Abstract
Introduction:
Diabetic nephropathy is leading cause of morbidity and mortality of type 1 diabetes mellitus (DM). Microalbuminuria is the first clinical sign of nephropathy.
Methods:
This was a cross-section study with longitudinal evaluation of urinary albumin xcretion in 199 children with type 1 diabetes attending CDiC Clinic in BIRDEM over a period of two years. The aim of the study was to assess the frequency of microalbuminuria and to determine other risk factors. We collected blood and early morning spot urinary sample and analyzed for HbA1c by Clover A1c and urinary microalbumin by a DCA analyzer. Children had urinary microalbumin 30-300 mg/L on at least two occasions were categorized as having persistent microalbuminuria. Demographic and clinical data were recorded including age at onset of diabetes, age during registration, gender and duration of diabetes which were compared between patients without microalbuminuria and with microalbuminuria.
Result:
Microalbuminuria developed in forty nine children and adolescents (25%). Among them 24% were Type 1, 27% were with Fibrocalculous pancreatic diabetes (FCPD) and 68% were Type 2 diabetes. Median HbA1c was higher 10.8 [9.4-12.4] vs 9.5 [8.0-11.2] (P.006) in adolescents with microalbuminuria. On logistic regression univariate analysis independent predictors of microalbuminuria were older age, systolic blood pressure, BMI SDS and mean HbA1c which remained significant in multivariate analysis as predictors of microalbuminuria.
Conclusion:
We found high prevalence of microalbuminuria which was associated with higher age, systolic blood pressure, BMI SDS and HbA1c.
Keywords: Adolescents, children, microalbuminuria, type 1 diabetes, type 2 diabetes
BACKGROUND
Diabetic nephropathy is the most common microvascular complication of type 1 diabetes which affects around 15%–40% patients, with a peak incidence after 15–20-year diabetes duration.[1] Microalbuminuria is the earliest clinical stage of clinical nephropathy, and its persistence is highly predictive of progression to overt proteinuria and of risk for cardiovascular diseases.[1] Several studies suggest that at these early stages, progression of diabetic nephropathy can be prevented.[2,3,4] In people with childhood-onset type 1 diabetes, the cumulative prevalence of microalbuminuria is around 12%–25% after 5 to 10 years of diabetes.[5,6,7,8,9,10] Within 2 decades of diabetes onset, a single episode of microalbuminuria is found in 2%–18% of children and adolescents with type 1 diabetes[11,12,13] but may be transient in up to half of cases.[10,14] Established risk factors for microalbuminuria in adolescents and adults include duration of diabetes,[14,15] suboptimal glycemic control,[16,17] and hypertension. In this study of 199 adolescents with diabetes, we examined the frequency of microalbuminuria in different types of diabetes and we specifically focused on the association between microalbuminuria and recognized risk factors – age, diabetes duration, body mass index (BMI), insulin dose, blood pressure, and hemoglobin A1c (HbA1c).
RESEARCH DESIGN AND METHODS
Children and adolescents with diabetes who were regularly attending the Changing Diabetes in Children (CDiC) clinic, a paediatric diabetes multidisciplinary clinic in BIRDEM, over 2-year period from February 2012 to February 2014 were included in this study. BIRDEM (Bangladesh Institute of Research and Rehabilitation of Diabetes Endocrine and Metabolic disorders) is a tertiary care diabetic hospital where patients are referred from all over Bangladesh. Clinical assessment including anthropometry and blood pressure measurement was done by pediatric endocrinologist during complications assessment. Diabetes mellitus was diagnosed according to WHO, ISPAD, and Mohan's criteria.[18,19,20] and all patients underwent abdominal X-Ray. Pancreatic autoantibodies were not available in our clinic. Patients were classified clinically as T1D with abrupt onset of typical symptoms of diabetes, usually who were nonobese, absence of signs of insulin resistance, severe diabetes with markedly elevated HbA1c, presenting with diabetic ketoacidosis (DKA), requiring insulin from time of onset. Patients were classified as fibrocalculus pancreatic diabetes (FCPD) if they had pancreatic calcification on X-ray or ultrasonography reported by radiologist and absence of alcohol intake hypercalcemia or biliary duct stones. Patients presenting with overweight (BMI more than 85th centile) or obesity (BMI more than 95th centile), at pubertal age, with signs of insulin resistance-acanthosis nigricans, hypertension, dyslipidemia, polycystic ovaries, and positive family history in 1st or 2nd degree relatives were classified as type 2 diabetes.
