Abstract
Background
Norepinephrine (NE), a neurotransmitter released from the sympathetic nerves, has been shown to be involved in rheumatoid arthritis (RA). However, its role in the sympathetic nervous system in RA is divergent. Herein, we demonstrate that the sympathetic neurotransmitter NE exerts an anti-inflammatory effect in collagen-induced arthritis (CIA), a mouse model of RA, by inhibiting Th17 cell differentiation and function via β2-adrenergic receptor (β2-AR) signaling.
Material/Methods
CIA was prepared by intradermal injection of collagen type II in the tail base of DBA1/J mice. On the 41st day post-immunization, the mice were used as CIA models. CD4+ T cells from the spleen were purified using magnetic cell sorting and activated with anti-CD3 anti-CD28 antibodies. Th17 cells were polarized from the CD4+ T cells using various antibodies and cytokines.
Results
Co-expression of CD4 and β2-AR was observed in spleens of both intact and CIA mice. The β2-AR expression in the ankle and spleen was downregulated in CIA mice. CIA induced increases in production of interleukin (IL)-17 and IL-22, CD25−IL-17+ cell percentage, and ROR-γt expression in CD4+ T cells. Importantly, NE reduced the CIA-induced CD4+ T cell shift towards Th17 phenotype, and the β2-AR antagonist ICI118551 blocked the NE effect. Moreover, the β2-AR agonist terbutaline (Terb) inhibited CIA-induced CD4+ T cell proliferation and shift towards Th17 phenotype, and the protein kinase A (PKA) inhibitor H-89 abolished the agonist effect. Terb also reduced CIA-induced Th17 enhancement, and H-89 impaired the Terb effect.
Conclusions
NE inhibits Th17 cell differentiation and function in CIA condition by activation of β2-AR/PKA signaling.
MeSH Keywords: Arthritis, Experimental; CD4-Positive T-Lymphocytes; Norepinephrine; Receptors, Adrenergic; Th17 Cells
Background
Rheumatoid arthritis (RA), a representative human autoimmune disease, is characterized by joint inflammation with subsequent destruction of cartilage, pannus formation, and infiltrates of immune cells [1–3]. Although the exact pathogenesis of RA is unknown, some observations suggest that CD4+ T lymphocytes play a pivotal role in induction or perpetuation of this chronic inflammatory disease [4]. CD4+ T cells, on activation and expansion, develop into different T cell subsets, including helper T (Th)1, Th2, Th17, and regulatory T (Treg) cells, with different cytokine profiles and distinct effector functions. It has become clear that balances between Th1 and Th2 cells, or their cytokines, are important in induction or prevention of RA [5–7]. Th17 cells are the most recently discovered members of the effector Th cell family and are characterized by high proinflammation via producing specific cytokines such as interleukin (IL)-17 and IL-22 [8,9]. In RA, earlier studies showed that Th1 cells are enriched in the joints of these patients [10–12]. Subsequent studies revealed similar findings for Th17 cells in the joints of RA subjects [13]. The Th17 cells are associated with immune pathology in arthritis [14]. Particularly, Th17 cells have been reported to be critical to the pathogenesis of collagen type II (CII)-induced arthritis (CIA), a murine model of autoimmune arthritis [15]. CIA shares many pathological and histological similarities with human RA [16]; therefore, it was used in this study as an animal model of RA.
Norepinephrine (NE), a neurotransmitter released from sympathetic nerve fibers in the peripheral nervous system, has been shown to be involved in RA and CIA. Synovial tissue is perfectly innervated with sympathetic nerve fibers, and the sympathetic neurotransmitter NE has anti-inflammatory effect when its concentration is high [17–19]. Unfortunately, sympathetic nerve fibers are lost in inflamed tissue of patients with RA [20] and in synovial tissue and lymph nodes of animals with CIA [21,22]. On the other hand, the effect of the sympathetic nervous system on the inflamed synovial tissue in RA is different, depending on the phase of inflammation. In the early acute phase of experimental arthritis, the sympathetic nervous system has a pro-inflammatory role [21,23,24], whereas in the late chronic phase anti-inflammatory effects have been described [21]. Therefore, clarifying the effect of the sympathetic neurotransmitter NE on Th17 cells, the crucial pro-inflammatory cells in RA pathogenetic process, is important for better understanding of the role of the sympathetic nervous system in RA.
NE acts on target cells by binding to the receptors, α-adrenergic receptor (α-AR) or β-AR. Immune cells express both α-AR and β-AR, and T and B lymphocytes express β2-AR almost exclusively [25]. Engagement of β2-AR activates a cascade of signaling intermediates, including cAMP and protein kinase A (PKA), which leads to the phosphorylation of cellular proteins [25]. We previously showed that NE promotes a shift of Th1/Th2 balance towards Th2 response by activating β2-AR [26]. However, it is not known whether the β2-AR-cAMP-PKA signaling pathway is involved in the action of NE on Th17 cells in CIA condition. Thus, in the present study, we firstly determined the expression of β2-AR by CD4+ T cells and the change of the expression in CIA condition; secondly, we assessed the effects of NE on Th17 cell differentiation and function by measurement of the percentage of Th17 cells, the expression of retinoic acid-related orphan receptor-γt (ROR-γt), a specific transcriptional factor of Th17 cells, the production of IL-17 and IL-22, and the proliferative response of CD4+ T cells in CIA condition; thirdly we showed that the β2-AR-cAMP-PKA signaling pathway mediated the NE effects on Th17 cells in CIA condition. This investigation thereby provides potentially useful information for treatment of RA with β2-AR signaling activators or stimulators targeting Th17 cells.
