Abstract
Background
The majority of rectal cancers are discovered in locally advanced forms (UICC stage II, III). Treatment consists of preoperative radiochemotherapy, followed by surgery 6–8 weeks later and finally by postoperative chemotherapy. The aim of this study was to find out if tumor regression affected long-term survival in patients with localy advanced rectal cancer, treated with neoadjuvant radiochemotherapy.
Patients and methods
Patients with rectal cancer stage II or III, treated between 2006 and 2010, were included in a retrospective study. Clinical and pathohistologic data were acquired from computer databases and information about survival from Cancer Registry. Survival was estimated according to Kaplan-Meier method. Significance of prognostic factors was evaluated in univariate analysis; comparison was carried out with log-rank test. The multivariate analysis was performed according to the Cox regression model; statistically significant variables from univariate analysis were included.
Results
Two hundred and two patients met inclusion criteria. Median follow-up was 53.2 months. Stage ypT0N0 (pathologic complete response, pCR) was observed in 14.8% of patients. Pathohistologic stage had statistically significant impact on survival (p = 0.001). 5-year survival in patients with pCR was>90%. Postoperative T and N status were also found to be statistically significant (p = 0.011 for ypT and p < 0.001 for ypN). According to multivariate analysis, tumor response to neoadjuvant therapy was the only independent prognostic factor (p = 0.003).
Conclusions
Pathologic response of tumor to preoperative radiochemotherapy is an important prognostic factor for prediction of long-term survival of patients with locally advanced rectal cancer.
Key words: rectal cancer, tumor regression, preoperative radiochemotherapy, prognosis
Introduction
Combined chemoradiotherapy (CRT) followed by total mesorectal excision (TME) is the standard treatment for patients with locally advanced rectal cancer.1 This approach led to significantly enhanced tumor control, with local recurrence rates of < 10%.
Preoperative chemotherapy induces changes in both gross appearance of the surgical specimen and its pathological features. Pathologic tumor response to therapy is an important prognostic factor for long-term prognosis. Moreover, patients with complete pathologic response to neoadjuvant treatment have much better prognosis than patients with less or no response.2 The rate of response is better in neoadjuvant CRT compared with long course RT, and possibly absent in short course RT with immediate surgery. In fact, maximal response of the radiation occurs only several weeks after its end.3 For that reason surgery has been delayed until 6–12 weeks following neoadjuvant CRT.4,5 The use of neoadjuvant CRT leading to tumor shrinkage increases the likelihood of performing a sphincter preserving surgery and increases circumferential and distal margins in surgical specimen with reduction of lymphatic and vascular invasion.6,7,8,9 Chemoradiation induces a tumor downstaging effect, which potentially improves the feasibility of a complete resection with benefits in local disease control. However, the type and remission rate to neoadjuvant CRT remains considerably variable. While some patients may not respond, others may even have progression of disease. Other group of patients experiences downstaging and 15–25% has surgical specimens without any viable tumor cells, a condition referred to as pathologic complete response (ypCR).10,11,12
The aim of this study was to find out if tumor regression affected long-term survival in patients with localy advanced rectal cancer, treated with neoadjuvant radiochemotherapy.
Patients and methods
Our retrospective research included patients with locally advanced rectal cancer (stage II, III), treated in Clinical Department of Abdominal Surgery, University Medical Centre Ljubljana between 2006 and 2010. The study was approved by institutional board, informed consent was obtained from all patients and all procedures were performed according to the guidelines of the Helsinki Declaration.
After analysing available medical documentation and considering exclusion criteria (stage I or IV at diagnosis; noninvasive tumors, tumors in situ, inoperable tumors, nonradical resection (R1, R2), reoperation because of tumor recurrence), two hundred and two patients were selected for analysis.
Relevant patients’ data were: age, sex, type of operation, survival, preoperative stage established by MRI (cTNM), type of neoadjuvant therapy and pathohistological findings. The latter allowed for a classification of the anatomical extent of the disease according to the 7th ed. of the UICC TNM classifiation.13 Histopathological regression grade of the primary tumor after neoadjuvant radiochemotherapy, was assessed according to Dworak regression scale.14
Survival data were provided by Cancer registry. Kaplan-Meier method was used to analyse survival. Significance of prognostic factors was evaluated with univariate analysis and log-rank test. Statistically sigificant variables from univariate analysis were used in multivariate analysis; with Cox regression model independent variables with effect on long-term survival of rectal cancer patients were pointed out.
