Abstract
Background
This study aimed to investigate the therapeutic effect of curcumin in lipopolysaccharide (LPS) induced neonatal acute lung injury (ALI) and the possibly associated molecular mechanisms.
Material/Methods
ALI neonatal animal model was established by using LPS. Curcumin and/or peroxisome proliferator-activated receptor γ (PPARγ) inhibitor BADGE (bisphenol A diglycidyl ether) were administrated to animals. Lung edema was evaluated by PaO2 and lung wet/dry weight ratio (W/D) measurements. EMSA was used to determine the PPARγ activity. Levels of high-mobility group box 1 (HMGB1), secretory receptor for advanced glycation end products (RAGE), tumor necrosis factor α (TNFα), interleukin 6 (IL6), and transforming growth factor β1 (TGFβ1) in bronchoalveolar lavage fluid (BALF) were examined by ELISA. Western blotting was used to evaluate the expression levels of HMGB1, RAGE, heme oxygenase 1 (HO1), TNFα, IL6, and TGFβ1 in lung tissue.
Results
Curcumin administration significantly improved lung function by increasing PaO2 and decreasing W/D in neonatal ALI rats. Curcumin treatment upregulated the PPARγ activity and expression level of HO1 which were suppressed in lung tissue of neonatal ALI rats. Elevated levels of HMGB1, RAGE, TNFα, IL6, and TGFβ1 in both lung tissue and BALF from neonatal ALI rats were decreased dramatically by curcumin treatment. PPARγ inhibitor BADGE administration impaired curcumin’s alleviation on lung edema, inhibitory effects on inflammatory cytokine expression and recovery of PPARγ/HO1 signaling activation.
Conclusions
Curcumin alleviated lung edema in LPS-induced ALI by inhibiting inflammation which was induced by PPARγ/HO1 regulated-HMGB1/RAGE pro-inflammatory pathway.
MeSH Keywords: Acute Lung Injury, Curcumin, Inflammation, PPAR gamma
Background
Acute lung injury (ALI) is one of the devastating situation needing intensive care, and is life threatening [1]. Pathologically, ALI is characterized by the impaired integrity, increased permeability and activated inflammation of alveolar epithelium, which leads to the pulmonary edema, hypoxemia, atelectasis, and hyaline membrane [2]. Neonates are extremely susceptible to ALI which is one of the most frequent causes of mortality in newborns [3]. Lipopolysaccharide (LPS) is known as the bacterial bio-active component involved in many pathological conditions by activating inflammatory cascade. It is implicated that LPS takes the responsibility as the inducer of ALI and thus has been used in establishing ALI animal models in literature [4].
The role of LPS in inducing ALI is depending on its pro-inflammatory activities. LPS could recruit monocytes infiltration and aggregation, promote inflammatory cytokines synthesis and secretion and induce alveolar epithelial apoptosis [5]. Peroxisome proliferator-activated receptor γ (PPARγ) is the member of nuclear hormone receptor family and one of the isoforms of PPARs. PPARγ activation exerted anti-inflammatory and anti-apoptotic effects in many inflammatory diseases models including ALI [6]. High-mobility group box 1 (HMGB1) is synthesized and secreted by activated immunocytes, such as monocytes and macrophages, and has been considered as one of the important inflammatory inducers. After binding with receptor for advanced glycation end products (RAGE), HMGB1 activates the nuclear factor κB signaling [7]. Thus, the expression levels pro-inflammatory cytokines including tumor necrosis factor α (TNFα), interleukin 6 (IL6), and transforming growth factor β1 (TGFβ1) are upregulated [8–10]. Notably, according to several previous studies, HMGB1/RAGE was considered as one of the downstream targets of PPARγ through modulating the mediator heme oxygenase 1 (HO1) [11].
As a natural polyphenol, curcumin is one of the bio-active extracts of the Chinese medicinal plant Curcuma longa linn which is also known as turmeric. Curcumin possesses a wide spectrum of biological activities such as antioxidant, antiproliferative, and anti-inflammatory effects [12]. Several previous investigations pointed out that curcumin acted partially as an agonist of PPARγ [13]. Moreover, administration of curcumin attenuated lung injuries in paraquot-, LPS-, and Staphylococcus aureus- induced ALI animal models [14–16]. However, very few studies have investigated the protective role of curcumin in ALI neonatal animal models. The involvement of PPARγ signaling has also been rarely studied. In the present study, the protective role of curcumin on an established LSP-induced ALI model in neonatal rats was studied. Furthermore, the molecular mechanism concerning PPARγ signaling was investigated. We believe that results from this study could not only suggest to us more information about the mechanism of neonatal ALI, but also provide the theoretical groundwork for potential application of curcumin on neonatal ALI.
