Abstract
Introduction:
Indian phenotype includes higher waist circumference despite lower body mass index, thereby making Indians more prone to diabetes and its complications.
Aim:
The present study aimed to analyze the serum levels of adiponectin and leptin in the participants with type 2 diabetes mellitus (T2DM) and obesity and their correlation with hypertension and dyslipidemia.
Materials and Methods:
In the study, 50 diabetics and 50 controls aged between 40 and 60 years were included in the study.
Results:
Adiponectin levels were significantly higher in diabetics than in nondiabetic participants irrespective of gender (P ≤ 0.04 in males, P ≤ 0.02 in females). Leptin levels were significantly higher in diabetics compared to nondiabetics (P ≤ 0.001) in both males and females.
Conclusion:
Adiponectin and leptin levels may be used as important clinical markers for T2DM and obesity.
Keywords: Adiponectin, dyslipidemia, hypertension, leptin, type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus is a leading cause of death and disability worldwide. Type 2 diabetes mellitus (T2DM) is the most common form of diabetes constituting 90% of the diabetic population.[1] India has earned the distinction of being the diabetes capital of the world.[2] The number of patients with diabetes in India is currently over 62 million.[3] It has been attributed to the so-called “Asian Indian phenotype” which refers to certain unique clinical and biochemical abnormalities in Indians including increased insulin resistance and higher waist circumference despite lower body mass index (BMI). This phenotype makes Indians more prone to diabetes and its complications.[4]
Adipocytokines are cytokines secreted by adipose tissue. Among other, they include adiponectin and leptin.[5] Adiponectin primarily modulates glucose regulation and fatty acid catabolism.[6] Despite being produced in adipose tissue, adiponectin is decreased in obesity and the circulating levels of adiponectin are inversely correlated with body fat percentage in adults showing significant increase after weight reduction.[7] Lower levels of adiponectin are considered as an independent risk factor for developing T2DM, dyslipidemia, and cardiovascular diseases.[8]
Leptin is a key hormone regulating energy intake and expenditure through controlling appetite and glucose metabolism.[9] It is primarily secreted by adipocytes and circulating leptin levels are directly proportional to the total amount of fat in the body.[10] The cellular target of leptin is the mediobasal nucleus of the hypothalamus, where it inhibits appetite. Leptin deficiency or resistance leads to uncontrolled food intake, obesity, and diabetes mellitus.[11] It may also lead to atherosclerosis, hypertension, and coronary vascular disease.[12] Adiponectin in combination with leptin has been shown to completely reverse insulin resistance in mice.[13]
Indians have a unique body structure characterized by increased abdominal fat deposition in spite of correspondingly low BMI.[14] There are many international studies correlating adiponectin and leptin levels in type 2 diabetics and obesity,[15] but only a few in the Indian population, where a large population of type 2 diabetics exist without a raised BMI, but rather an increased waist-to-hip ratio (WHR).[16] This combination of anthropometric characters makes this population highly predisposed to metabolic syndrome including T2DM. This particular phenotype is termed as Asian Indian phenotype.[2] Earlier studies have indicated that the long-term intake of oral hypoglycemic agents in T2DM participants favorably alters the levels of circulating adipocytokines. Regular analysis of circulatory adipocytokines is also useful to select the best treatment with specific reference to these important therapeutic markers.[17] The present study was undertaken to find if T2DM in this phenotype, with or without obesity, displays any significant correlation with circulating levels of adiponectin and leptin.
MATERIALS AND METHODS
Study design
The present study was an observational, cross-section study.
Subjects
A total of 50 (27 men and 23 women) type 2 diabetics, registered and undergoing treatment at Bharati Vidyapeeth Medical College and Hospital, were recruited for the study. Fifty (27 men and 23 women) age- and gender-matched controls were also recruited.
Inclusion and exclusion criteria
The clinically diagnosed T2DM participants as per the American Diabetes Association guidelines[18] aged between 40 and 60 years, with either a fasting (no caloric intake for at least 8 h) plasma glucose ≥126 mg/dl or HbA1C ≥6.5 mg/dl, were included in the study. The diabetic patients with pregnancy, kidney disease, liver disease, and malignancy were excluded from the study.
