alpha-tocopherol inhibits IL-8 synthesis induced by thrombin and high glucose in endothelial cells. Horm Metab Res. 2002;34:49–54. doi: 10.1055/s-2002-20523. [DOI] [PubMed] [Google Scholar]
- 23.Waring WS. Anti-oxidants in prevention and treatment of cardiovascular diseases. Proc R Coll Physicians Edinb. 2001;31:288–92. [Google Scholar]
- 24.Surapaneni KM, Vishnu Priya V. Antioxidant enzymes and vitamins in gestational diabetes. J Clin Diagn Res. 2008;2:1081–5. [Google Scholar]
- 25.Kharb S. Ascorbic acid and uric acid levels in gestational diabetes mellitus. J Obstet Gynecol India. 2007;57:401–2. [Google Scholar]
- 26.Rasika C, Samal S, Ghose S. Association of elevated first trimester serum uric acid levels with development of GDM. J Clin Diagn Res. 2014;8:OC01–5. doi: 10.7860/JCDR/2014/8063.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25:78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Zhao X, Wang Z, Hu Y. Combination of lipids and uric acid in mid-second trimester can be used to predict adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;25:2633–8. doi: 10.3109/14767058.2012.704447. [DOI] [PubMed] [Google Scholar]