Material and Methods
Animals and ALI model establishment
Newborn Sprague-Dawley rats (3–8 day old, 8–14 g bodyweight) were provided by the Animal Experimental Center of Zhejiang University. All experimental procedures were performed by following the Recommended Guideline for the Care and Use of Laboratory Animals issued by Chinese Council on Animal Research. Protocols for animal experiments were approved by Animal Ethics Committee of Zhejiang Yongkang Women and Children’s Health Service Hospital. Rat pups were maintained in polypropylene cages with their nursing mothers. Animals were housed in an artificial environment providing 25±5°C temperature, 50% humidity and a 12-hour dark/light lighting circle. Intraperitoneal injections of LPS (3 mg/kg bodyweight, Sigma-Aldrich) were administrated to rats to induce ALI. Rats also received intraperitoneal injections of curcumin (Sigma-Aldrich) at various concentrations (1.5, 3.0, and 6.0 mg/kg bodyweight daily for 7 consecutive days) after LPS exposure. The PPARγ inhibitor bisphenol A diglycidyl ether (BADGE) (Sigma-Aldrich) at 30 mg/kg bodyweight daily for 7 consecutive days after LPS exposure.
Lung edema evaluation
In this study, lung edema was evaluated by both PaO2 and lung wet/dry weight ratio (W/D). Isoflurane inhalation was used to anesthetize the animals. Blood samples were harvested from abdominal aorta. PaO2 was measured by an automatic blood gas analyzer (Bobas B123, Roche). Right lungs were weighted to get measurements of wet weight (W). The right lung was dried at 70°C for 48 hours then the dry weight (D) was measured. The W/D was then calculated.
Bronchoalveolar lavage fluid (BALF) harvest and ELISA
Bronchoalveolar lavage fluid (BALF) was acquired by lavaging the lung with sterile PBS by intratracheal injection 3 times. Supernatant of BALF was separated by centrifugation at 800 g at 4°C for 10 minutes. ELISA kits were used to detect the concentrations of HMGB1 (Shino-Test Corporation), secretory RAGE (R&D), TNFα (R&D), TGFβ1 (R&D), and IL6 (R&D) in BALF. The protocols of ELSIA were carried out per manufacturers’ instructions.
Western blotting
Lung tissue was homogenized with ice-cold RIPA lysis buffer system (Santa Cruz) supplemented with PMSF (Santa Cruz). The supernatant was collected after the lysates were centrifuged at 1 4000 g at 4°C for 20 minutes. The cytoplasmic protein was extracted by Cytoplasmic Protein Extraction kit (Beyotime) and the nuclear protein was acquired by using Nuclear Protein Extraction kit (Beyotime). Proteins were then subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then electronically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). The membranes were incubated with blocking buffer (Abcam), washed and then incubated with primary antibodies of HMGB1 (1: 2000, Abcam), RAGE (1: 2000, Abcam), HO1 (1: 2000, Sigma-Aldrich), TNFα (1: 2500, Sigma-Aldrich), TGFβ1 (1: 2500, Sigma-Aldrich), IL6 (1: 2500, Sigma-Aldrich), and GAPDH (1: 4000, Sigma-Aldrich). Horseradish peroxidase (HRP)-conjugated secondary antibodies (1: 4000, Abcam; 1: 4000, Sigma-Aldrich) were used to incubate the membranes, which were then developed by SuperSignal West Pico Chemiluminescent Reagent (Pierce). The immunoblots were visualized on x-ray films.