The controls between 40 and 60 years with nondiabetic history were included in the study while those with pregnancy, kidney disease, liver disease, and malignancy were excluded from the study.
Ethical standards
The study was initiated after approval by the Institutional Ethics Committee of Bharati Vidyapeeth Medical College, Bharati Vidyapeeth Deemed University, Pune-Satara Road, Pune, Maharashtra 411043, India (Ref. No. BVDU/MC/2/2012–2013). The participants were recruited for the study after receiving informed written consent.
Data recording
All the participants included in the study were delivered a questionnaire to record detailed clinical history and physical examination.
Anthropometric measurements
After giving informed consent, anthropometric measurements such as height, weight, waist circumference, and hip circumference were taken. Waist circumference was measured halfway between the last rib and the iliac crest with full abdominal relaxation. Height was measured to the nearest 0.1 cm with the subject standing barefoot. Body weight was measured to the nearest 0.1 kg on a balanced scale. Blood pressure was measured using standard mercury sphygmomanometer, with the participant in sitting position for at least 10 min.
Blood collection
Fasting blood samples (8 ml) from the participants were collected by venipuncture and collected in plain and EDTA vacutainers.
Biochemical investigations
Serum glucose and cholesterol, triglyceride, high-density lipoproteins (HDL), and low-density lipoproteins (LDL) were estimated using commercially available kits (Coral Clinical Systems, Goa, India). The serum levels of circulating adiponectin and leptin were measured by commercially available ELISA kits (Invitrogen, USA).
Statistical analysis
Data were analysed using the Statistical Package of the Social Sciences (SPSS version 20.0). Data are presented as mean ± standard deviation. Independent sample t-test, Chi-square test, and Fisher's exact test were used to compare means of different parameters. The differences among the means were considered significant if P < 0.05.
RESULTS
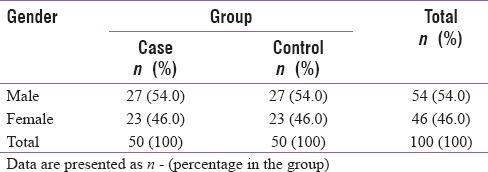
In the study, 50 diabetics (case) and 50 nondiabetic (control) people in the age bracket of 40–60 years were included in the study. The mean age of the case group was 50.02 ± 5.43 years, whereas the mean age of control group was 49.5 ± 6.07 years. There was no significant (P = 0.653) difference in age between these two groups. In both the study groups, 27 males and 23 females were included in the study, and there was no significant difference between the two groups with respect to gender [Table 1].
Table 1.
Distribution of study groups according to gender
Out of all diabetic participants, 36% (18) of the diabetic participants had diabetes for <1 year, 32% (16) had for 1–5 years, 16% (8) had diabetes for 5–10 years and 16% (8) had diabetes for >10 years.
Mean serum adiponectin was 8.76 ± 4.69 μg/ml in males compared to 10.28 ± 3.80 μg/ml in females of control group. This was statistically not significant (P = 0.219). Mean serum adiponectin was 4.97 ± 3.80 μg/ml in males compared to 5.49 ± 4.12 μg/ml in females of case group. This was statistically not significant (P = 0.535). Thus, serum adiponectin levels did not vary significantly between genders although significant differences were observed in adiponectin content between diabetics and nondiabetics irrespective of gender (P ≤ 0.04 in males, P ≤ 0.02 in females), by independent sample t-test [Table 2].
Table 2.
Serum adiponectin and leptin levels according to gender in control group and case group
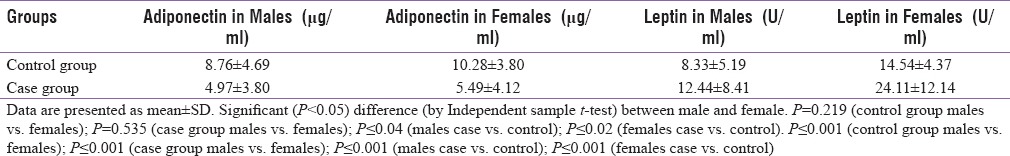
Mean serum leptin was 8.33 ± 5.19U/ml in males compared to 14.54 ± 4.37U/ml in females of control group. This was statistically highly significant (P ≤ 0.001). Mean serum leptin was 12.44 ± 8.41U/ml in males compared to 24.11 ± 12.14U/ml in females of case group. This too was statistically highly significant (P ≤ 0.001). Thus, serum leptin levels were higher in females than males. Furthermore, serum leptin levels were significantly higher in diabetics compared to nondiabetics in both males and females (P ≤ 0.001), by independent sample t-test [Table 2].
