Abstract
Fryns syndrome (FS) is a multiple malformations syndrome with major features of congenital diaphragmatic hernia, pulmonary hypoplasia, craniofacial dysmorphic features, distal digit hypoplasia, and a range of other lower frequency malformations. FS is typically lethal in the fetal or neonatal period. Inheritance is presumed autosomal recessive. Although no major genetic cause has been identified for FS, biallelic truncating variants in PIGN, encoding a component of the glycosylphosphatidylinositol (GPI)-anchor biosynthesis pathway, have been identified in a limited number of cases with a phenotype compatible with FS. Biallelic variants in PIGN, typically missense or compound missense with truncating, also cause multiple congenital anomalies-hypotonia-seizures syndrome 1 (MCAHS1). Here we report six further patients with FS with or without congenital diaphragmatic hernia and recessive loss of function PIGN alleles, including an intragenic deletion with a likely founder effect in La Réunion and other Indian Ocean islands. Our results support the hypothesis that a spectrum of phenotypic severity is associated with recessive PIGN variants, ranging from FS at the extreme end, caused by complete loss of function, to MCAHS1, in which some residual PIGN function may remain. Our data add FS resulting from PIGN variants to the catalog of inherited GPI deficiencies caused by the disruption of the GPI-anchor biosynthesis pathway.
Introduction
Fryns syndrome (FS; OMIM 229850) is a multiple congenital anomaly syndrome with presumed autosomal recessive inheritance. The clinical presentation includes congenital diaphragmatic hernia (CDH) with pulmonary hypoplasia, craniofacial dysmorphic features, cleft lip/palate, brachytelephalangy with nail hypoplasia, and various possible internal malformations [1]. The phenotype of FS has been reviewed and diagnostic guidelines proposed [2, 3]. Lin et al. [3] suggested a definition of the Fryns phenotype according to the combination of six criteria based on previously published articles: diaphragmatic defects, characteristic facies, distal digital hypoplasia, pulmonary hypoplasia, associated anomalies (polyhydramnios, cloudy corneas, cleft lip/palate and genitourinary, cardiovascular and cerebral malformations), and affected sibs. The presence of four among the six criteria provides a strict definition of FS, whereas the presence of three criteria constitutes a broad definition of FS. Until recently, no gene had been clearly associated with FS. The diagnosis of FS typically remains clinical, after exclusion of chromosomal aberrations using array comparative genomic hybridization (CGH). In particular, phenotypes similar to FS have been associated with chromosomal deletions at 15q26.2 and 8p23.1 or mosaic trisomy 1q [4, 5].
Recently, recessive variants in PIGN (Phosphatidyl Inositol Glycan Anchor Biosynthesis, Class N) have been reported in four patients with syndromic CDH, all of which meet strict diagnostic criteria for FS [6, 7]. Genetic heterogeneity was suspected by McInerney-Leo et al. [6], as two FS cases were negative for PIGN variants. We performed whole-exome sequencing of one patient from La Réunion Island with a clinical diagnosis of FS [8] and of one FS patient with consanguineous parents of North African origin. A homozygous loss of function PIGN allele (an intragenic deletion or a frameshift) was identified in each case. Investigation of two additional families from La Réunion Island and Mayotte Island led to the confirmation of a founder effect in this region for the PIGN intragenic deletion.
Clinical reports
Written informed consent for genetic testing and publication of patient photographs were obtained from the families.
Family 1 (patients 1 and 2)
A clinical description of patients 1 and 2 (individuals II-1 and II-2, respectively, of family 1 in Fig. 1) has been previously published (patients 1 and 2, respectively, in Alessandri et al. [8]) and the key features are reproduced here as follows:
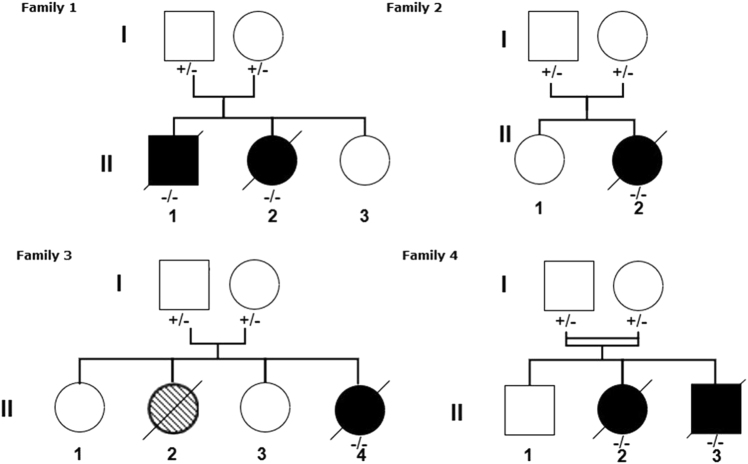
Fig. 1.
Pedigrees of families in which Fryns syndrome segregates with homozygous PIGN loss of function alleles. The hatched shading for individual II: 2 of family 3 indicates a probably affected patient. PIGN genotype: +/+, wildtype; +/−, heterozygous variant or deletion; −/−, homozygous variant or deletion
Patient 1 was a male, the first child of unrelated parents from La Réunion Island. Ultrasonographic examination during pregnancy indicated a cleft lip, a cystic dilatation of the fourth ventricle, and polyhydramnios. Magnetic resonance imaging (MRI) of the brain showed moderate hypoplasia of the cerebellar vermis. At birth, he had coarse facial features, bilateral cataracts, dysplastic ears, a unilateral cleft lip and palate, a small penis, bilateral cryptorchidism, brachytelephalangia of the toes and digits, absent nails of the fifth digits, and a ventricular septal defect. X-rays revealed normal diaphragmatic shadows. The baby died at 8 days of life. Array CGH with 60 kb resolution (Agilent Technologies) was normal.