PPARγ binding activity
The DNA-binding activity of PPARγ was evaluated by electrophoretic mobility shift assay (EMSA) in the current study. The oligonucleotide (sequence: 5′-CAAATCAGGTCAAAGGTCA-3′) for peroxisome proliferator response element was synthesized by TaKaRa. The oligonucleotide was then labeled by γ32P-ATP by using a T4 Polynucleotide Kinase kit (Promega). EMSA/Gel-shift binding buffer (Beyotime) was used to accomplish the binding between nuclear protein and the oligonucleotide. Then the protein-oligonucleotide complex was subjected to EMSA/Gel-shift running buffer (Beyotime) and separated from free probes by electrophoresis with a 5% native polyacrylamide gel. The resulted gel was then transferred to 3MM filter paper (GE Health Care) and dried. The probes were visualized on x-ray films after exposure for 20 hours at −80°C
Statistics
Data acquired in this study was presented as (mean ±SD) and were analyzed by using software SPSS (version 16.0, SPSS). Student’s t-tests and one-way ANOVA were performed to analyze the differences between groups. NSK tests were carried out as post-hoc tests. P<0.05 was considered statistically significant.
Results
Curcumin alleviated pulmonary edema in neonatal rats with ALI
The results are shown in Figure 1. The PaO2 decreased while the W/D increased significantly in neonatal rats with ALI. However, administration of curcumin dramatically increased PaO2 and decreased W/D in neonatal rats with ALI in a concentration-dependent manner. The PPARγ inhibitor BAGDE, however, impaired the attenuating effects of curcumin on lung edema in neonatal rats with ALI.
Figure 1.
(A) Columns indicate the detected PaO2 of arterial blood samples collected from neonatal rats that received treatments of LPS/curcumin/BADGE. (B) Columns indicate the lung wet/dry weight ratio (W/D) in neonatal rats that received treatments of LPS/curcumin/BADGE. * P<0.05.
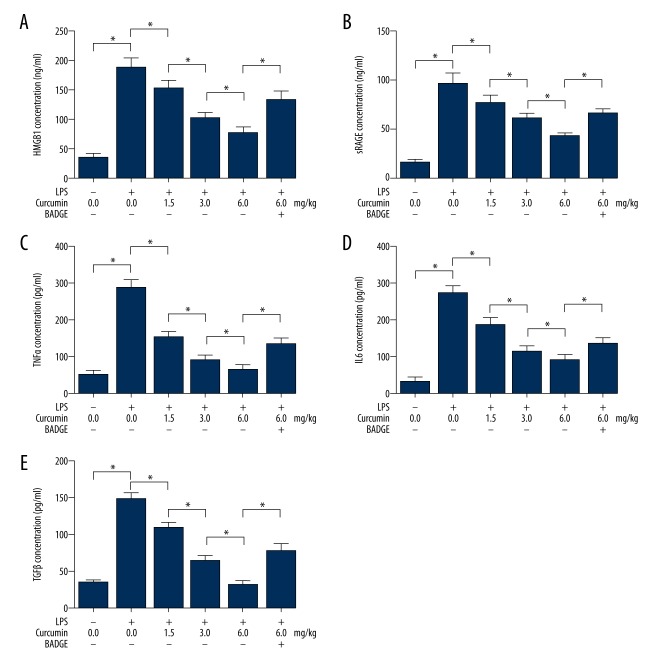
Curcumin suppressed airway inflammation by inhibiting HMGB1/RAGE in neonatal with ALI
As demonstrated in Figure 2, the concentrations of inflammatory factors in BALF were determined by ELISA. The concentrations of HMGB1 (Figure 2A), secretory RAGE (Figure 2B), TNFα (Figure 2C), IL6 (Figure 2E), and TGFβ1 (Figure 2E) were elevated in BALF from neonatal rats with ALI. Concentrations of HMGB1, secretory RAGE, TNFα, IL6, and TGFβ1 were found elevated in BALF from neonatal rats with ALI. The administration of curcumin dramatically decreased concentrations of these inflammatory factors in BALF from neonatal rats with ALI. However, co-administration of BADGE significantly impaired curcumin’s inhibitory effects on inflammatory factors in BALF from neonatal rats with ALI.
Figure 2.
Columns on A, B, C, D, and E indicate detected concentrations of HMGB1, RAGE, TNFα, IL6, and TGFβ in BALF collected from neonatal rats that received treatments of LPS/curcumin/BADGE, respectively. * P<0.05.