Low serum adiponectin (<3 μg/ml) was seen in 58% diabetics whereas it was only seen in 6% of nondiabetics. This was statistically highly significant (P ≤ 0.001), by Fisher's exact test [Table 3].
Table 3.
Association of low serum adiponectin and leptin levels in diabetics versus control group
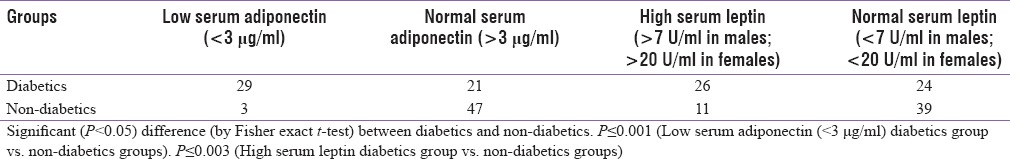
Significantly high serum leptin (>7 U/ml in males; >20 U/ml) was seen in 52% diabetics (P ≤ 0.003) whereas it was seen in 22% of nondiabetics [Table 3].
Serum adiponectin levels were compared between diabetics (case) and nondiabetics (control). Mean serum adiponectin was 9.46 ± 4.33 μg/ml in nondiabetics. Comparatively, mean serum adiponectin was lower in diabetics (5.19 ± 3.89 μg/ml). This was statistically significant (P ≤ 0.004), by Chi-square test [Table 4].
Table 4.
Comparison of mean serum adiponectin and leptin among diabetics and non-diabetics
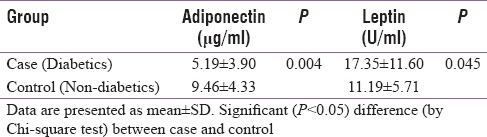
Serum leptin levels were significantly higher (17.35 ± 11.60 U/ml) in diabetics than in nondiabetics (11.19 ± 5.71 U/ml) (P ≤ 0.045) [Table 4].
In the diabetic group of 50 cases, low serum adiponectin (<3 μg/ml) was associated with obesity (>25 kg/m2) in 22 out of 29 cases (76%) (P ≤ 0.001) [Table 5] while high serum leptin (>7U/ml in males; >20U/ml in females) was associated with obesity (>25 kg/m2) in 18 out of 26 (69%) cases (P ≤ 0.025) [Table 5].
Table 5.
Comparison of serum adiponectin and leptin levels with BMI in diabetic group
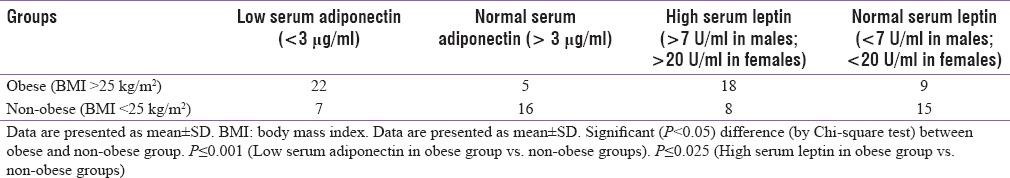
Low serum adiponectin (<3 μg/ml) in diabetics was associated with central obesity (WHR > 0.90 in males; >0.85 in females) in 22 (71%) out of 31 cases (P ≤ 0.001) [Table 6] while high serum leptin (>7U/ml in males; >20U/ml in females) was associated with central obesity (WHR > 0.90 in males; >0.85 in females) in 15 (83%) out of 18 cases (P ≤ 0.001) [Table 6].
Table 6.