Patient 2 was the sister of patient 1. The pregnancy was complicated by polyhydramnios. Following birth, she presented with refractory hypoxemia and died at 24 h of life. She had dysmorphic facial features, cloudy corneas and terminal hypoplasia of the fifth digits with absence of fingernails (Figs. 2 and 3). A chest X-ray showed right-sided diaphragmatic hernia. Array CGH (60 kb resolution, Agilent Technologies) was normal.
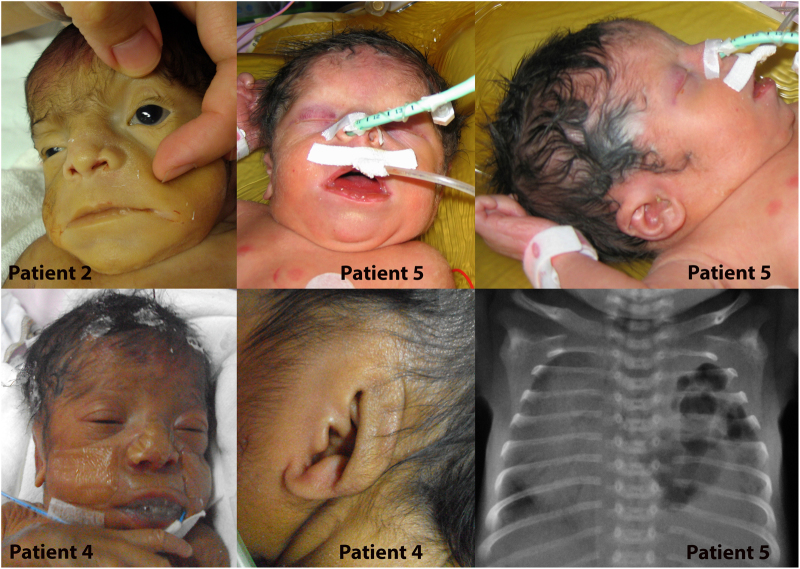
Fig. 2.
Clinical and radiological features of patients 2, 4, and 5. Facial dysmorphism with coarse features and macrostomia is shown for all three. Low set and dysplastic ears are shown for patients 4 and 5. The X-ray indicates left CDH in patient 5
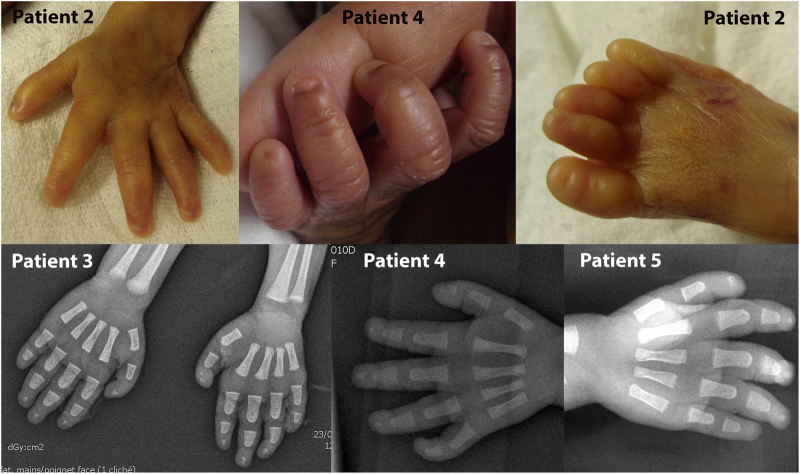
Fig. 3.
Brachytelephalangy in patients 2–5. Short distal phalanges, hypoplastic nails, and absent nail of the fifth digit are shown for the hands of patients 2 and 4. Absent or hypoplastic toenails are shown for patient 2. Hand X-rays of patients 3, 4, and 5 indicate absent distal phalanx of the fifth digit and hypoplastic phalanges of the other fingers
Family 2 (patient 3)
Patient 3 (individual II-2, family 2 in Fig. 1), a female, was the second child of unrelated parents from La Réunion Island. She is an unpublished patient. Family history was unremarkable. Mother and father were respectively aged 22 and 23 years old at the beginning of pregnancy. Ultrasonographic examination performed at 16 weeks of gestation (WG) revealed bilateral pyelectasia in a female fetus (left: 7.7 mm, right: 7.6 mm). At 19 WG, tetralogy of Fallot was diagnosed and at 23 WG a right club foot, cleft palate and polyhydramnios were identified. Semi-circular canals were present. Karyotype analysis performed at 19 WG on amniotic fluid showed normal chromosomes (46, XX) and no 22q11.2 deletion by in situ hybridization. MRI of the fetal brain at 28 WG was normal.