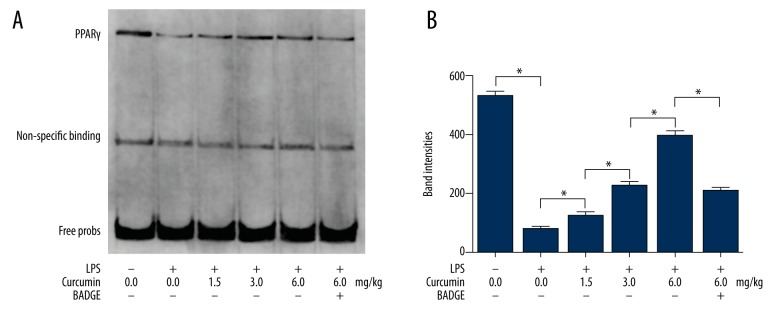
Curcumin administration increased PPARγ activity in lungs from neonatal rats with ALI
EMSA was used to evaluate the PPARγ activity and the results are shown in Figure 3. In lungs from neonatal rats with ALI, the PPARγ activity decreased significantly. The curcumin administration increased the PPARγ activity in lungs of neonatal rats with ALI in a concentration-dependent manner. However, the PPARγ activity inhibitor BADGE prevented curcumin in increasing the activity of PPARγ in lungs of neonatal rats with ALI.
Figure 3.
(A) Results of EMSA ware shown. Nuclear protein samples of lung tissue harvested from neonatal rats that received treatments of LPS/curcumin/BADGE were probed. (B) Columns indicate the probing band intensities of PPARγ. * P<0.05.
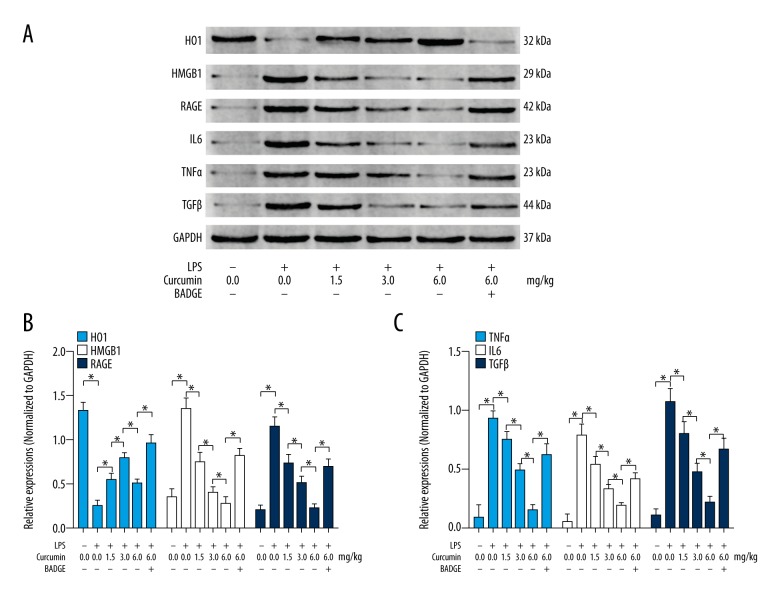
Curcumin inhibited HMGB1/RAGE- induced inflammation by activating PPARγ/HO1 signaling in lungs from neonatal rats with ALI
The immunoblots of HO1, HMGB1, RAGE, IL6, TNFα, TGFβ, and GAPDH in lungs of neonatal rats with ALI are shown in Figure 4A. The expression levels of HMGB1 (Figure 4B, white columns), RAGE (Figure 4B, deep blue columns), TNFα (Figure 4C, blue columns), IL6 (Figure 4C, white columns) and TGFβ1 (Figure 4C, deep blue columns) increased while the expression level of HO1 (Figure 4B, blue columns) was downregulated in lungs of neonatal rats with ALI. The administration of curcumin dramatically decreased the expression levels of HMGB1, RAGE, TNFα, TGFβ1, and IL6 and upregulated expression level of HO1 in lungs of neonatal rats in a concentration-dependent manner. However, co-administration of BADGE impaired curcumin’s effects on decreasing expression levels of RAGE, TNFα, TGFβ1, and IL6 and on increasing expression level of HO1 in lungs from neonatal rats with ALI.
Figure 4.