Comparison of serum adiponectin and leptin levels with waist hip ratio (WHR) in diabetic group
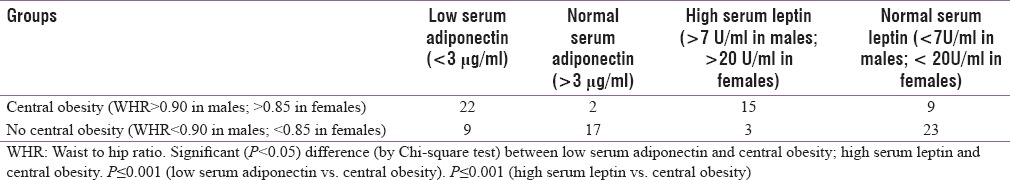
Serum adiponectin and leptin levels were also compared with the incidence of hypertension in the entire study population (40 persons out of 100 were hypertensive, of which 32 were from diabetic group). It was found that low serum adiponectin (<3 μg/ml) was associated with a higher incidence of hypertension (25 out of 31 = 81%) (P ≤ 0.002) [Table 7] while high serum leptin (>7U/ml in males; >20U/ml in females) was associated with higher incidence of hypertension (24 out of 37 = 65%) (P ≤ 0.025) [Table 7].
Table 7.
Comparison of serum adiponectin levels with incidence of hypertension in study population
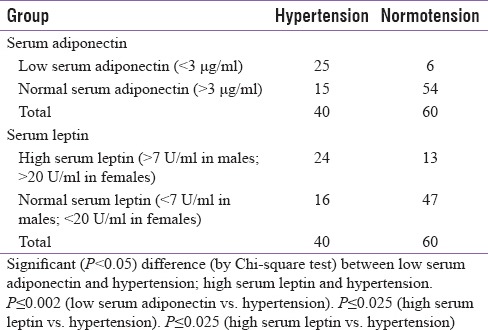
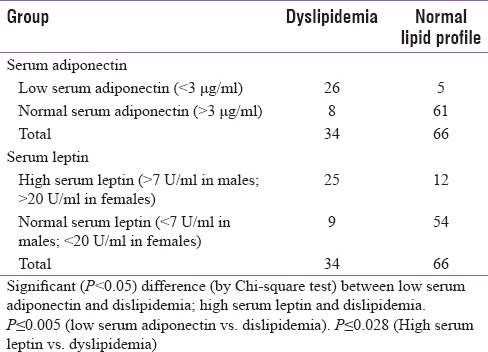
Serum adiponectin and leptin levels were compared with the incidence of dyslipidemia in the entire study population (34 persons out of 100 were dyslipidemic (LDL/HDL ratio >3 or patient already on antihyperlipidemic therapy), of which 26 were from diabetic group). It was found that low serum adiponectin (<3 μg/ml) had a higher incidence of dyslipidemia (26 out of 31 = 84%) (P ≤ 0.005) [Table 8] while high serum leptin (>7U/ml in males; >20U/ml in females) was associated with a higher incidence of dyslipidemia (25 out of 37 = 68%) (P ≤ 0.028) [Table 8].
Table 8.
Comparison of serum adiponectin levels with incidence of dyslipidemia in study population
Serum adiponectin and leptin levels exhibited an inverse relationship [Figure 1].
Figure 1.
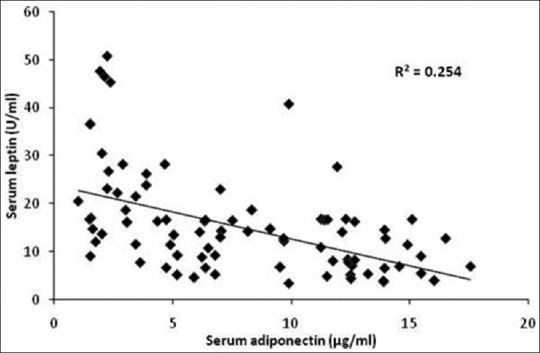
Relation of serum adiponectin to serum leptin in the study group
DISCUSSION
In the present study, the mean age for cases was 50.02 years and controls was 49.50 years with no significant difference. Hence, the two groups were age matched. In both, the diabetic and nondiabetic group, 27 males and 23 females were included in the study. Hence, both the groups were gender matched. According to the WHO, middle-aged and older adults are still at the highest risk for developing T2DM. According to IDF 2009,[19] adults aged 45–64 were the most diagnosed age group for diabetes.