Spontaneous fetal demise occurred at 36 WG. Birth weight was 2260 g (3–10th centile), length 46 cm (10–25th centile) and head circumference 31 cm (10–25th centile). The malformations identified in utero were confirmed. Necropsy showed in addition extreme brachytelephalangy with hypoplastic nails of all fingers (Fig. 3). Post-mortem array CGH (60 kb resolution, Agilent Technologies) on DNA extracted from a blood sample was normal, as was testing of the TBC1D24 gene (associated with DOORS syndrome, OMIM 220500).
Family 3 (patient 4)
Patient 4 (individual II-4, family 3 in Fig. 1), a female, was the fourth child of unrelated parents from Mayotte Island. She is an unpublished patient. Two previous children were healthy. One previous child (individual II-2, family 3 in Fig. 1) died 28 min after delivery. She had bilateral cleft lip and palate, bilateral talipes, oligodactyly of one foot, and an abdominal wall defect. Severe polyhydramnios was present. Amniocyte chromosome analysis performed at 29 WG revealed a normal 46, XX karyotype. Necropsy or other investigations were declined. Ultrasonographic examination of Patient 4 performed at 27 WG revealed polyhydramnios and a thin and short corpus callosum. Amniocyte chromosome analysis performed at 27 WG revealed a normal 46, XX karyotype. Spontaneous vaginal delivery occurred at 33 weeks and 5 days. Apgar score was 6 at 1 min and 7 at 3 min. Birth weight was 2130 g (75th centile), length 47 cm (75th centile) and head circumference 30 cm (25th centile). The infant needed non-invasive respiratory support. She had coarse facial features, anteverted nares, macrostomia, low set and dysplastic ears, and a cleft palate (Fig. 2). The terminal phalanges of the digits and toes were hypoplastic (Fig. 3). The fifth digits were short with absent nails. Ophthalmologic examination was normal. Skeletal radiographs showed marked hypoplasia of the distal digital phalanges (Fig. 3). Chest X-ray was normal, without diaphragmatic hernia. Echocardiography showed a large conotruncal ventricular septal defect and a large patent ductus arteriosus. Cerebral MRI revealed agenesis of the olfactory bulbs, a thin corpus callosum, and hypoplasia of the vermis. Serial electroencephalograms showed frontal and temporal notches and significant asynchrony of the patterns. Neurological clinical examination revealed abnormal spontaneous movements, poor reactivity, weakness, and absent sucking reflex. Alkaline phosphatase level was normal (250 U/L). Clinical care was complicated by progressive cardiac and respiratory failure despite medical treatment. The patient died at 39 days of life. Autopsy was declined. Array CGH (60 kb resolution, Agilent Technologies) on DNA extracted from a blood sample was normal.
Family 4 (patients 5 and 6)
Patients 5 and 6 are unpublished patients. The family 4 parents are first cousins (f = 1/16) of North African origin. Their first child is healthy and was born after an uneventful pregnancy and delivery. During the second pregnancy, patient 5 (individual II-2, family 4 in Fig. 1) was diagnosed at 22 WG with a left diaphragmatic hernia associated with bilateral pyelectasis and polyhydramnios. The parents did not want amniocentesis to be performed and continued the pregnancy. A female was delivered at 36 WG and 5 days and died at 2 days of life. Birth weight was 2530 g (40th centile), length 49 cm (85th centile), and head circumference 33 cm (60th centile). Necropsy was denied. She presented with hypoplasia of distal phalanges and nails of hands and feet, hirsutism and facial dysmorphic features, small, low set and dysplastic ears, a small nose with a flat nasal bridge, and macrostomia (Figs. 2 and 3). Array CGH (500 kb resolution, Agilent) was normal. Fryns syndrome was suspected.
During the following pregnancy, omphalocele and recurrence of left diaphragmatic hernia and polyhydramnios, with dilated and hyperechogenic kidneys, were observed in patient 6 (II-3, family 4 in Fig. 1). Amniocyte chromosome analysis revealed a normal 46, XY karyotype. Array CGH on DNA extracted from amniotic fluid was normal (500 kb resolution, Agilent). The parents continued the pregnancy and fetal death was discovered at 29 WG. Birth weight was 1175 g (45th centile), length 33.5 cm (3rd centile) and head circumference 22 cm (below 3rd centile). Necropsy showed a male fetus presenting symmetric growth retardation, hypoplastic nails, and terminal phalanges of hands and feet, micropenis, and a wide and short neck associated with facial dysmorphic features: anteverted nares, macrostomia, a posterior cleft palate, and dysplastic ears. Visceral examination confirmed the left diaphragmatic hernia and revealed a ventricular septal defect and two bilobed and hypoplastic lungs.