Material and Methods
Induction of CIA mice
The induction of CIA mice was as described previously [7]. Briefly, male DBA1/J mice (8–10 weeks old, from Slack Experimental Animal Center, Shanghai, China) were intradermally injected in the tail base with 100 μl emulsion containing 100 μg CII (Sigma-Aldrich, USA) on day 0. On day 21, the mice were intraperitoneally boosted with 100 μg CII emulsified with an equal volume of incomplete Freund’s adjuvant (Sigma-Aldrich, USA). An intraperitoneal injection of 20 μg lipopolysaccharide dissolved in 20 μl PBS was executed to the mice on day 28. Mice were sacrificed on the 41st day after the first immunization with CII as CIA models for subsequent in vivo and in vitro experiments.
Immunofluorescence staining
The spleens were fixed in 4% paraformaldehyde for 24 h. The spleen sections (25 μm thick) were mounted on glass slides and processed for immunofluorescence staining. To block nonspecific binding sites, the spleen sections were exposed to phosphate-buffered saline (PBS) containing 3% goat serum and 1% Triton X-100 for 30 min at room temperature. The sections were stained doubly with rat anti-CD4 antibody (1: 400; Serotec, UK) and rabbit anti-β2-AR antibody (1: 200; Abcam, UK), which were incubated with Alexa Fluor-conjugated secondary antibodies (1: 200; Molecular Probes, USA). A confocal microscope (Leica, Germany) was used to view and acquire the images.
CD4+ T cell purification and activation, and Th17 cell polarization
Naive CD4+ T cells were obtained using magnetic cell sorting from the spleens of DBA1/J mice. Sorted cells were suspended in RPMI 1640 medium containing 10% heat-inactivated calf serum at the final concentration of 5×106 cells/ml and stimulated with anti-CD3 antibody (2 μg/ml; BD Pharmingen, USA) and anti-CD28 antibody (2 μg/ml; BD Pharmingen, USA) for 24 h. Subsequently, the activated CD4+ T cells were exposed to various treatments.
For Th17 cell polarization, as described previously [27], the purified CD4+ T cells were activated with anti-CD3 and anti-CD28 antibodies and stimulated with anti-IL-4-neutralizing and anti-interferon (IFN)-γ-neutralizing antibodies (both 10 μg/ml; BD Pharmingen, USA) plus a Th17 ‘cocktail’ containing transforming growth factor (TGF)-β1 (3 ng/ml; R&D Systems, USA), IL-6 (30 ng/ml; R&D Systems, USA), tumor necrosis factor (TNF)-α (10 ng/ml; Peprotech, USA), IL-1β (10 ng/ml; Peprotech, USA), and IL-23 (20 ng/ml; Peprotech, USA) for 48 h. Subsequently, the polarized Th17 cells were exposed to various treatments.
Drug treatments
The activated CD4+ T cells were exposed to NE (10−5 M; Sigma-Aldrich, USA) for 24 h. To show that β2-AR mediates the NE effect, a highly selective β2-AR antagonist ICI118551 (ICI, 10−5 M; Sigma-Aldrich, USA) was applied to the activated CD4+ T cells for 30 min, and then NE acted on the cells for 24 h. The activated CD4+ T cells were also treated with the specific β2-AR agonist terbutaline (Terb, 10−6 or 10−5 M; Sigma-Aldrich, USA) for 24 or 72 h according to different experiments, or treated combined with the PKA inhibitor H-89 (10−5 or 10−4 M; Sigma-Aldrich, USA) 30 min earlier and the β2-AR agonist Terb for 72 h. Subsequent analyses as described below were performed.
In addition, the polarized Th17 cells were exposed to the β2-AR agonist Terb for 24 h, or exposed combinedly to H-89 at 30 min earlier and Terb for 24 h, followed by the subsequent analyses.
Western blot analysis
Total proteins were extracted from the spleens and ankle joints of mice or from in vitro cultured CD4+ T cells and Th17 cells. Briefly, tissues or cells were homogenized in lysis buffer, which contained 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, and 10 μl/ml protease inhibitor. By centrifuging at 4°C at 12,000 rpm for 15 min, the supernatants were obtained. The proteins were separated and transferred to membranes according to our previous description [7]. After blocking nonspecific binding, the membranes were incubated with rabbit antibodies against β2-AR (1: 200; Abcam, UK), ROR-γt (1: 500; Abcam, UK), IL-17 (1: 200; Santa Cruz Biotechnology, USA), IL-22 (1: 200; Santa Cruz Biotechnology, USA), PKA (1: 200; Santa Cruz Biotechnology, USA), or mouse anti-β-actin antibody (1: 5000; Sigma, USA) at 4°C overnight. Following incubation with the corresponding secondary antibodies (1: 5,000; Rockland Immunochemicals, USA), the membranes were visualized using Odyssey laser scanning system (LI-COR Inc, USA), and the protein band intensities were quantified by an image analysis system (Odyssey 3.0 software).