All statistical analyses were carried out with statistical program SPSS 19.0.0 (SPSS Inc, Chicago, USA). A p value < 0.05 was considered statistically significant.
Results
Two hundred and two rectal cancer patients were included in the research. There were 114 (56.4%) male and 88 (43.6%) female. The median age was 62.5 years (range 33–86). Median follow up was 53.2 months (range 29–88). According to preoperative diagnostics (physical examination, laboratory tests, chest radiography, ultrasound of abdomen and MRI of pelvis) TNM stage was established. Thyrty eight patients (18.5%) had stage II and 164 (81.5%) stage III of the disease. They all received neoadjuvant treatment: long-course radiotherapy (radiation of totally 50.4–54 Gy) and most of them additional chemotherapy (5-fluorouracil or capecitabine). Six to eight weeks after finishing preoperative treatment all patients underwent total mesorectal excision (TME) surgery. One hundred and fifty-two (75%) patients had low anterior resection, of which 2 were without creating anastomosis (Hartmann resection) and 1 was laparoscopic. Fifty-two (25%) patients underwnet abdominoperineal excision. One hundred and sixty-eight (83%) patients received postoperative 5-FU based chemotherapy. The rest 17% of patients did not receive adjuvant therapy because of postoperative complications, preexisting comorbidities or favourable patohistological results.
Pathohistological findings of resected specimens revealed
31 patients (15.3%) with complete tumour response in rectal wall (ypT0). Other results were: ypT1 in 13 patients (6%), ypT2 in 46 (23%), ypT3 in 104 (52%) and ypT4 in 7 patients (4%), respectively.
Lymph nodes in resected specimens
in 133 patients (66%) no tumor cells were found in them (ypN0) and in the 69 patients (34%), the lymph nodes were positive.
After neoadjuvant therapy, TNM stage was reassessed. thirty patients (14.8%) achieved final stage 0 (ypT0N0), which means complete pathologic response to preoperative treatment. Other tumors responded as follows: pooperative stage I was achieved in 45 patients (22.3%), stage II in 52 (25.8%), stage III in 63 (31.2%) and stage IV in 12 patients (5.9%).
Analysing closely the group of patients with complete pathological response (ypT0N0), 17 of them (57%) had preoperatively stage II disease and 13 (43%) stage III. Preoperative stage T was following
cT2 in 6 patients (20%), cT3 23 (77%) and cT4 1 patient (3%). Lymh nodes were preoperatively negative in 17 patients (57%) and cN1 was established in 13 (43%). In none of the patients with pathological complete response cN2 was detected preoperatively, (Table 1, Figure 1).
Table 1.
Results of survival analysis
| Median survival [years] | 95% confidence interval | p (log rank) | |
|---|---|---|---|
| Pooperative stage 0 | 6.6 | 6.1–7.1 | 0.001 |
| pooperative stage I | 6.4 | 5.8–6.9 | |
| Pooperative stage II | 5.5 | 4.9–6.1 | |
| Pooperative stage III | 4.9 | 4.3–5.6 | |
| Pooperative stage IV | 3.7 | 2.8–4.6 | |
| ypT0 | 6.6 | 6.1–6.7 | 0.011 |
| ypT1 | 6.0 | 5.2–6.9 | |
| ypT2 | 6.1 | 5.5–6.7 | |
| ypT3 | 5.3 | 4.8–5.8 | |
| ypT4 | 3.9 | 2.0–5.8 | |
| ypN0 | 6.1 | 5.8–6.5 | < 0.001 |
| ypN1 | 5.2 | 4.4–6.0 | |
| ypN2 | 3.7 | 3.0–4.4 | |
| Preoperative stage II | 5.8 | 5.0–6.6 | 0.389 |
| Preoperative stage III | 5.6 | 5.1–6.0 |
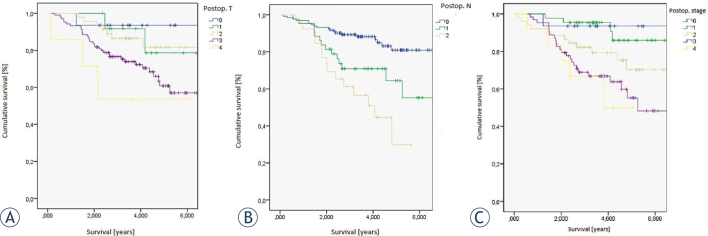
Figure 1.