Methods
Families 1, 2, and 3
Exome sequencing was performed according to the standard procedures using the Agilent Clinical Research Exome kit, from 5 µg of genomic DNA from patient 1. The resulting exome-capture libraries underwent 2 × 76-bp paired-end sequencing on an Illumina HiSeq 2000. Raw data were processed as previously described [9]. Briefly, reads were aligned to the human reference genome (GRCh37/hg19) with the Burrows-Wheeler Aligner (BWA) and potential duplicate paired-end reads removed using Picard Tools. The Genome Analysis Toolkit (GATK) 3.5 was used for base quality score recalibration, indel realignment, and variant calling. All variants identified in the patient were annotated with SeattleSeq SNP annotation (http://snp.gs.washington.edu/SeattleSeqAnnotation138/). Copy number variants were detected using the XHMM software [10]. Sanger sequencing was performed to confirm the candidate variants, as follows. PCR fragments were purified with the multiscreen Vacuum Manifold system (Millipore). Sequencing was performed with the ABI BigDye Terminator Cycle Sequencing kit (v.3.1) (Applied Biosystems) in an ABI 3130 sequencer 7 (Applied Biosystems) according to the manufacturer’s instructions. Sequence data were analyzed with Variant Surveyor (Softgenomics). Sequences of primers used for amplification of a fragment spanning the PIGN exon 5–7 deletion are: GTTACATTTTAAAAGCGCCCTGG and TTTCATGATTCTGTGCAGTGGTT for 5′ and 3′, respectively (primers CTTCCCCTTATGGCCTGAATC and GGTAGATGCTAGGAATGGGCTC were also used with similar results). Sequences of primers for amplification of PIGN exons 6 and 7 (in family 2) are: exon 6 (5′-CTGTAATTAAACATCCCTATTTCAGA-3′ and 5′-TGGGTTGGATGGAAGCATA-3′), exon 7 (5′-CGAAAAACAGGTTTCCTAAATCC-3′ and 5′-CCCCATCATAATGAACAATGC-3′).
Family 4
Exome sequencing was performed on patient 5, following protocols similar to those previously published [11]. Briefly, libraries were prepared from 3 µg of sheared genomic DNA. Exome capture was performed with the SureSelect All Exons 51 Mb V5 kit (Agilent) followed by sequencing on a HiSeq2500 (Illumina), generating 2 × 75 bp paired-end reads. Sequences were aligned to the reference human genome (GRCh37/hg19) using BWA and variant calling was performed with the GATK Unified Genotyper. Variants were filtered against public SNP databases (dbSNP, 1000 genomes, EVS, ExAC, and gnomAD) and more than 9000 in-house exomes performed at the Institut Imagine.
Results
Families 1, 2, and 3
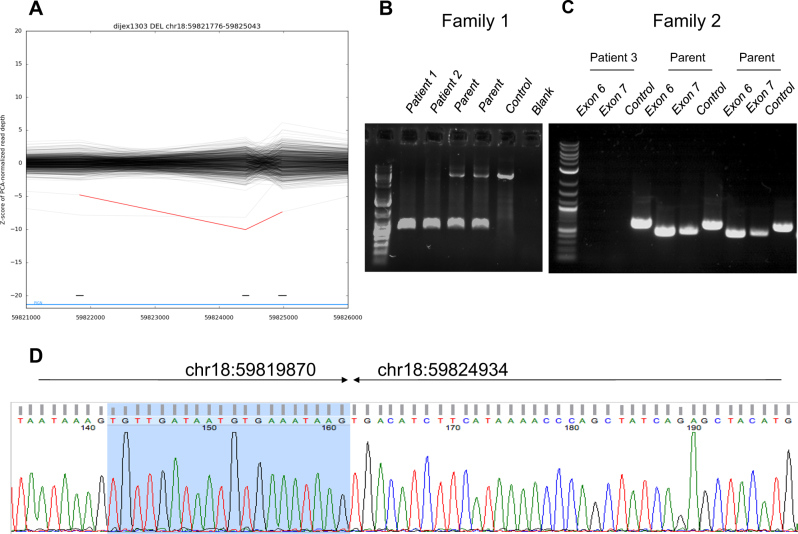
Overall, 2.7 gigabases of whole-exome sequencing data from patient 1 were analyzed, resulting in a median coverage of 79-fold, with 95.6% of bases sequenced by at least 10 reads. Because of the recurrence of similar symptoms in a brother and a sister, the interpretation focused on a suspected autosomal recessive mode of inheritance. No clinically relevant single nucleotide variant was evidenced. A deletion of exons 5–7 of PIGN (NM_176787.4) was predicted by the XHMM software, with a −7.40 normalized z-score for the read-depth (Fig. 4a). The deletion interval had a predicted size of 3.27 kb. The z-score suggested the presence of a deletion in the homozygous state. Direct PCR amplification of a fragment surrounding the deletion and Sanger sequencing confirmed this suspicion, with deletion co-ordinates of chr18:59819870–59824934 (Fig. 4d); this 5.07 kb interval spans from within exon 5 to within intron 7. Although the effect of this deletion on splicing of the PIGN transcript is difficult to predict, even if in-frame, it would result in the loss of at least 90 amino acids of the protein, and therefore is likely to result in a null allele. The deletion was confirmed by PCR as heterozygous in both parents (I-1 and I-2 in family 1), and as homozygous in both affected siblings (patients 1 and 2, II-1 and II-2 in family 1) (Fig. 4b).
Fig. 4.