Quantitative real-time PCR measurement
Total RNA was extracted from CD4+ T cells or Th17 cells using Trizol reagent (Invitrogen, USA), and cDNA was subsequently generated by reverse transcription kit (Roche, Germany), following the manufacturers’ instructions. The PCR reactions (94°C, 20 s; 60°C, 20 s; 72°C, 20 s) were performed on a Rotor-Gene 3000 Real-Time Cycler (Corbett Research, Australia) using Universal SYBR Green Master Mix (Roche, Germany). Species-specific mouse primers were used as follows:
5′-GCTCCAGAAGGCCCTCAGA-3′ and
5′-AGCTTTCCCTCCGCATTGA-3′ for IL-17;
5′-GGCCAGCCTTGCAGATAACA-3′ and
5′-GCTGATGTGACAGGAGCTGA-3′ for IL-22; 5′-ACCCACACTGTGCCCATCTA-3′ and
5′-GCCACAGGATTCCATACCCA-3′ for β-actin.
Relative gene expression was calculated as fold-change over control using the 2−ΔΔCt method after normalization to β-actin.
Flow cytometric assay for CD25−IL-17+ cell percentage
This method referred to our previous work [27]. Activated CD4+ T cells were incubated with 50 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich, USA), 1 μM ionomycin (Sigma-Aldrich, USA), and 2 μM monensin (BD PharMingen, USA) for 5 h at 37°C. After surface staining with FITC-conjugated anti-CD25 antibody (BD PharMingen, USA), the cells were resuspended in Fixation/Permeabilization solution (Cytofix/Cytoperm kit; BD Pharmingen, USA), and then stained intracellularly with phycoerythrin-conjugated anti-IL-17 antibody (eBioscience, USA). All samples were analyzed using a FACSCalibur flow cytometer with CellQuest software (BD Biosciences, USA).
Flow cytometric assay for CD4+ T cell proliferation
Carboxy fluorescein diacetate succinimidyl ester (CFSE; Invitrogen, USA) labeling was used to measure CD4+ T lymphocyte proliferation. Briefly, purified CD4+ T cells were incubated with CFSE (2 μM) for 15 min at 37°C in the dark. The labeling reaction was terminated by adding an equal volume of calf serum for 1 min. The CD4+ T cells were stimulated with anti-CD3 and anti-CD28 antibodies and treated with H-89 and Terb as described above, which was incubated for 72 h. Percentage of proliferative CD4+ T cells was analyzed on a flow cytometer.
Enzyme-linked immunosorbent assay (ELISA)
The culture supernatants of either CD4+ T cells or Th17 cells were harvested and analyzed for IL-17 and IL-22 concentrations with ELISA (eBioscience, USA) following the manufacturer’s instructions. The lysates of cultured Th17 cells were harvested and quantified for cAMP concentration with ELISA (Biovision, USA) according to the manufacturer’s instructions.
PKA activity assay
The lysates of cultured Th17 cells were harvested and assessed for PKA activity using the assay kit (Enzo Life Sciences, USA) in accordance with the manufacturer’s instructions.
Statistical analysis
The Statistical Package for the Social Science (SPSS, 16.0) was used in the statistical analyses. The data were expressed as the mean ± standard deviation. Comparisons between 2 groups were assessed using Student’s t tests. Multiple comparisons among the groups were evaluated by one-way analysis of variance, followed by a post hoc analysis. The results were considered statistically significant at p<0.05.
Results
β2-AR is expressed by CD4+ T cells and CIA downregulates this expression
On the 41st day following the first immunization with CII, the mice reached a peak in clinical score of 4 paws and manifested 4 inflamed paws, elevated anti-CII-IgG antibody level in the serum, destruction of articular cartilages in the ankle joints, and infiltrated inflammatory cells in the synovial tissue of the ankle joints (data not shown). These results confirmed that CII induced an RA-like arthritis. Thus, the mice that were on the 41st day following the first immunization with CII were used as CIA models in subsequent experiments.