Screening for microalbuminuria was done during the visit. Children with febrile illness, DKA, urinary tract infection, and preexistent renal disease were excluded from the study. Patients were included in this study if albumin concentration had been measured two or more times within 6 months. Spot early morning urinary albumin concentration was measured by DCA analyzer. Normoalbuminuria was defined as urinary albumin concentration <30 mg/L in all urine samples collected. Microalbuminuria was defined as urinary albumin concentration 30–300 mg/L on at least two of three samples. Macroalbuminuria was defined if it was >300 mg/L in at least two samples. Height was measured using a Harpenden stadiometer and weight using the bathroom scales. Obesity was defined as a BMI standard deviation [SD] score ≥2.[21] Blood pressure was measured by auscultation after 5 min of rest, and percentiles and SD score were calculated based on age and gender.[22] HbA1c was assessed by Clover A1c using photoelectric method. HbA1c done during the screening was taken for analysis. Patients and their families gave informed consent for the results of the complications’ assessment to be analyzed. The project was approved by local Diabetic Association of Bangladesh.
Statistical analysis
Descriptive statistics is presented as mean (_SD) score for normally distributed data and median (interquartile range or range) for skewed data. Continuous data were compared using parametric test ANOVA and skewed data using the nonparametric test Kruskal–Wallis test.
Risk factors for the development of microalbuminuria from the last complication assessment were examined using logistic regression. Potential variables from the last complication assessment used in the regression model were age during the examination, BMI, HbA1c, and blood pressure.
RESULTS
Majority of the patients (88%) were type 1, 10.6% FCPD, and 1.5% were type 2 diabetes. Microalbuminuria was found in forty-nine children and adolescents (25%). Among them, seven patients (3.5%) had macroalbuminuria (≥300 mg/L). Considering the prevalence in different types of diabetes, microalbuminuria was found in 24% of type 1 diabetes, 27% of FCPD, and 68% of type 2 diabetes patients. We also analyzed the duration of diabetes among different types of diabetes in microalbuminuric patients. The median duration was longer in FCPD patients, i.e., 4.4 [3.1–5.60] compared to type 1 3.8 [2.4–5.7] and type 2 patients 3.4 [1.8–5.0)] years (P = 0.139). Seven patients (14%) developed microalbuminuria within 2 years of diagnosis. Among them, six (13%) were type 1 and one (50%) was type 2 diabetes. The shortest diabetes duration to development of microalbuminuria was 7 months. Compared with the patients with normoalbuminuria, microalbuminuric patients had significantly higher BMI SD score - 0.47 ± 1.30 versus 0.10 ± 1.35 (P = 0.010) and waist height ratio 0.45 [IQR: 0.43–0.56] versus 0.43 [IQR: 0.40–0.48] (P = 0.031) [Table 1]. Systolic and diastolic blood pressure was significantly higher in patients with microalbuminuria.
Table 1.
Characteristics in normoalbuminuric versus microalbuminuric patients
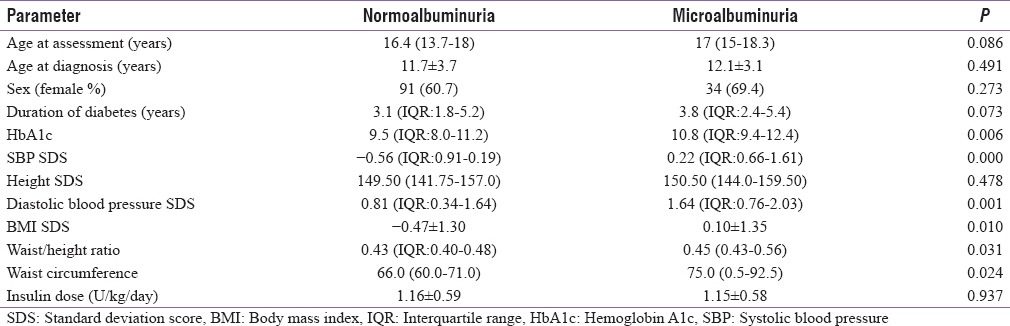
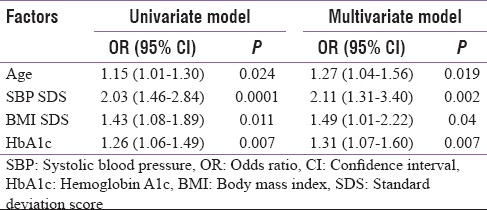
Median HbA1c was higher 10.8 (9.4–12.4) v rsus 9.5 (8.0–11.2) (P = 0.006) in adolescents with microalbuminuria. More than 81% patients had high HbA1c >9% and only 8% patients had <7.5% in microalbuminuric group (P < 0.014) [Figure 1]. On logistic regression, univariate analysis-independent predictors of microalbuminuria were older age, systolic blood pressure, BMI SDS, and mean HbA1c which remained significant in multivariate analysis as predictors of microalbuminuria [Table 2].