Characterization of the recurrent intragenic deletion of PIGN identified in patients from La Réunion. a Prediction of a deletion-spanning exons 5–7 of PIGN using the XHMM software in whole-exome sequencing data of patient 1 (red line). Note the presence of a similar deletion in another patient, also from La Réunion island, present in the heterozygous state (gray line). b Direct PCR amplification of a deletion-spanning product from genomic DNA in family 1, demonstrating the presence of a homozygous deletion of PIGN, with a size of ~5.0 kb. c Direct PCR amplification of exons 6 and 7 for patient 3 and her parents from family 2, consistent with a homozygous deletion in patient 3. A PCR product from TBC1D24 was taken as a positive control for this experiment. d Direct Sanger sequencing of the deletion breakpoints, defining the precise genomic co-ordinates of the deletion in patient 1
A founder effect for the PIGN intragenic deletion was suspected because of the accumulation of several lines of evidence: (i) the over-representation of patients with FS in La Réunion Island, (ii) the presence in family 1 of a homozygous deletion in children from apparently unrelated parents, and (iii) the incidental detection, by exome sequencing, of the same deletion in the heterozygous state in an unrelated patient from La Réunion Island, with a classical autosomal recessive polycystic kidneys disorder. Given the geographic origins of families 2 and 3 (La Réunion and Mayotte Islands, respectively), we searched for the same PIGN intragenic deletion in these families. PCR amplification suggested the absence of exons 6 and 7 in patient 3 (family 2, Fig. 4c), consistent with the presence of a homozygous deletion. PCR and Sanger sequencing of a deletion-spanning product confirmed the recurrent PIGN deletion in family 3. The familial segregation of the deletion was demonstrated as homozygous in the affected individual and heterozygous in the parents (data not shown).
Family 4
Given the consanguinity of the parents in family 4, we performed singleton whole-exome sequencing for patient 5, with the goal of identifying homozygous variants. The mean depth of coverage obtained was 73-fold, with 97% of the exome covered at least 15-fold. After retaining only homozygous variants predicted to alter protein sequence and with a frequency below 0.001 in in-house and public SNP databases, we identified variants in seven genes: QSOX1, TMEM44, FOS, CHMP4B, C20orf194, CST7, and PIGN. All were missense except for a stop variant in CST7 (NM_003650.3:c.[95C>G], p.(Ser32*)) and a frameshift variant in PIGN (NM_176787.4:c.[421dup], p.(Ile141Asnfs*10)). CST7 codes for cystatin F, which is thought to have a role in immune cell regulation. As noted above, variants in PIGN have been associated with polymalformative disorders and the PIGN frameshift was therefore considered the most likely causal variant for the phenotype of patient 5. Sanger sequencing confirmed that the PIGN variant was homozygous in both affected sibs of family 4 and heterozygous in each parent.
Results were submitted to the ClinVar database (intragenic deletion: ID SCV000609493; frameshift variant: ID SCV000609492).
Discussion
In this report, using exome sequencing we have detected a 5.07 kb homozygous intragenic deletion in PIGN in two siblings who had been previously reported in a series of six patients clinically diagnosed as FS or Fryns-like syndrome [8]. The homozygous deletion was also identified in two new unrelated patients having a severe MCA condition with extreme brachytelephalangia. All four patients were native to Indian Ocean islands (La Réunion and Mayotte). Finally, in a family of North African descent, we identified a homozygous frameshift variant in the PIGN gene in two siblings with FS. All six patients reported here with disruption of PIGN died during the fetal or neonatal periods.
PIGN encodes glycosylphosphatidylinositol (GPI) ethanolamine phosphate (EtNP) transferase 1, which is involved in the GPI-anchor biosynthesis pathway and adds EtNP to the first mannose of GPI [12]. GPI-anchors are glycolipids sharing a common core consisting of phosphatidylinosytol, glucosamine, mannose, and EtNP; they anchor proteins to the outer leaflet of the cell membrane. The synthesis of GPI is followed by transfer (mediated by GPI-transamidase) to proteins bearing a GPI-attachment signal sequence. Then, the GPI-anchored proteins (GPI-APs) are modified and finally expressed on the cell surface. GPI-APs are involved in signal transduction, cell adhesion and antigen presentation. Biosynthesis, protein-attachment, and remodeling of the GPI involve the activity of at least 26 genes. Variants in nearly half of these genes have been linked to a group of developmental diseases termed inherited GPI deficiencies (IGDs). IGDs share an overlapping phenotype often including profound intellectual disability, seizures, dysmorphic features, and congenital anomalies [13].
Biallelic variants in PIGN were first identified in MCAHS1 (OMIM 614080) in a large consanguineous Israeli-Arab family [14]. Further reports have delineated the phenotype of this disorder [15–20]. The condition is characterized by global hypotonia, mostly severe developmental delay, early seizures, and a combination of congenital anomalies. Perinatal features include polyhydramnios, excessive neonatal growth, and non-specific facial dysmorphism with mainly coarse face, arched palate, depressed nasal bridge, and dysplastic ears. Some patients have visceral congenital anomalies involving the genitourinary, gastrointestinal, and cardiac systems. Brachytelephalangy and hypoplastic fingernails are reported in some families [14, 16, 17]. The clinical course is marked by nystagmus and visual impairment, feeding difficulties, and severe and/or intractable epilepsy. Early deaths due to recurrent aspiration and pneumonia have been reported [14, 16]. Structural abnormalities are not observed on brain MRI during the first months of life, but in survivors repeat brain MRI after 2 years of age revealed cerebellar atrophy [16, 18–20]. Almost all PIGN variants causing MCAHS1 are biallelic missense or compound missense/loss of function.