Survival according to (A) postoperative T (ypT), (B) postoperative N (ypN) and (C) postoperative stage (yS).
The results show that patients with complete pathological response (ypT0N0) have excellent prognosis, as 5-year survival rate exceeds 90%, (72% in postoperative stage II ad 57% in postoperative stage III). Statistically significant are also differences in survival according to preoperative T stage (p = 0.011) and preoperative N stage (p < 0.001). If tumor cells are found in resected specimens, it means worse prognosis, as 5-year survival rate falls from 80 % in ypN0 to 65% in ypN1 and only 30% in ypN2.
According to univariate analysis, statistically important variables were pooperative stage and pooperative T and N. We used proportional hazards model or the Cox regression to check, if any of aforementioned variables, including response to preoperative therapy (considered as postoperative downstaging), act as independent prognostic factors in predicting survival in patients after neoadjuvant therapy. The results are shown in Table 2. ypT, ypN and postoperative stage do not act as independent variables. The only statistically significant independent prognostic factor is the response to neoadjuvant therapy (p < 0.003).
Table 2.
Results of multivariate analysis
| Hazard ratio | 95% confidence interval | p | |
|---|---|---|---|
| ypT | 1.307 | 0.847–2.014 | 0.226 |
| ypN | 1.507 | 0.935–2.428 | 0.092 |
| Postoperative stage | 1.268 | 0.793–2.027 | 0.793 |
| Downstaging (response to preoperative therapy) | 2.725 | 1.4–5.3 | 0.003 |
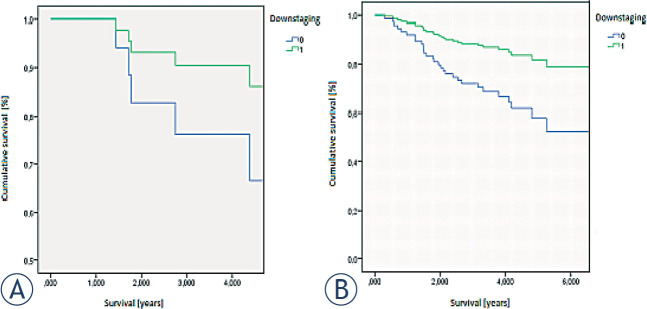
Figure 2 shows differences in survival according to response to neoadjuvant therapy in group of patients with preoperative stage II, compared to group of patients with preoperative stage III. Survival is statistically significantly better if patients respond to neoadjuvant therapy.
Figure 2.
Survival according to response to neoadjuvant therapy (0: no response,1: response): (A) group of patients with preoperative stage II, (B) group of patients with preoperative stage III.
Discussion
Evaluating tumor response to neoadjuvant radiochemotherapy only on the basis of downstaging can be misleading. Tumor can decrease in size significantlly (for example from preoperative T3 to postoperative T2), but there may be no evident tumor regression, which means considerable mass of tumor cells in macroscopically small tumor. On the other hand, despite of no downsizing after neoadjuvant therapy, there may be good regression and very few or no tumor cells are found in the resected surgical specimen.10,14
Complete pathologic response (pCR), which means stage ypT0N0 or in other words no tumor cells in resected surgical specimen, can be detected in 7–30% of patients with locally advanced rectal cancer, treated with neoadjuvant therapy.2,7,8 Our results are comparable with those studies, as we detected 14.8% of pCR. Using statistical analysis, we found out that pCR means excellent prognosis, as 5-year survival rate turned out to be >90% (p = 0.001). Meta analysis of 12 larger studies worldwide reports 90.2% 5-year survival rate in pCR patients (p = 0.0001)15; similar percentage (90% or more) is mentioned in various other studies2,4,16, while others failed to prove relation between pCR and better survival.15 In the literature, strong evidence exists that patients with pCR have very few local recurrences (2–5% in 5 years) and that there are statistically significant differences, if groups of patients with pCR are compared to those who failed to respond to preoperative treatment.4,16 It is important to state that in some no local recurrences at all were found in groups of pCR patients.6,17 Nevertheless, regardless of no local recurrences, chance of distant metastases still exists. Primary tumor can completely respond to neoadjuvant therapy, but the problem is distant micrometastatic focuses, which can stay undetected in the time of primary diagnostics. They can respond to neoadjuvant therapy or not, in the latter case they remain the source of tumor cells even after successful neoadjuvant treatment at the site of primary tumor.15,18
According to our research, pT, pN and postoperative stage all importantly affect survival. Lower pT, no tumor cells in resected lymph nodes and lower postoperative stage mean better prognosis (p = 0.011; < 0.001 and 0.001 for pT, pN and postoperative stage, respectivelly). Nevertheless, none of mentioned variables proved to be statistically significant in multivariate analysis. The only prognostic factor, which acts as independend variable, was response to neoadjuvant therapy, in other words downstaging (p = 0.003). Tumor deposits in local lymph nodes almost invariably mean worse prognosis.