In our study, the serum adiponectin in nondiabetic males was lower as compared to nondiabetic females. In the diabetic males, serum adiponectin was lower than in diabetic females. Circulating adiponectin levels, though statistically nonsignificant, were higher in females than males, irrespective of the glycemic status, but serum adiponectin levels are lower in diabetics of both sexes compared to nondiabetics, and the difference is statistically highly significant in both the sexes.
A study by Saltevo et al., 2009[20] showed that adiponectin concentrations in women decreased relatively more compared with men across individuals with prediabetes and T2DM, and absolute adiponectin concentrations were significantly higher in women, but the gender ratio (women to men) for adiponectin concentrations decreased linearly.
In the present study, serum leptin levels in nondiabetic males were significantly lower compared to nondiabetic females. Similarly, serum leptin in diabetic males was significantly lower compared to diabetic females. Irrespective of the glycemic status, circulating leptin levels were significantly higher in females compared to males. Furthermore, serum leptin levels were significantly higher in diabetics of both sexes compared to nondiabetics.
It has been reported that serum leptin gradually declines during aging and leptin reduction is higher in women than in men, in adult humans of different body weight.[21]
Adiponectin levels in type 2 diabetics and nondiabetics
Low serum adiponectin (<3 μg/ml) was seen in diabetics which was significantly lower as compared to nondiabetics. Furthermore, the serum adiponectin was significantly lower than the age- and sex-matched nondiabetics. A meta-analysis of multiethnic population for genome-wide association study reports low circulating adiponectin levels in individuals with increased risk of T2DM.[22,23] A recent study of newly identified diabetic cases in Japan showed that serum adiponectin levels are inversely associated with incidence of diabetes.[24]
In an Indian study conducted in Chennai, the mean baseline adiponectin level was found to be lower in the diabetic participants than in the nondiabetic participants. Low adiponectin levels have been identified as a strong predictor of future development of diabetes and HbA1c also showed a positive predictive association.[25]
Leptin levels in type 2 diabetics and nondiabetics
High serum leptin (>7U/ml in males and >20U/ml in females) was recorded in diabetics, compared to incidence of high serum leptin in nondiabetics. The leptin levels were lower in the diabetic population and were higher in the females of diabetic and nondiabetic groups.[26] McNeely et al., 1999,[27] showed that among Japanese Americans, increased baseline leptin levels were associated with increased risk of developing T2DM in men but not in women. In accordance with these studies, incidence of high serum leptin was higher in diabetics than nondiabetics.
Adiponectin and obesity
Obesity in terms of BMI >25 kg/m2 was associated with low adiponectin, after adjusting for age, sex, and diabetes, which was statistically highly significant as compared to nonobese group. Comparison of high WHR of >0.90 in males and >0.85 in females indicates central obesity. Individuals with high WHR had significantly lower adiponectin after adjusting for age, sex, and diabetic status. In normal WHR participants, we observed normal serum adiponectin levels, suggesting that serum adiponectin has an inverse correlation with obesity and WHR.
The plasma adiponectin levels have been shown to be negatively correlated with BMI and WHR[28] and with waist circumference.[29] A study in Mumbai, India, showed that serum adiponectin was lower in obese participants compared to nonobese participants, and adiponectin is inversely associated with BMI and waist circumference.[30]
Leptin and obesity
Obesity in terms of BMI >25 kg/m2 was associated with high serum leptin level in males and females. Maffei et al., 1995,[31] reported that plasma leptin levels in rodents and in lean and obese humans are highly correlated with BMI. Al-Maskari and Alnaqdy 2006[32] studied obese and nonobese healthy participants and found that there was a significant difference in serum leptin and a significant positive correlation between leptin levels in obese participants with weight, body fat percentage, and BMI. Moreover, a significant positive correlation of leptin with BMI and waist circumference has been demonstrated among diabetic and healthy controls.[30]
Comparison of high WHR of >0.90 in males and >0.85 in females indicates central obesity where diabetic participants with high WHR had significant high serum leptin. Furthermore, in normal WHR participants, we observed normal serum leptin levels.