Until now PIGN variants have been identified in four patients presenting syndromic CDH. Brady et al. [6] reported a homozygous donor splice site variant in a fetus with bilateral diaphragmatic hernia and multiple congenital anomalies. The pregnancy was terminated at 16 WG. He presented with facial dysmorphic features with broad nose, anteverted nostrils, low set and dysplastic ears, hygroma colli, and a cleft palate. Associated malformations were present: ventricular septum defect, overriding aorta, hypoplastic pulmonary trunk, aberrant retro-esophageal right subclavia, gut malrotation, and cryptorchidism. He had oligodactyly of the left foot, with absence of rays 3 to 5, hypoplasia of the remaining toes, and absent toenails. The authors emphasized that the combination of the described features overlapped with FS. Parents were consanguineous of North African descent. Recently, recessive variants in PIGN were identified in three foetuses with normal chromosomes by karyotype and features of FS [7]. Two foetuses, the pregnancy terminated in each case, presented with unilateral CDH, pulmonary hypoplasia, echogenic kidneys, omphalocele, and cleft lip. Extremities appeared normal prenatally, but were not examined postnatally. Compound heterozygous variants in PIGN were identified in the siblings: a stop variant and a donor splice site variant. A third fetus from non-consanguineous parents presented with bilateral diaphragmatic hernia, facial dysmorphism (macrostomia, anteverted nostrils and flat nasal bridge), cleft palate, omphalocele, abnormal pulmonary segmentation, and agenesis of corpus callosum. The fingers were short, with marked brachytelephalangia of the fifth fingers. A homozygous stop variant was detected in PIGN. Notably, all four of the above cases fulfill strict clinical criteria for FS and have biallelic truncating variants in PIGN. These FS genotypes, along with the biallelic loss of function PIGN variants in the patients presented here, appear to correlate with the overall more severe phenotype of FS compared to MCAHS1, which is associated with biallelic missense or compound missense/loss of function variants, and in which some residual PIGN protein function may remain [7, 16] (Table 1).
Table 1.
Frequency of clinical phenotypes in patients with PIGN-associated Fryns syndrome versus MCAHS1
| PIGN-associated Fryns syndromea (with presumed PIGN loss of function variants). | PIGN-associated MCAHS1b (with presumed residual PIGN function) | |||
|---|---|---|---|---|
| Polyhydramnios | 60% | (6/10) | 23% | (3/13) |
| Macrosomiac | 33% | (2/6) | 47% | (8/17) |
| Facial dysmorphism | 80% | (8/10) | 76% | (13/17) |
| Diaphragmatic hernia | 70% | (7/10) | 0% | (0/17) |
| Brachytelephalangiad | 80% | (8/10) | 23.5% | (4/17) |
| Orofacial cleft | 70% | (7/10) | 0% | (0/17) |
| Cloudy cornea/cataract | 33% | (2/6) | 5.8% | (1/17) |
| Cardiovascular malformations | 60% | (6/10) | 35% | (6/17) |
| Genitourinary anomalies | 50% | (5/10) | 17.6% | (3/17) |
| Congenital cerebral malformations | 30% | (3/10) | 0% | (0/12) |
| Progressive cerebellar atrophy | — | 41.6% | (5/12) | |
| Abnormal EEG/seizures | 100% | (2/2) | 94% | (16/17) |
| Neurological featurese | 50% | (1/2) | 100% | (17/17) |
| Perinatal deathf | 100% | (10/10) | 0% | (0/17) |
| Lethal courseg | — | 52.9% | (9/17) | |
Features were not available for all patients
a10 Patients from Brady et al., McInerney-Leo et al., and our series
b17 Patients from Maydan et al., Ohba et al., Couser et al., Fleming et al., Khayat et al., Nakagawa et al., Jezela-Stanek et al
cBirth weight on or above 95th centile
dClinical (phalanges, nails) and/or radiological features
eHypotonia, developmental delay when survival beyond first week of life
fTermination of pregnancy or death during first weeks of life
gDeath beyond one month of life
The two siblings from family 1 in this study presented with coarse face, abnormal ears, cleft lip and palate, eye anomalies (cataract, cloudy cornea), and congenital malformations with lethal neonatal outcome. Massive polyhydramnios was noted in the second and third trimester of pregnancy. Fetal growth was normal or excessive. Brachytelephalangy and nail hypoplasia were present, with marked involvement of the fifth digits. Patient 1 had abnormal electroencephalogram. Although CDH was absent in the first sibling (patient 1), the combination of the above features with CDH in a second sibling (patient 2) was consistent with the diagnosis of FS, according to the current diagnostic guidelines [2, 3]. The siblings were native to La Réunion Island. They were previously published in a series of six patients from Indian Ocean islands having a MCA syndrome with hypoplasia/absence of the distal phalanges. Their shared condition was thought to represent a distinctive entity, overlapping FS, DOORS, or Schinzel–Giedion syndromes [8].