Figure 1.
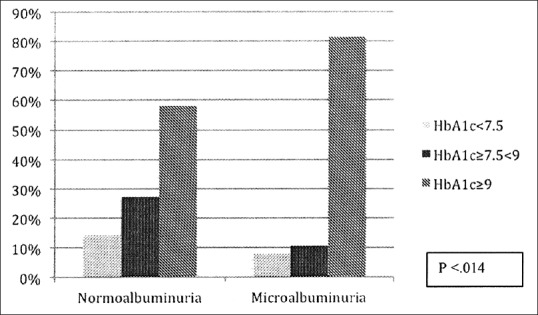
Frequency of hemoglobin A1c in two groups
Table 2.
Factors associated with microalbuminuria in adolescents with diabetes
DISCUSSION
In this cohort of 199 youth with diabetes, the prevalence of microalbuminuria was 25%, and among them, seven patients (3.5%) had macroalbuminuria (≥300 mg/L).
The prevalence of microalbuminuria in children with type 1 diabetes varies between 3%–30%.[23,24,25,26] The wide range of prevalence in various studies may be due to multiple genetic factors in different ethnic groups. The prevalence of microalbuminuria was highest in type 2 diabetes in our population. Higher prevalence was found in adolescents with type 2 diabetes in comparison with type 1 diabetes in different reports. Microalbuminuria was found in 28% of youth with type 2 diabetes after a relatively short duration of diabetes in a study done in Australia.[27] Similarly, the incidence of nephropathy among type 2 diabetic patients is as twice as high than in type 1 diabetic patients in a study done in Japan.[28] Microalbuminuria was observed in 18% of Korean patients with type 2 diabetes compared with 11% with type 1 diabetes.[29] Increasing age in our population increased the risk of microalbuminuria and all patients were at pubertal age. Older age and puberty were determined as risk factors for complications in previous reports.[30,31,32,33] Although longer duration of diabetes, older age, and puberty were determined as risk factors for complications in previous reports,[31,32,33] seven patients in our population developed microalbuminuria within 2 years of diagnosis of diabetes. Among them, two developed earliest within 1 year. One study done in India has demonstrated urine microalbuminuria in five children with <2 years of diabetes duration.[34] Screening from age 11 years with 2 years diabetes duration and from 9 years with 5 years duration are recommended according to ISPAD 2009 guideline.[35] Seven children in our study would have not been diagnosed with microalbuminuria based on the current ISPAD criteria for screening. Obesity at the first complications assessment was associated with the development of persistent microalbuminuria in one study.[36] BMI SDS was significantly (p. 018) higher and waist/height ratio was also higher (p. 035) in our microalbuminuric children. An increase in blood pressure is associated with increase in urinary albumin excretion in both adults and children.[17,33] Though there is controversy as to whether an increase in blood pressure precedes or is a result of the development of microalbuminuria. In our population, we found that systolic blood pressure was associated with development of microalbuminuria. Our data indicate that the modifiable predictors for the development of microalbuminuria were poor glycemic control, high blood pressure, and high BMI which are consistent with findings in different studies.[33,34,35,36] Both baseline and more recent lack of glycemic control predict risk of microalbuminuria, 5–16, and follow-up data from the diabetes control, and complications trial showed that previous intensive treatment of patients with diabetes and near normal glycemia has an extended benefit in delaying progression of diabetic nephropathy.[17]
CONCLUSION
We found there is high prevalence of microalbuminuria which was associated with higher age, systolic blood pressure, BMI SDS, and HbA1c. The earlier, after 2–5-year diabetes duration evaluation microalbuminuria in childhood diabetes much earlier than the current recommendations. Evaluation of risk factors and early screening for microvascular complications in children and adolescents are imperative to assist in preventive strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 2.Defronzo RA. Diabetic nephropathy. In: Porte D, Sherwin R, editors. Ellenberg and Rifklin's Diabetes Mellitus. 6th ed. New York: Mc Graw-Hill Companies; 2002. pp. 723–40. [Google Scholar]