Correlation of adiponectin with hypertension and dyslipidemia
Low serum adiponectin (<3 μg/ml) was associated with a higher incidence of hypertension, as compared to normal adiponectin (>3 μg/ml). These results are in accordance with Adamczak et al., 2003,[33] where they reported plasma adiponectin concentration to be lower in hypertensive patients and is negatively associated with blood pressure. Low serum adiponectin levels were found to be independently associated with a higher risk for the development of hypertension.[34]
Adiponectin was also associated with HDL and plasma triglyceride concentrations.[35] Low adiponectin levels were associated with higher triglycerides, LDL, and lower HDL.[36] In our study, serum adiponectin levels were also compared with the incidence of dyslipidemia in the entire study population. It was found that low serum adiponectin levels were related to a higher incidence of dyslipidemia, as compared to normal adiponectin.
Correlation of leptin with hypertension and dyslipidemia
Incidence of higher serum leptin was associated with a higher incidence of hypertension and dyslipidemia. A recent study showed that leptin has a strong association with hypertension in hypertensive patients in India.[37] An another recent study showed elevated leptin levels with increased risk of obesity in men, regardless of the presence of T2DM than in women.[38]
Limitation
One of the limitations of the study was that it was a cross-sectional study, which can simply bring association but cannot predict the effect of individual variables. Therefore, prospective studies should be carried out to assess whether low adiponectin levels and high leptin levels can be used to predict the development of T2DM and obesity in the population and also to prevent its complications. Our study had a small sample size. Large sample size needs to be studied to have better inferences.
In addition, these adipocytokines have not been studied yet at epidemiological scale to determine the normal levels of these parameters in healthy population. Although their imbalance is seen in diabetes and obesity, lack of normal range for these parameters makes it difficult to use them as markers of risk for obesity and Type 2 DM in an individual case.
CONCLUSION
The study showed that adiponectin and leptin concentrations vary between diabetic and nondiabetic participants. Low serum adiponectin and high serum leptin levels are associated with increased risk of T2DM, obesity, increased WHR, hypertension, and dyslipidemia. In the future, these adipocytokines may also be used to prevent or to treat T2DM, obesity, and its complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Bharati Vidyapeeth Deemed University for financial support.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian Scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 3.World Health Organization. Fact Sheet No. 312: What is Diabetes? 2014. [Last accessed on 2015 Jul 22]. Available from: http://www.who.iny/mediacentre/factsheets/fs312/en .
- 4.Sosale A, Prasanna Kumar KM, Sadikot SM, Nigam A, Bajaj S, Zargar AH, et al. Chronic complications in newly diagnosed patients with type 2 diabetes mellitus in India. Indian J Endocrinol Metab. 2014;18:355–60. doi: 10.4103/2230-8210.131184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF, et al. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2012;109:14568–73. doi: 10.1073/pnas.1211611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 8.Coppola A, Marfella R, Coppola L, Tagliamonte E, Fontana D, Liguori E, et al. Effect of weight loss on coronary circulation and adiponectin levels in obese women. Int J Cardiol. 2009;134:414–6. doi: 10.1016/j.ijcard.2007.12.087. [DOI] [PubMed] [Google Scholar]
- 9.Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: Pathways and genes. Obesity (Silver Spring) 2008;16(Suppl 3):S11–22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 11.Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;7:51. doi: 10.3389/fnins.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams KW, Scott MM, Elmquist JK. From observation to experimentation: Leptin action in the mediobasal hypothalamus. Am J Clin Nutr. 2009;89:985S–990S. doi: 10.3945/ajcn.2008.26788D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 14.Chandalia M, Lin P, Seenivasan T, Livingston EH, Snell PG, Grundy SM, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One. 2007;2:e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajkovic N, Zamaklar M, Lalic K, Jotic A, Lukic L, Milicic T, et al. Relationship between obesity, adipocytokines and inflammatory markers in type 2 diabetes: Relevance for cardiovascular risk prevention. Int J Environ Res Public Health. 