An interesting finding is that in approximately 17% of patients with ypT0, tumor cells in perirectal lymph nodes can still be found. These patients act similar as group of patients with no response to neoadjuvant therapy.6,19
There remains an open question why achieving pCR means good prognosis. pCR is achieved in tumors, which themselves have a favourable biological profile with lesser susceptibility to local recurrences or distant metastases. Various trials tried to find possible biological markers for pCR.2,11,19
Considering data exist about excellent prognosis in patients with pCR but a question about most appropriate therapy in patients with pCR is still unanswered. Could neoadjuvant radiochemotherapy without surgery suffice or might less extensive operation, for example transanal local excision be a better option for them?1,19,20 There are many reasons against TME: it is a mutilating procedure with significant mortality and many long-term consequences (fecal incontinence, urinary and sexual dysfunction). But on the other hand, without surgery we can not reliably assess pCR as accuracy of other methods for assesment of tumour response to preoperative treatment is low.12
Is there any possibility to assess preoperatively, whether patients responded to treatment completely and all tumor cells were destroyed? Clinical complete response (cCR) represents a list of clinical and endoscopic characteristics: whitening of rectal wall mucosa, telangiectasias within mucosa, scars in rectal wall, seen as light stiffness of the wall during the insuflation. If an ulceration, palpable node or stenosis are found during examination, it means incomplete clinical response.12 Two different terms are used: initial cCR, which is assessed immediately after neoadjuvant therapy, and sustained cCR, when cCR is mantained for 10 weeks – 12 months after completing chemoradiotherapy. The problem of this approach is that we do not know anything about nodal status. Namely, in lymph nodes residual tumor cells may still be present.18 Brasilian researchers were the first to introduce so-called »wait-and-see« approach in selected group of patients.16,19 Those patients were not operated, yet were closely followed. Follow up consisted of clinical examination, rigid proctoscopy, biopsies and measurements of serum CEA levels. In this trial only 99 patients with sustained cCR were included. 5-year overall survival was 92.7% and 5-year disease free survival 85%, which is comparable with results in operated patients. According to the results of exsistent trial they concluded that »wait-and-see« is safe and successfull method, but only in carefully selected patients with low-rectal carcinoma and good response to neadjuvant therapy.16,19
Dutch research group defined cCR on the basis of MRI and endoscopy as follows
on MRI no residual tumor is detected or only fibrosis is present; there are no suspicious lymh nodes; endoscopically there can be no residual tumor seen; biopsy must be negative; if in the beginning tumor is palpable at the digitorectal examination, it should be undetectable at the same examination after neoadjuvant therapy. Their testing group numbered 21 patients: oncological outcome was comparable to the outcome in operated patients, 2-year survival was 100%, local recurrence was detected in 2%. Moreover, unoperated patients had significantly less functional complications. Researchers put stress on the importance of assessing nodal status after neoadjuvant therapy when making a decision whether certain patient is appropriate for »wait-and-see« approach. They used MRI to assess nodal status, which was not the case in Brasilian trial. Consequently, the latter included more patients with undetected residual tumor cells in lymph nodes. It might be the reason why oncological outcome in brasilian trial is worse than in the Dutch one.21 Other trials did not present such good results of »wait-and-see« approach, in fact they noted significantly more local recurrences (23–83%) while long term survival could be compared to long term survival in operated patients.