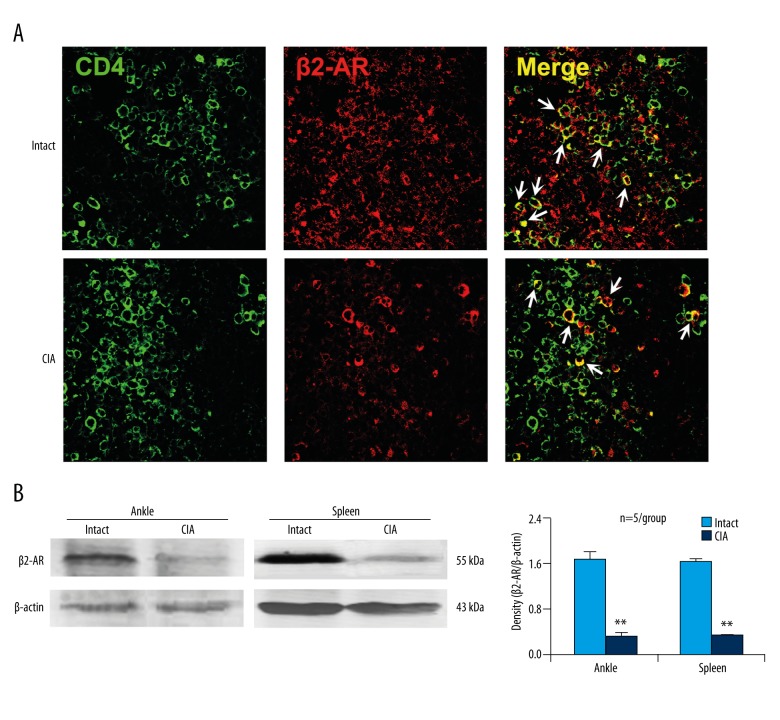
Co-expression of CD4 and β2-AR was observed in the spleens of both intact and CIA mice (Figure 1A). Further experiments found that the β2-AR expression was downregulated in both the ankle and spleen tissues of CIA mice compared with that of intact mice (Figure 1B). The data showed that CD4+ T cells expressed β2-AR, and the expression was downregulated in CIA condition.
Figure 1.
β2-AR is expressed by CD4+ T cells and CIA downregulates this expression. (A) Immunofluorescence staining of the spleen sections. The arrows point to the typical cells of co-expressing CD4 and β2-AR. (B) β2-AR expression levels in the ankle and spleen assessed by Western blot analysis. ** p<0.01, versus intact mice.
NE reduces CIA-induced CD4+ T cell shift towards Th17 phenotype and β2-AR antagonist blocks the NE effect
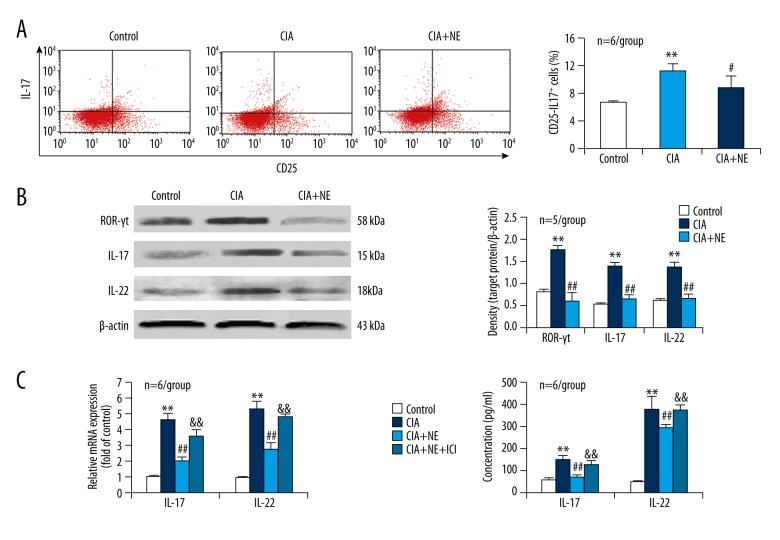
CD4+ T cells were separated from the spleens of both CIA and intact mice by magnetic beads and activated with anti-CD3 and anti-CD28 antibodies. Percentage of CD25−IL-17+ cells, which represent Th17 cells, in CD4+ T cells was more in CIA condition than in intact condition (control) (Figure 2A). Simultaneously, expression of the Th17-related transcriptional factor ROR-γt and cytokines IL-17 and IL-22 was upregulated in CD4+ T cells derived from CIA mice compared with controls (Figure 2B). Importantly, NE treatment of CD4+ T cells from CIA mice reduced the percentage of CD25−IL-17+ cells and the expression of ROR-γt, IL-17, and IL-22, with respect to NE-untreated CD4+ T cells from CIA mice (Figure 2A, 2B). The results indicated that NE inhibited CIA-induced CD4+ T cell shift towards Th17 phenotype.
Figure 2.
NE reduces CIA-induced CD4+ T cell shift towards Th17 phenotype and this effect is blocked by the β2-AR antagonist ICI. (A) Flow cytometric assay for CD25−IL-17+ cell percentage in CD4+ T cells from the spleen. (B) Protein expression levels of the Th17 cell specific transcriptional factor ROR-γt and the Th17 cell-related cytokines IL-17 and IL-22 in CD4+ T cells assessed by Western blot analysis. (C) mRNA expression of IL-17 and IL-22 in CD4+ T cells tested by real-time PCR. (D) Levels of IL-17 and IL-22 in the supernatants of CD4+ T cell cultures determined by ELISA. ** p<0.01, versus control; # p<0.05; ## p<0.01, versus CIA group; && p<0.01, versus CIA + NE group.