Our patients, especially patient 3 (family 2), also fulfilled some diagnostic criteria for DOORS syndrome (characterized by deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures): coarse facies, anteverted nares, hypoplastic or absent distal phalanges of the digits and toes, and hypoplastic or absent fingernails or toenails. Polyhydramnios is commonly reported in DOORS syndrome [21]. Variants in TBC1D24 are identified in 50% of affected patients [22]. One patient demonstrating this overlap is the patient 4 from the series of patients with PIGN variants reported by Fleming et al. [16]; he had coarse facies, overfolded helices, depressed nasal bridge, brachytelephalangy, and absent or hypoplastic nails of digits and toes with marked involvement of the fifth digits. Radiographs of the right hand showed absence of the fifth distal phalanx and hypoplasia of the second to fourth distal phalanges. Clinical course was marked by neonatal hypotonia, feeding problems, early seizures, nystagmus, and visual impairment. Ophthalmologic evaluation at 2 weeks of life revealed bilateral cloudy corneas. Although deafness was absent, the authors emphasized that the combination of the features overlaps with DOORS syndrome. However, ophthalmological anomalies (cloudy corneas, cataracts) are rarely reported in DOORS syndrome [21], and are frequent features (17%) in FS [2]. Therefore, FS (without CDH) could be considered as a differential diagnosis for this patient. Interestingly, Fleming et al. [16] consider their patient 4 to have one of the most severe phenotypes reported in the PIGN-associated MCAHS1 literature. The patient has a maternally inherited frameshift variant in PIGN and a paternally inherited intragenic deletion of PIGN. The paternal deletion is quite similar to the La Réunion deletion, encompassing exon 5 partially and exons 6 and 7. The father is African-American. It would be interesting to know the exact deletion boundaries and precise origins of the father. The patient has probable biallelic loss of function variants, reinforcing the hypothesis of a genotype–phenotype correlation [6, 7, 16].
CDH is a major feature in FS, present in more than 80% of reported cases [2]. In our series, CDH is present in 50% of the patients. Molecular testing of PIGN in further patients may lead to diagnosis of PIGN-associated FS in other patients without CDH. CDH and associated pulmonary hypoplasia are responsible for early death in most patients. However, prolonged survival in FS has been reported, mainly among patients without CDH. Developmental delay with intellectual deficiency and seizures is almost constant in survivors [23, 24]. Very few survivors with FS have had repeat cerebral MRI after 2 years of age. Among them, we note the patient reported by Riela et al. [25] with FS features without CDH, with neonatal hypotonia, areflexia, early myoclonic epilepsy and progressive cerebral atrophy on repeated cerebral MRI. This patient displays a significant overlap with MCAHS1.
Finally, IGDs can result in the failure of the GPI-anchor to regulate attachment of proteins such as alkaline phosphatase to the cell surface, resulting in hyperphosphatasia with mental retardation syndrome (Mabry syndrome) (OMIM 239300) [26]. It is worth noting that hyperphosphatasia has not yet been associated with MCAHS1 or FS resulting from PIGN variants.
FS is likely to be genetically heterogeneous [27]. Two other patients with FS from the study by McInerney-Leo et al. [7] showed no damaging variant in PIGN. The series reported here supports the conclusion that a subset of FS is caused by autosomal recessive loss of function variants in PIGN. Systematic evaluation for variants (including non-coding variants) in PIGN or in other genes of the GPI-anchor biosynthesis pathway should be considered in patients with syndromic CDH, FS or Fryns-like syndrome; and also in some patients having a MCA condition with hypoplasia/absence of the distal phalanges, onychodystrophy, and neurological impairment or epilepsy (e.g., patients with atypical presentations of DOORS syndrome and without molecular etiology).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Jean-Luc Alessandri and Christopher T. Gordon contributed equally to this work.
References
- 1.Fryns JP. A variable MCA syndrome with diaphragmatic defects, coarse face, and distal limb hypoplasia. J Med Genet. 1987;24:271–4. doi: 10.1136/jmg.24.5.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slavotinek AM. Fryns syndrome: a review of the phenotype and diagnosis guidelines. Am J Med Genet. 2004;124A:427–33. doi: 10.1002/ajmg.a.20381. [DOI] [PubMed] [Google Scholar]
- 3.Lin AE, Pober BR, Mullen MP, Slavotinek AM. Cardiovascular malformations in Fryns syndrome: is there a pathogenic role for neural crest cells? Am J Med Genet A. 2005;139:186–93. doi: 10.1002/ajmg.a.31023. [DOI] [PubMed] [Google Scholar]
- 4.Slavotinek A, Lee SS, Davis R, et al. Fryns syndrome phenotype caused by chromosome microdeletions at 15q26.2 and 8p23.1. J Med Genet. 2005;42:730–6. doi: 10.1136/jmg.2004.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bone KM, Chernos JE, Perrier R, et al. Mosaic trisomy 1q: a recurring chromosome anomaly that is a diagnosis challenge and is associated with a Fryns-like phenotype. Prenat Diag. 2017;37:602–10. doi: 10.1002/pd.5058. [DOI] [PubMed] [Google Scholar]