(A) Immunoblots of HO1, HMGB1, RAGE, IL6, TNFα, TGFβ, and GAPDH. (B) Columns indicate the relative expression levels of HO1 (blue), HMGB1 (white), and RAGE (deep blue) in lung tissue collected from neonatal rats that received treatments of LPS/curcumin/BADGE, respectively. (C) Columns indicate the relative expression levels of TNFα (blue), IL6 (white), and TGFβ (deep blue) in lung tissue collected from neonatal rats that received treatments of LPS/curcumin/BADGE respectively. * P<0.05.
Discussion
Resulted from severe bacterial infection, ALI is taking responsibility for mortality in newborns that are more vulnerable than adults [17,18]. The onset of ALI is considered as one of the early manifests of multiple organ failure which is correlated with endotoxin or LPS in circulation [18]. It has been established that inflammation plays a critical role in initiation and maintenance of ALI [19]. The inflammation cytokines take responsibility of increasing permeability of pulmonary epithelium, inducing lung tissue damage and accumulation of neutrophils which characterize ALI and lead to lung edema. Elevation of TNFα level was correlated with ALI in septic pediatric critically ill patients and animal models [20,21]. IL6 is identified as one of the biomarkers in monitoring ALI [22]. Changes of TGFβ1 would affect the synthesis and deposition of collagens which was especially important for developing lungs [23]. Monitoring the changes of TGFβ1 was important for assessments of therapeutic outcomes and prognosis of neonatal ALI [24]. In this study, we found that the indicators of lung edema changed significantly in neonatal rats with ALI: PaO2 decreased while the W/D increased dramatically. Moreover, the concentrations of inflammatory cytokines, namely HMGB1, RAGE, TNFα, IL6, and TGFβ elevated significantly in both BALF and lung tissue of neonatal rats with ALI.
Biological extracts from Chinese medicinal herbs have been attracting attention from both investigators and doctors due to the various pharmacological effects on many pathological conditions. Curcumin is one of the typical polyphenol extracted the roots of Curcuma longa linn. Modern pharmacological investigations have revealed the anti-inflammatory effects of curcumin in many inflammation-associated diseases [25]. Several recent investigations indicated the protective and therapeutic effects of curcumin in animal models of multiple organ distress syndrome (MODS) [26]. In this study, we administrated curcumin to neonatal rats with ALI. As a result, the PaO2 decrease and W/D elevation were dramatically attenuated, indicating that lung edema was relieved by curcumin. We also found that in BALF and lung tissue the levels of the inflammatory cytokines HMGB1, RAGE, IL6, and TGFβ were suppressed by curcumin administration.
It was evidenced that the activation of PPARγ played a role as an inflammatory suppressor by interacting with several signaling pathways [27]. For instance, HO1 is one of the downstream effectors of PPARγ which conducts the anti-inflammatory signal of PPARγ [28]. It was suggested that the activation of PPARγ/HO1 lead to inhibition of the inflammatory HMGB1/RAGE axis, which facilitates the transcriptional initiation of IL6, TNFα, and TGFβ1 [29]. In the current study, our results showed that the activation of PPARγ/HO1 was significantly suppressed in neonatal rats with ALI, which was evidenced by decreased PPARγ activity and HO1 expression level. As a result, the HMGB1/RAGE signaling was activated, leading to inflammation. Previous studies have indicated the involvement of curcumin in activating PPARγ [30]. In this study, we found that curcumin administration dramatically increased PPARγ activity in lungs harvested from neonatal rats with ALI. As a result, the expression level of HO1 was elevated, indicating the activation of PPARγ/HO1 signaling. Thus, the signaling transduction of pro-inflammatory HMGB1/RAGE pathway was blocked and the generation of IL6, TNFα, and TGFβ were downregulated by curcumin.
We investigated whether the specific PPARγ inhibitor BADGE was used to treat neonatal rats with ALI as a co-administration of curcumin. BADGE is a synthetic PPARγ inhibitor, acting as a ligand for PPARγ which was been reported to antagonize the ability of ligands such as rosiglitazone [31]. Our study results showed that BADGE impaired the curcumin-induced activation of the PPARγ/HO1 pathway. Thus, the inhibition of HMGB1/RAGE activation was absolved in neonatal rats with ALI that received co-administration of curcumin and BADGE. These results further suggested that PPARγ/HO1 was the molecular target for curcumin in neonatal ALI.