To show that the NE effect is mediated by β2-AR, we added the β2-AR antagonist ICI to NE-treated CD4+ T cells. The reduction of CIA-induced expression of IL-17 and IL-22 in CD4+ T cells by NE was blocked by the β2-AR antagonist ICI (Figure 2C). In addition, IL-17 and IL-22 levels in the supernatants of CD4+ T cell cultures were higher in CIA condition than in controls (Figure 2D). Importantly, NE reduced the CIA-induced secretion of IL-17 and IL-22 from CD4+ T cells (Figure 2D). More importantly, the β2-AR antagonist ICI abolished the NE effect of reducing IL-17 and IL-22 secretion from CD4+ T cells in CIA condition (Figure 2D). The data showed that β2-AR mediated the NE effect of inhibiting CIA-induced CD4+ T cell shift towards Th17 phenotype.
β2-AR agonist inhibits CIA-induced CD4+ T cell shift towards Th17 phenotype and this agonist effect is abolished by PKA inhibitor
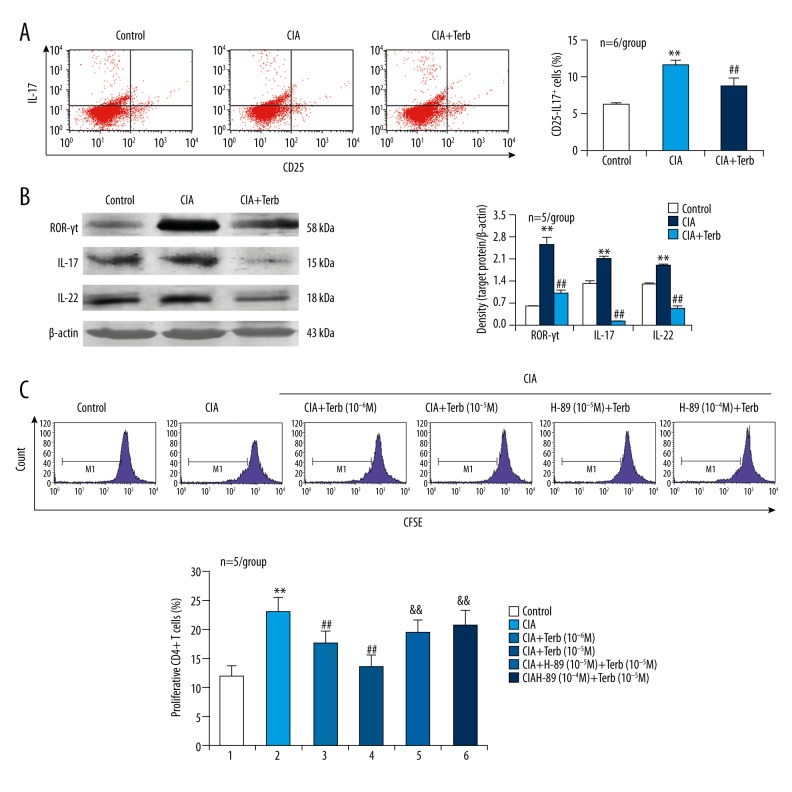
To further demonstrate the role of β2-AR expressed on CD4+ T cells in CIA-induced Th17 cell differentiation and function, we applied the β2-AR agonist Terb to the activated CD4+ T cells derived from CIA mice. The increased percentage of CD25−IL-17+ cells in CD4+ T cells in CIA condition was reduced by the β2-AR agonist Terb (Figure 3A). Simultaneously, the upregulated expression of ROR-γt, IL-17, and IL-22 in CD4+ T cells in CIA condition was inhibited by the β2-AR agonist Terb (Figure 3B). In addition, CIA induced CD4+ T cell proliferation and this effect was also inhibited by the β2-AR agonist Terb (Figure 3C). Notably, the PKA inhibitor H-89 blocked the effect of Terb inhibiting CIA-induced CD4+ T cell proliferation (Figure 3C). These data demonstrated that activation of β2-AR impaired CIA-induced CD4+ T cell shift towards Th17 phenotype via PKA signaling.
Figure 3.
The β2-AR agonist Terb inhibits CIA-induced CD4+ T cell shift towards Th17 phenotype and this effect is abolished by the PKA inhibitor H-89. (A) Flow cytometric assay for CD25−IL-17+ cell percentage in CD4+ T cells from the spleen. (B) Protein expression of the Th17 cell-specific transcriptional factor ROR-γt and the Th17 cell-related cytokines IL-17 and IL-22 in CD4+ T cells. (C) Flow cytometric assay for CD4+ T cell proliferative response. The cells in M1 area represent proliferative CD4+ T cells. ** p<0.01, versus control; ## p<0.01, versus CIA group; && p<0.01, versus CIA + Terb group.