- 6.Brady PD, Moerman P, De Catte L, Deprest J, Devriendt K, Vermeesch JR. Exome sequencing identifies a recessive PIGN splice mutation as a cause of syndromic congenital diaphragmatic hernia. Eur J Med Genet. 2014;57:487–93. doi: 10.1016/j.ejmg.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 7.McInerney-Leo AM, Harris JE, Gattas M, et al. Fryns syndrome associated with recessive mutations in PIGN in two separate families. Hum Mutat. 2016;37:695–702. doi: 10.1002/humu.22994. [DOI] [PubMed] [Google Scholar]
- 8.Alessandri JL, Cuillier F, Malan V, et al. Fryns syndrome without diaphragmatic hernia, DOOR syndrome or Fryns-like syndrome? Report on patients from Indian Ocean islands. Am J Med Genet. 2014;164A:648–54. doi: 10.1002/ajmg.a.36323. [DOI] [PubMed] [Google Scholar]
- 9.Thevenon J, Duffourd Y, Masurel-Paulet A, et al. Diagnostic odyssey in severe neurodevelopmental disorders: toward clinical whole-exome sequencing as a first-line diagnostic test. Clin Genet. 2016;89:700–7. doi: 10.1111/cge.12732. [DOI] [PubMed] [Google Scholar]
- 10.Poultney CS, Goldberg AP, Drapeau E, et al. Identification of small exonic CNV from whole-exome sequence data and application to autism spectrum disorder. Am J Hum Mut. 2013;93:607–19. doi: 10.1016/j.ajhg.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon CT, Petit F, Kroisel PM, et al. Mutations in endothelin 1 cause recessive auriculocondylar syndrome and dominant isolated question-mark ears. Am J Hum Genet. 2013;93:1118–25. doi: 10.1016/j.ajhg.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita T. Biosynthesis and deficiencies of glycosylphosphatidylinositol. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90:130–43. doi: 10.2183/pjab.90.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone DL, Nguyen TTM, Murakami Y, et al. Compound heterozygous mutations in the gene PIGP are associated with early infantile epileptic encephalopathy. Hum Mol Genet. 2017;26:1706–15. doi: 10.1093/hmg/ddx077. [DOI] [PubMed] [Google Scholar]
- 14.Maydan G, Noyman I, Har-Zahav A, et al. Multiple congenital anomalies-hypotonia-seizures syndrome is caused by a mutation in PIGN. J Med Genet. 2011;48:383–9. doi: 10.1136/jmg.2010.087114. [DOI] [PubMed] [Google Scholar]
- 15.Couser NL, Masood MM, Strande NT, et al. The phenotype of multiple congenital anomalies-hypotonia-seizures syndrome 1: report and review. Am J Med Genet. 2015;167A:2176–81. doi: 10.1002/ajmg.a.37129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming L, Lemmon M, Beck N, et al. Genotype–phenotype correlation of congenital anomalies in multiple congenital anomalies hypotonia seizures syndrome (MCAHS1)/PIGN-related epilepsy. Am J Med Genet. 2016;170A:77–86. doi: 10.1002/ajmg.a.37369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khayat M, Tilghman JM, Chervinsky I, Zalman L, Chakravarti A, Shalev SA. A PIGN mutation responsible for multiple congenital anomalies-hypotonia-seizures syndrome 1 (MCAHS1) in an Israeli-Arab family. Am J Med Genet. 2016;170A:176–82. doi: 10.1002/ajmg.a.37375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, Taniguchi-Ikeda M, Murakami Y, et al. A novel PIGN mutation and prenatal diagnosis of inherited glycosylphosphatidylinositol deficiency. Am J Med Genet. 2016;170A:183–8. doi: 10.1002/ajmg.a.37397. [DOI] [PubMed] [Google Scholar]
- 19.Ohba C, Okamoto N, Murakami Y, et al. PIGN mutations cause congenital anomalies, developmental delay, hypotonia, epilepsy, and progressive cerebellar atrophy. Neurogenetics. 2014;15:85–92. doi: 10.1007/s10048-013-0384-7. [DOI] [PubMed] [Google Scholar]
- 20.Jezela-Stanek A, Ciara E, Piekutowska-Abramczuk D, et al. Congenital disorder of glycosylphosphatidylinositol (GPI)-anchor biosynthesis—the phenotype of two patients with novel mutations in the PIGN and PGAP2 genes. Eur J Paediat Neurol. 2016;20:462–73. doi: 10.1016/j.ejpn.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 21.James AW, Miranda SG, Culver K, Hall BD, Golabi M. DOOR syndrome: clinical report, literature review and discussion of natural history. Am J Med Genet. 2007;143A:2821–31. doi: 10.1002/ajmg.a.32054. [DOI] [PubMed] [Google Scholar]
- 22.Campeau PM, Kasperaviciute D, Lu JT, et al. The genetic basis of DOORS syndrome: an exome-sequencing study. Lancet Neurol. 2014;13:44–58. doi: 10.1016/S1474-4422(13)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hove JL, Spiridigliozzi GA, Heinz R, McConkie-Rosell A, Iafolla AK, Kahler SG. Fryns syndrome survivors and neurologic outcome. Am J Med Genet. 2002;59:334–40. doi: 10.1002/ajmg.1320590311. [DOI] [PubMed] [Google Scholar]
- 24.Dentici ML, Brancati F, Mingarelli R, Dallapiccola B. A 6-year-old child with Fryns syndrome: further delineation of the natural history of the conditions in survivors. Eur J Med Genet. 2009;52:421–5. doi: 10.1016/j.ejmg.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Riela AR, Thomas LT, Gonzales AR, Ifft RD. Fryns syndrome: neurologic findings in a survivor. J Child Neurol. 1995;10:110–3. doi: 10.1177/088307389501000208. [DOI] [PubMed] [Google Scholar]
- 26.Cole DE, Thompson MD. Neurogenetic aspects of hyperphosphatasia in Mabry syndrome. Subcell Biochem. 2015;76:343–61. doi: 10.1007/978-94-017-7197-9_16. [DOI] [PubMed] [Google Scholar]
- 27.Thompson MD, Cole DE. Recessive PIGN mutations in Fryns syndrome: evidence for genetic heterogeneity. Hum Mut. 2016;37:621. doi: 10.1002/humu.23016. [DOI] [PubMed] [Google Scholar]