- 3.Vats A, Derubertis F. Diabetic nephropathy. In: Sperling MA, editor. Type I Diabetes, Etiology and Treatment. 1st ed. Totowa, NJ: Human Press Inc; 2003. pp. 409–27. [Google Scholar]
- 4.Francis J, Rose SJ, Raafat F, Milford DV. Early onset of diabetic nephropathy. Arch Dis Child. 1997;77:524–5. doi: 10.1136/adc.77.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bojestig M, Arnqvist HJ, Karlberg BE, Ludvigsson J. Glycemic control and prognosis in type I diabetic patients with microalbuminuria. Diabetes Care. 1996;19:313–7. doi: 10.2337/diacare.19.4.313. [DOI] [PubMed] [Google Scholar]
- 6.Joner G, Brinchmann-Hansen O, Torres CG, Hanssen KF. A nationwide cross-sectional study of retinopathy and microalbuminuria in young Norwegian type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35:1049–54. doi: 10.1007/BF02221680. [DOI] [PubMed] [Google Scholar]
- 7.Olsen BS, Sjølie A, Hougaard P, Johannesen J, Borch-Johnsen K, Marinelli K, et al. A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. Danish Study Group of Diabetes in Childhood J Diabetes Complications. 2000;14:295–300. doi: 10.1016/s1056-8727(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 8.Jones CA, Leese GP, Kerr S, Bestwick K, Isherwood DI, Vora JP, et al. Development and progression of microalbuminuria in a clinic sample of patients with insulin dependent diabetes mellitus. Arch Dis Child. 1998;78:518–23. doi: 10.1136/adc.78.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudberg S, Ullman E, Dahlquist G. Relationship between early metabolic control and the development of microalbuminuria – A longitudinal study in children with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:1309–14. doi: 10.1007/BF00400811. [DOI] [PubMed] [Google Scholar]
- 10.Gorman D, Sochett E, Daneman D. The natural history of microalbuminuria in adolescents with type 1 diabetes. J Pediatr. 1999;134:333–7. doi: 10.1016/s0022-3476(99)70459-2. [DOI] [PubMed] [Google Scholar]
- 11.Svensson M, Sundkvist G, Arnqvist HJ, Björk E, Blohmé G, Bolinder J, et al. Signs of nephropathy may occur early in young adults with diabetes despite modern diabetes management: Results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS) Diabetes Care. 2003;26:2903–9. doi: 10.2337/diacare.26.10.2903. [DOI] [PubMed] [Google Scholar]
- 12.Donaghue KC, Fairchild JM, Chan A, Hing SJ, Howard NJ, Silink M, et al. Diabetes complication screening in 937 children and adolescents. J Pediatr Endocrinol Metab. 1999;12:185–92. doi: 10.1515/jpem.1999.12.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Mohsin F, Craig ME, Cusumano J, Chan AK, Hing S, Lee JW, et al. Discordant trends in microvascular complications in adolescents with type 1 diabetes from 1990 to 2002. Diabetes Care. 2005;28:1974–80. doi: 10.2337/diacare.28.8.1974. [DOI] [PubMed] [Google Scholar]
- 14.Schultz CJ, Konopelska-Bahu T, Dalton RN, Carroll TA, Stratton I, Gale EA, et al. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford regional prospective study group. Diabetes Care. 1999;22:495–502. doi: 10.2337/diacare.22.3.495. [DOI] [PubMed] [Google Scholar]
- 15.Donaghue KC, Fairchild JM, Craig ME, Chan AK, Hing S, Cutler LR, et al. Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care. 2003;26:1224–9. doi: 10.2337/diacare.26.4.1224. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:177–88. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 17.Couper JJ, Clarke CF, Byrne GC, Jones TW, Donaghue KC, Nairn J, et al. Progression of borderline increases in albuminuria in adolescents with insulin-dependent diabetes mellitus. Diabet Med. 1997;14:766–71. doi: 10.1002/(SICI)1096-9136(199709)14:9<766::AID-DIA467>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabet Care. 1999;20(Supp11):S5. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 19.ISPAD Clinical Practice Consensus Guidelines 2014 Compendium. Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes. 2014;15(Suppl. 20):4–17. doi: 10.1111/pedi.12186. [DOI] [PubMed] [Google Scholar]