Conclusions
Our study demonstrated the potent therapeutic effects of curcumin in a neonatal ALI animal model. This effect was conducted by curcumin’s ability to activate the PPARγ/HO1 pathway which further inhibited the pro-inflammatory HMGB1/RAGE pathway. Results in this study provided evidence for activation of PPARγ as a new strategy of neonatal ALI treatment. Additionally, the potential value of the application of curcumin and curcumin-related compounds in neonatal ALI treatment could be foreseen.
Footnotes
Source of support: Departmental sources
References
- 1.De Luca D, Piastra M, Tosi F, et al. Pharmacological therapies for pediatric and neonatal ALI/ARDS: an evidence-based review. Curr Drug Targets. 2012;13(7):906–16. doi: 10.2174/138945012800675687. [DOI] [PubMed] [Google Scholar]
- 2.Cheifetz IML Year in review 2015: Pediatric ARDS. Respir Care. 2016;61(7):980–85. doi: 10.4187/respcare.05017. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty M, McGreal EP, Kotecha S. Acute lung injury in preterm newborn infants: Mechanisms and management. Paediatr Respir Rev. 2010;11(3):162–70. doi: 10.1016/j.prrv.2010.03.002. quiz 170. [DOI] [PubMed] [Google Scholar]
- 4.Ding Q, Liu GQ, Zeng YY, et al. Role of IL-17 in LPS-induced acute lung injury: An in vivo study. Oncotarget. 2017;8(55):93704–11. doi: 10.18632/oncotarget.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Song S, Hu Z, et al. Activation of Epac alleviates inflammation and vascular leakage in LPS-induced acute murine lung injury. Biomed Pharmacother. 2017;96:1127–36. doi: 10.1016/j.biopha.2017.11.110. [DOI] [PubMed] [Google Scholar]
- 6.Li A, Liu Y, Zhai L, et al. Activating peroxisome proliferator-activated receptors (PPARs): A new sight for chrysophanol to treat paraquat-induced lung injury. Inflammation. 2016;39(2):928–37. doi: 10.1007/s10753-016-0326-2. [DOI] [PubMed] [Google Scholar]
- 7.Imbalzano E, Quartuccio S, Di Salvo E, et al. Association between HMGB1 and asthma: A literature review. Clin Mol Allergy. 2017;15:12. doi: 10.1186/s12948-017-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luan ZG, Zhang H, Yang PT, et al. HMGB1 activates nuclear factor-kappaB signaling by RAGE and increases the production of TNF-alpha in human umbilical vein endothelial cells. Immunobiology. 2010;215(12):956–62. doi: 10.1016/j.imbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.He L, Sun F, Wang Y, et al. HMGB1 exacerbates bronchiolitis obliterans syndrome via RAGE/NF-kappaB/HPSE signaling to enhance latent TGF-beta release from ECM. Am J Transl Res. 2016;8(5):1971–84. [PMC free article] [PubMed] [Google Scholar]
- 10.Plazyo O, Romero R, Unkel R, et al. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol Reprod. 2016;95(6):130. doi: 10.1095/biolreprod.116.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Wang Q. Activation of PPARgamma by baicalin attenuates pulmonary hypertension in an infant rat model by suppressing HMGB1/RAGE signaling. FEBS Open Bio. 2017;7(4):477–84. doi: 10.1002/2211-5463.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewlings SJ, Kalman DS. Curcumin: A review of its’ effects on human health. Foods (Basel, Switzerland) 2017;6(10) doi: 10.3390/foods6100092. pii: E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng Z, Yu XH, Chen J, et al. Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-gamma activation. Acta Pharmacol Sin. 2014;35(10):1247–56. doi: 10.1038/aps.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Liang D, Dong L, et al. Anti-inflammatory effects of novel curcumin analogs in experimental acute lung injury. Respir Res. 2015;16:43. doi: 10.1186/s12931-015-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lelli D, Sahebkar A, Johnston TP, Pedone C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharmacol Res. 2017;115:133–48. doi: 10.1016/j.phrs.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Xu F, Lin SH, Yang YZ, et al. The effect of curcumin on sepsis-induced acute lung injury in a rat model through the inhibition of the TGF-beta1/SMAD3 pathway. Int Immunopharmacol. 2013;16(1):1–6. doi: 10.1016/j.intimp.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Franco ML, Waszak P, Banalec G, et al. LPS-induced lung injury in neonatal rats: Changes in gelatinase activities and consequences on lung growth. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L491–500. doi: 10.1152/ajplung.00140.2001. [DOI] [PubMed] [Google Scholar]