2014;11:4049–65. doi: 10.3390/ijerph110404049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdullah N, Attia J, Oldmeadow C, Scott RJ, Holliday EG. The architecture of risk for type 2 diabetes: Understanding asia in the context of global findings. Int J Endocrinol. 2014;2014:593982. doi: 10.1155/2014/593982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghadge AA, Harke SM, Khadke SP, Diwan AG, Pankaj M, Kulkarni OP, et al. Circulatory adipocytokines and lipid profile variations in type-2 diabetic subjects: Desirable side-effects of antidiabetic drugs. Diabetes Metab Syndr. 2014;8:230–2. doi: 10.1016/j.dsx.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes舑2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. Diabetes Atlas. 4th ed. Brussesls: International Diabetes Federation; 2009. [PubMed] [Google Scholar]
- 20.Saltevo J, Kautiainen H, Vanhala M. Gender differences in adiponectin and low-grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gend Med. 2009;6:463–70. doi: 10.1016/j.genm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Isidori AM, Strollo F, Morè M, Caprio M, Aversa A, Moretti C, et al. Leptin and aging: Correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85:1954–62. doi: 10.1210/jcem.85.5.6572. [DOI] [PubMed] [Google Scholar]
- 22.Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: A multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Matsushita Y, Nakagawa T, Hayashi T, Noda M, Mizoue T, et al. Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutr Diabetes. 2014;4:e130. doi: 10.1038/nutd.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A, et al. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26:3226–9. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 26.Tatti P, Masselli L, Buonanno A, Di Mauro P, Strollo F. Leptin levels in diabetic and nondiabetic subjects. Endocrine. 2001;15:305–8. doi: 10.1385/ENDO:15:3:305. [DOI] [PubMed] [Google Scholar]
- 27.McNeely MJ, Boyko EJ, Weigle DS, Shofer JB, Chessler SD, Leonnetti DL, et al. Association between baseline plasma leptin levels and subsequent development of diabetes in Japanese Americans. Diabetes Care. 1999;22:65–70. doi: 10.2337/diacare.22.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, et al. Plasma adiponectin levels in overweight and obese Asians. Obes Res. 2002;10:1104–10. doi: 10.1038/oby.2002.150. [DOI] [PubMed] [Google Scholar]
- 29.Milewicz A, Jedrzejuk D, Dunajska K, Lwow F. Waist circumference and serum adiponectin levels in obese and non-obese postmenopausal women. Maturitas. 2010;65:272–5. doi: 10.1016/j.maturitas.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Lele RD, Joshi SR, Gupte A. Association of adipocytokines (leptin, adiponectin TNF-alpha), insulin and proinsulin with diabetes – The mumbai obesity project [MOP] J Assoc Physicians India. 2006;54:689–96. [PubMed] [Google Scholar]
- 31.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 32.Al Maskari MY, Alnaqdy AA. Correlation between serum leptin levels, body mass index and obesity in omanis. Sultan Qaboos Univ Med J. 2006;6:27–31. [PMC free article] [PubMed] [Google Scholar]
- 33.Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y, et al. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16:72–5. doi: 10.1016/s0895-7061(02)03197-7. [DOI] [PubMed] [Google Scholar]
- 34.Imatoh T, Miyazaki M, Momose Y, Tanihara S, Une H. Adiponectin levels associated with the development of hypertension: A prospective study. Hypertens Res. 2008;31:229–33. doi: 10.1291/hypres.31.229. [DOI] [PubMed] [Google Scholar]
- 35.von Enyatten M, Hamann A, Twardella D, Nawroth P, Brenner H, Rotehbacher D. Relationship of adiponectin with markers of systemic inflammation, atherogenic dyslipidemia and heart failure in patients with coronary artery disease. Clin Chem. 2006;52:853–9. doi: 10.1373/clinchem.2005.060509. [DOI] [PubMed] [Google Scholar]
- 36.Marso SP, Mehta SK, Frutkin A, House JA, McCrary JR, Kulkarni KR, et al. Low adiponectin levels are associated with atherogenic dyslipidemia and lipid-rich plaque in nondiabetic coronary arteries. Diabetes Care. 2008;31:989–94. doi: 10.2337/dc07-2024. [DOI] [PubMed] [Google Scholar]
- 37.Mahadik SR. Association between adipocytokines and insulin resistance in Indian hypertensive patients. Indian Heart J. 2012;64:35–9. doi: 10.1016/S0019-4832(12)60008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirrakhimov EM, Kerimkulova AS, Lunegova OS, Mirrakhimov AE, Nabiev MP, Neronova KV, et al. The association of leptin with dyslipidemia, arterial hypertension and obesity in kyrgyz (Central Asian Nation) population. BMC Res Notes. 2014;7:411. doi: 10.1186/1756-0500-7-411. [DOI] [PMC free article] [PubMed] [Google Scholar]