β2-AR agonist reduces CIA-induced Th17 cell enhancement and the agonist effect is impaired by PKA inhibitor
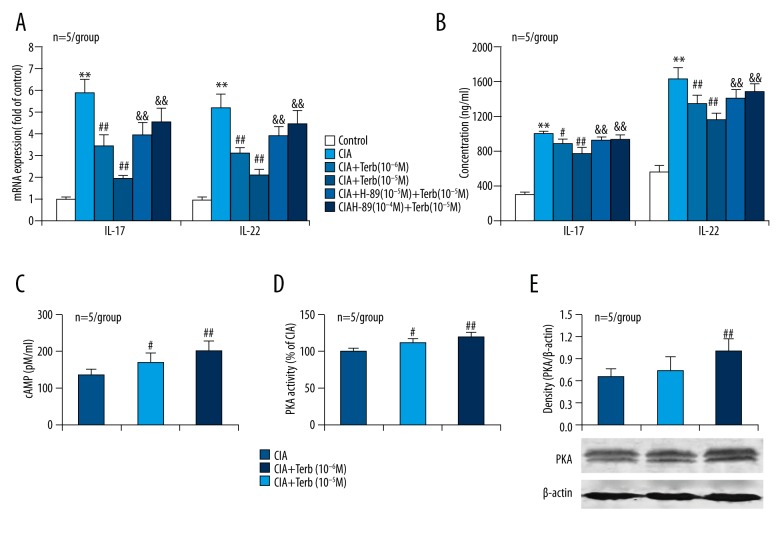
To determine whether β2-AR/PKA signaling has a direct effect on Th17 cells, we applied the β2-AR agonist Terb and the PKA inhibitor H-89 to Th17 cells that were activated and polarized from CD4+ T cells using various antibodies and cytokines. Both the mRNA expression of IL-17 and IL-22 in Th17 cells and the concentrations of IL-17 and IL-22 in the supernatants of Th17 cell cultures were increased in CIA condition compared to controls (Figure 4A, 4B). Importantly, the β2-AR agonist Terb reduced the CIA-induced IL-17 and IL-22 expression in and secretion from Th17 cells (Figure 4A, 4B). More importantly, the PKA inhibitor H-89 impaired the effects of Terb reducing CIA-induced IL-17 and IL-22 expression in and secretion from Th17 cells (Figure 4A, 4B). These findings demonstrated that activation of β2-AR attenuated CIA-induced Th17 cell enhancement via PKA signaling.
Figure 4.
The β2-AR agonist Terb reduces CIA-induced Th17 cell enhancement and this effect is impaired by the PKA inhibitor H-89. (A) mRNA expression levels of the pro-inflammatory cytokines in Th17 cells. (B) Concentrations of the pro-inflammatory cytokines in the culture supernatants of Th17 cells. (C) cAMP concentration in the lysates of cultured Th17 cells determined by ELISA. (D) PKA activity in the lysates of cultured Th17 cells. (E) PKA expression in Th17 cells. ** p<0.01, versus control; # p<0.05, ## p<0.01, versus CIA group; && p<0.01, versus CIA + Treb group.
To confirm that the cAMP-PKA signaling pathway in Th17 cells is activated by the β2-AR agonist Terb, we tested cAMP and PKA levels in response to Terb in Th17 cells from CIA mice. Exposure to the β2-AR agonist Terb increased cAMP production (Figure 4C), PKA activity (Figure 4D) and expression (Figure 4E) in Th17 cells. These data confirmed that the cAMP-PKA signaling pathway in Th17 cells from CIA mice was activated by the β2-AR agonist Terb.
Discussion
CD4+ T cells expressed β2-AR and particularly Th17 cells produced a response to the β2-AR agonist Terb in this study, suggesting that Th17 cells functionally express β2-AR. Although it is thought that β2-AR is expressed on all CD4+ T cells, we demonstrated that naive CD4+ T cells and effector Th1 cells express β2-AR, while effector Th2 cells do not [28–30]. Here, we provide new evidence for the expression of β2-AR on effector Th17 cells. More importantly, β2-AR expression was downregulated in both the ankle and spleen in CIA condition in this study. Consistent with our results, in rats challenged to induce adjuvant arthritis, a model of RA, we show that during severe disease, β2-AR affinity and density decrease in the spleen and draining lymph nodes for the arthritic limbs, indicating receptor downregulation [31]. The decreased β2-AR expression in CIA suggests a disease-specific internalization and degradation of receptors, as explained by Lorton et al. [31].