- 18.Miotla JM, Teixeira MM, Hellewell PG. Suppression of acute lung injury in mice by an inhibitor of phosphodiesterase type 4. Am J Respir Cell Mol Biol. 1998;18(3):411–20. doi: 10.1165/ajrcmb.18.3.2913. [DOI] [PubMed] [Google Scholar]
- 19.Gando S, Kameue T, Matsuda N, et al. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: Role of neutrophil and endothelial activation. Inflammation. 2004;28(4):237–44. doi: 10.1023/b:ifla.0000049049.81688.fe. [DOI] [PubMed] [Google Scholar]
- 20.Azevedo ZM, Moore DB, Lima FC, et al. Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) single nucleotide polymorphisms: Importance in ARDS in septic pediatric critically ill patients. Hum Immunol. 2012;73(6):661–67. doi: 10.1016/j.humimm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Rabelo MAE, Lucinda LMF, Reboredo MM, et al. Acute lung injury in response to intratracheal instillation of lipopolysaccharide in an animal model of emphysema induced by elastase. Inflammation. 2018;41(1):174–82. doi: 10.1007/s10753-017-0675-5. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Shi L, Li Y, et al. Disease-specific dynamic biomarkers selected by integrating inflammatory mediators with clinical informatics in ARDS patients with severe pneumonia. Cell Biol Toxicol. 2016;32(3):169–84. doi: 10.1007/s10565-016-9322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chimenti L, Camprubi-Rimblas M, Guillamat-Prats R, et al. Nebulized heparin attenuates pulmonary coagulopathy and inflammation through alveolar macrophages in a rat model of acute lung injury. Thromb Haemost. 2017;117(11):2125–34. doi: 10.1160/TH17-05-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LP, Mao QH, Yang L. Effect of pulmonary surfactant combined with mechanical ventilation on oxygenation functions and expressions of serum transforming growth factor-beta1 (TGF-beta1) and bone morphogenetic protein 7 (BMP-7) of neonatal respiratory distress syndrome. Eur Rev Med Pharmacol Sci. 2017;21(19):4357–61. [PubMed] [Google Scholar]
- 25.Abdollahi E, Momtazi AA, Johnston TP, Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J Cell Physiol. 2018;233(2):830–48. doi: 10.1002/jcp.25778. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Zhang J, Pang Q, et al. The protective role of curcumin in zymosan-induced multiple organ dysfunction syndrome in mice. Shock (Augusta, Ga) 2016;45(2):209–19. doi: 10.1097/SHK.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo H, Fan Z, Xiang D, et al. The protective effect of umbelliferone ameliorates myocardial injury following ischemiareperfusion in the rat through suppression NLRP3 inflammasome and upregulating the PPAR-gamma. Mol Med Rep. 2018;17(2):3404–10. doi: 10.3892/mmr.2017.8208. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Li Z, Sun X, et al. Heme oxygenase-1/p21WAF1 mediates peroxisome proliferator-activated receptor-gamma signaling inhibition of proliferation of rat pulmonary artery smooth muscle cells. FEBS J. 2010;277(6):1543–50. doi: 10.1111/j.1742-4658.2010.07581.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Liu L, Zhang Y, et al. Activation of PPARgamma attenuates LPS-induced acute lung injury by inhibition of HMGB1-RAGE levels. Eur J Pharmacol. 2014;726:27–32. doi: 10.1016/j.ejphar.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Jin H, Lian N, Zhang F, et al. Activation of PPARgamma/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 2016;7:e2189. doi: 10.1038/cddis.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duque G, Li W, Vidal C, et al. Pharmacological inhibition of PPARgamma increases osteoblastogenesis and bone mass in male C57BL/6 mice. J Bone Min Res. 2013;28(3):639–48. doi: 10.1002/jbmr.1782. [DOI] [PubMed] [Google Scholar]