- 20.Mohan V, Nagalotimath SJ, Yajnik CS, Tripathy BB. Fibrocalculous pancreatic diabetes. Diabetes Metab Rev. 1998;14:153–70. doi: 10.1002/(sici)1099-0895(199806)14:2<153::aid-dmr212>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood Institute. Report of the second task force on blood pressure control in children-1987: Task force on blood pressure control in children. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 23.Schultz CJ, Neil HA, Dalton RN, Konopelska Bahu T, Dunger DB, Oxford Regional Prospective Study Group et al. Blood pressure does not rise before the onset of microalbuminuria in children followed from diagnosis of type 1 diabetes. Oxford Regional Prospective Study Group. Diabetes Care. 2001;24:555–60. doi: 10.2337/diacare.24.3.555. [DOI] [PubMed] [Google Scholar]
- 24.Moayeri H, Dalili H. Prevalence of microalbuminuria in children and adolescents with diabetes mellitus Type I. Acta Med Iran. 2006;44:105–10. [Google Scholar]
- 25.Viswanathan V, Snehalatha C, Shina K, Ramachandran A. Persistent microalbuminuria in type 1 diabetic subjects in South India. J Assoc Physicians India. 2002;50:1259–61. [PubMed] [Google Scholar]
- 26.Downie E, Craig ME, Hing S, Cusumano J, Chan AK, Donaghue KC, et al. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: Role of insulin therapy and glycemic control. Diabetes Care. 2011;34:2368–73. doi: 10.2337/dc11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300–6. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–11. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 29.Yoo EG, Choi IK, Kim DH. Prevalence of microalbuminuria in young patients with type 1 and type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1423–7. doi: 10.1515/jpem.2004.17.10.1423. [DOI] [PubMed] [Google Scholar]
- 30.Daneman D. Early diabetes-related complications in adolescents: Risk factors and screening. Horm Res. 2005;63:75–85. doi: 10.1159/000083692. [DOI] [PubMed] [Google Scholar]
- 31.Olsen BS, Johannesen J, Sjølie AK, Borch-Johnsen K, Hougarrdss P, Thorsteinsson B, et al. Metabolic control and prevalence of microvascular complications in young Danish patients with type 1 diabetes mellitus. Danish Study Group of Diabetes in Childhood. Diabet Med. 1999;16:79–85. doi: 10.1046/j.1464-5491.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 32.Salgado PP, Silva IN, Vieira EC, Simões e Silva AC. Risk factors for early onset of diabetic nephropathy in pediatric type 1 diabetes. J Pediatr Endocrinol Metab. 2010;23:1311–20. doi: 10.1515/jpem.2010.205. [DOI] [PubMed] [Google Scholar]
- 33.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, et al. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: Effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30:2523–8. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- 34.Poovazhagi V, Senguttuvan P, Padmaraj R. Prevalence of microalbuminuria in children with type 1 diabetes mellitus. Diabet Clin. 2012 DOI: 10.7199/ped.oncall. 2012.43. [Google Scholar]
- 35.Donaghue KC, Charelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. Microvascular and macrovascular complicattions associated with diabetes in children and adolescents. Paediatr Diabet. 2014;15(Suppl 20):257–69. doi: 10.1111/j.1399-5448.2009.00576.x. [DOI] [PubMed] [Google Scholar]
- 36.Stone ML, Craig ME, Chan AK, Lee JW, Verge CF, Donaghue KC, et al. Natural history and risk factors for microalbuminuria in adolescents with type 1 diabetes: A longitudinal study. Diabetes Care. 2006;29:2072–7. doi: 10.2337/dc06-0239. [DOI] [PubMed] [Google Scholar]