Th17 cells produce IL-17 and IL-22 and express ROR-γt [32–36]. By testing IL-17, IL-22, and ROR-γt levels in CD4+ T cells, we found that NE reduced CIA-induced CD4+ T cell shift towards Th17 phenotype and β2-AR antagonist reversed the NE effect. Further investigation showed that activating β2-AR with the agonist Terb attenuated CIA-induced CD4+ T cell shift towards Th17 phenotype. These findings suggest that NE, via activating β2-AR on CD4+ T cells, inhibits Th17 cell differentiation and function and thereby has an anti-inflammatory property in CIA. The cellular activity of naive and effector CD4+ T cells is regulated primarily by cytokines and costimulatory molecules, as well as by neurotransmitters and hormones [37]. The neurotransmitter NE is stored in sympathetic nerve terminals that reside within the parenchyma of lymphoid tissue [38] and are in close association with CD4+ T cells [39,40]. NE is released from these nerve terminals after antigen enters the system and binds to β2-AR expressed on immune cells [41]. Other studies support our present hypothesis that CIA stimulates sympathetic nerves to release NE, which acts on CD4+ T cells by binding to β2-AR to inhibit inflammatory response. However, sympathetic nerve fibers are lost in inflamed tissue of patients with RA [20] and in synovial tissue and lymph nodes of animals with CIA [21,22]. As a compensatory mechanism for this deprivation of sympathetic neurotransmitter in the inflamed joints and lymphoid tissues, cells that are capable of producing catecholamine neurotransmitters accumulate [7,42–44]. This tyrosine hydroxylase-positive catecholamine-producing cells have an anti-inflammatory property in human and experimental arthritis [7,43–46]. Our present findings support these reported results and provide further evidence for the anti-inflammatory property of the sympathetic neurotransmitter NE in CIA.
A direct inhibitory effect of the β2-AR agonist Terb on CIA-induced Th17 cell response was also observed in the current study. This effect of the agonist was reduced by the PKA inhibitor H-89. These data suggest that NE can directly inhibit Th17 cell function via β2-AR/PKA signaling. Indeed, cAMP production and PKA expression and activity in Th17 cells were increased by the β2-AR agonist Terb in CIA condition in this study, confirming that in Th17 cells, β2-AR also is coupled with the downstream signaling pathway cAMP-PKA. It has been reported that cAMP-PKA signaling in CD4+ T cells is important in the differentiation of Th subsets and their subsequent inflammatory responses [47]. Here, we propose that activating β2-AR-cAMP-PKA signaling in Th17 cells reduces the cell inflammatory response induced by CIA. Thus, an agonist or activator of β2-AR-cAMP-PKA signaling may be helpful for alleviation of RA.
Conclusions
β2-AR is expressed by CD4+ T cells and this expression is downregulated in CIA condition. NE reduces CIA-induced CD4+ T cell shift towards Th17 phenotype by activation of β2-AR on the CD4+ T cells. Activating β2-AR-cAMP-PKA signaling in Th17 cells impairs the cell inflammatory response induced by CIA. These results suggest that the sympathetic neurotransmitter NE exerts an anti-inflammatory effect by inhibition of Th17 cell differentiation and function via β2-AR/PKA signaling in CIA disease.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by grant 31371182 from the National Natural Science Foundation of China, MS12015104 and MS12015096 from the Nantong Applied Research Program of China, and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions
References
- 1.Mcinnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smeets TJ, Dolhain RJ, Breedveld FC, Tak PP. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J Pathol. 1998;186:75–81. doi: 10.1002/(SICI)1096-9896(199809)186:1<75::AID-PATH142>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miltenburg AM, Van Laar JM, De Kuiper R, et al. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603–10. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 6.Aarvak T, Chabaud M, Thoen J, et al. Changes in the Th1 or Th2 cytokine dominance in the synovium of rheumatoid arthritis (RA): A kinetic study of the Th subsets in one unusual RA patient. Rheumatology (Oxford) 2000;39:513–22. doi: 10.1093/rheumatology/39.5.513. [DOI] [PubMed] [Google Scholar]
- 7.Zhao XY, Cui SW, Wang XQ, et al. Tyrosine hydroxylase expression in CD4(+) T cells is associated with joint inflammatory alleviation in collagen type II-induced arthritis. Rheumatol Int. 2013;33:2597–605. doi: 10.1007/s00296-013-2788-y. [DOI] [PubMed] [Google Scholar]
- 8.Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–57. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-β are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–52. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1998;25:1466–71. [PubMed] [Google Scholar]
- 11.Van Der Graaff WL, Prins AP, Niers TM, et al. Quantitation of interferon gamma- and interleukin-4-producing T cells in synovial fluid and peripheral blood of arthritis patients. Rheumatology (Oxford) 1999;38:214–20. doi: 10.1093/rheumatology/38.3.214. [DOI] [PubMed] [Google Scholar]
- 12.Berner B, Akca D, Jung T, et al. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27:1128–35. [PubMed] [Google Scholar]
- 13.Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–85. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 14.Nistala K, Adams S, Cambrook H, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci USA. 2010;107:14751–56. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–57. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Lorton D, Bell Inger DL. Molecular mechanisms underlying beta-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci. 2015;16:5635–65. doi: 10.3390/ijms16035635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pongratz G, Straub RH. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat Rev Rheumatol. 2013;9:117–26. doi: 10.1038/nrrheum.2012.181. [DOI] [PubMed] [Google Scholar]
- 20.Weidler C, Holzer C, Harbuz M, et al. Low density of sympathetic nerve fibres and increased density of brain derived neurotrophic factor positive cells in RA synovium. Ann Rheum Dis. 2005;64:13–20. doi: 10.1136/ard.2003.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Härle P, M bius D, Carr DJ, et al. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 2005;52:1305–13. doi: 10.1002/art.20987. [DOI] [PubMed] [Google Scholar]
- 22.Del Rey A, Wolff C, Wildmann J, et al. Disrupted joint-immune-brain communication during experimental arthritis. Arthritis Rheum. 2008;58:3090–99. doi: 10.1002/art.23869. [DOI] [PubMed] [Google Scholar]
- 23.Bellinger DL, Millar BA, Perez S, et al. Sympathetic modulation of immunity: Relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebbinghaus M, Gajda M, Boettger MK, et al. The anti-inflammatory effects of sympathectomy in murine antigen-induced arthritis are associated with a reduction of Th1 and Th17 responses. Ann Rheum Dis. 2012;71:253–61. doi: 10.1136/ard.2011.150318. [DOI] [PubMed] [Google Scholar]
- 25.Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: Do we understand it yet? Brain Behav Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang HW, Fang XX, Wang XQ, et al. Regulation of differentiation and function of helper T cells by lymphocyte-derived catecholamines via α1- and β2-adrenoceptors. Neuroimmunomodulation. 2015;22:138–51. doi: 10.1159/000360579. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Huang Y, Cao BB, et al. Th17 cells induce dopaminergic neuronal death via LFA-1/ICAM-1 interaction in a mouse model of Parkinson’s disease. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0249-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Ramer-Quinn DS, Baker RA, Sanders VM. Activated Th1 and Th2 cells differentially express the beta-2-adrenergic receptor: A mechanism for selective modulation of Th1 cell cytokine production. J Immunol. 1997;159:4857–67. [PubMed] [Google Scholar]
- 29.Sanders VM, Baker RA, Ramer-Quinn DS, et al. Differential expression of the beta-2-adrenergic receptor by Th1 and Th2 clones: Implications for cytokine production and B cell help. J Immunol. 1997;158:4200–10. [PubMed] [Google Scholar]
- 30.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naiven CD4(+) T cells exposed to norepinephrine. J Immunol. 2001;166:232–40. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 31.Lorton D, Bellinger DL, Schaller JA, et al. Altered sympathetic-to-immune cell signaling via β2-adrenergic receptors in adjuvant arthritis. Clin Dev Immunol. 2013;2013:764395. doi: 10.1155/2013/764395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 33.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–66. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–38. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Astry B, Venkatesha SH, Moudgil KD. Temporal cytokine expression and the target organ attributes unravel novel aspects of autoimmune arthritis. Indian J Med Res. 2013;138:717–31. [PMC free article] [PubMed] [Google Scholar]
- 36.Moudgil KD. Interplay among cytokines and T cell subsets in the progression and control of immune-mediated diseases. Cytokine. 2015;74:1–4. doi: 10.1016/j.cyto.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–81. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 38.Kohm AP, Tang Y, Sanders VM, Jones SB. Activation of antigen-specific CD4+ Th2 cells and B cells in vivo increases norepinephrine release in the spleen and bone marrow. J Immunol. 2000;165:725–33. doi: 10.4049/jimmunol.165.2.725. [DOI] [PubMed] [Google Scholar]
- 39.Felten SY, Madden KS, Bellinger DL, et al. The role of the sympathetic nervous system in the modulation of immune responses. Adv Pharmacol. 1998;42:583–87. doi: 10.1016/s1054-3589(08)60818-2. [DOI] [PubMed] [Google Scholar]
- 40.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synaptic-like contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 41.Mcalees JW, Smith LT, Erbe RS, et al. Epigenetic regulation of beta2-adrenergic receptor expression in T(H)1 and T(H)2 cells. Brain Behav Immun. 2011;25:408–15. doi: 10.1016/j.bbi.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther. 2014;16:504. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capellino S, Weber K, Gelder M, et al. First appearance and location of catecholaminergic cells during experimental arthritis and elimination by chemical sympathectomy. Arthritis Rheum. 2012;64:1110–18. doi: 10.1002/art.33431. [DOI] [PubMed] [Google Scholar]
- 44.Wang XQ, Liu Y, Cai HH, et al. Expression of tyrosine hydroxylase in CD4(+) T cells contributes to alleviation of Th17/Treg imbalance in collagen-induced arthritis. Exp Biol Med (Maywood) 2016;241:2094–103. doi: 10.1177/1535370216660635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capellino S, Cosentino M, Wolff C, et al. Catecholamine-producing cells in the synovial tissue during arthritis: Modulation of sympathetic neurotransmitters as new therapeutic target. Ann Rheum Dis. 2010;69:1853–60. doi: 10.1136/ard.2009.119701. [DOI] [PubMed] [Google Scholar]
- 46.Jenei-Lanzl Z, Capellino S, Kees F, et al. Anti-inflammatory effects of cell-based therapy with tyrosine hydroxylase positive catecholaminergic cells in experimental arthritis. Ann Rheum Dis. 2015;74:444–51. doi: 10.1136/annrheumdis-2013-203925. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Murray F, Koide N, et al. Divergent requirement for Gαs and cAMP in the differentiation and inflammatory profile of distinct mouse Th subsets. J Clin Invest. 2012;122:963–73. doi: 10.1172/JCI59097. [DOI] [PMC free article] [PubMed] [Google Scholar]