One must point out the limitations of current researches
many of them are small, retrospective studies with relative short follow-up, therefore more extensive trial should be carried out in the future. The most appropriate would be a prospective randomised clinical trial to compare »wait-and-see« approach to standard neoadjuvant radiochemotherapy with total mesorectal excision of rectal cancer. However, random patient assignment to either of research groups could be questionable. An American retrospective trial, which assessed the percentage of patients with preoperatively determined cCR that actually achieved pCR, determined postoperatively. Only a fourth of cCR patients achieved also pCR, what points out, how important is careful selection of patients, suitable for nonoperative treatment.12,22
Our research allowed us to demonstrate that patients with good response to preoperative radiochemotherapy have better prognosis and less recurrences or distant metastases. For them, benefits of neoadjuvant therapy are indisputable. Existent research should be a basis for further researches, with which predictive factors of good or poor respose to radiochemotherapy in a population of patients with locally advanced rectal cancer could be defined. In a population there are always patients with poor or no response to neodjuvant therapy. It is proven that preoperative radiochemotherapy generally (except for patients with pCR) does not improve overall survival. It certainly diminishes possibility of local recurrences, but the main cause of death in rectal cancer patients are usually distant metastases, which can not always be prevented by neoadjuvant therapy.18 Many studies show that high quality of radical total mesorectal excisions overweights multimodal treatment. The question remains whether chemotherapeutics and radiation are really so vital for rectal cancer patients. The fact is that with quality radical mesorectal excision all tumor tissue and lymph nodes are removed.23 TME is mutilating procedure which causes many functional disabilities, but on the other hand radiochemotherapy also has its side effects. One of them are long-term effects because of nerve and vascular damage in perirectal area, which means worsening of anorectal function. It can be much worse after radiochemotherapy than after TME alone.24,25 In the future surveys on posttreatment quality of life is necessary to define most appropriate approach with best oncological and functional outcome in patients, who respond to treatment poorly or do not respond at all.
Footnotes
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.Trakarnsanga A, Ithimakin S, Weiser MR.. Treatment of locally advanced rectal cancer: controversies and questions. World J Gastroenterol. 2012;18:5521–32. doi: 10.3748/wjg.v18.i39.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari L. Fichera A Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterology Report. 2015;3:277–88. doi: 10.1093/gastro/gov039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters KC, van de Velde CJH, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK. et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients – a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 4.Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W. et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–36. doi: 10.1097/01.sla.0000161980.46459.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Aguilar J, Hernandez de Anda E, Sirivongs P., Lee SH, Madoff RD, Rothenberger DA.. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298–304. doi: 10.1097/01.DCR.0000054637.75996.DF. [DOI] [PubMed] [Google Scholar]
- 6.Hughes R, Glynne-Jones R, Grainger J, Richman P, Makris A, Harrison M. et al. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? Int J Colorectal Dis. 2006;21:11–7. doi: 10.1007/s00384-005-0749-y. https://doi.org/10.1007/s00384-005-0749-y [DOI] [PubMed] [Google Scholar]
- 7.Huebner M, Wolff BG, Smyrk TC, Aakre J, Larson DW.. Partial pathologic response and nodal status as most significant prognostic factors for advanced rectal cancer treated with preoperative chemoradiotherapy. World J Surg. 2012;36:675–83. doi: 10.1007/s00268-011-1409-8. [DOI] [PubMed] [Google Scholar]
- 8.Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC. et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250:582–9. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 9.Huh JW, Kim HR, Kim YJ.. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum. 2013;56:698–703. doi: 10.1097/DCR.0b013e3182837e5b. [DOI] [PubMed] [Google Scholar]
- 10.Hermanek P, Merkel S, Hohenberger W.. Prognosis of rectal carcinoma after multimodal treatment: ypTNM classification and tumor regression grading are essential. Anticancer Res. 2013;33:559–66. doi: 10.4143/crt.2015.254. [DOI] [PubMed] [Google Scholar]
- 11.Kawai K, Ishihara S, Nozawa H, Hata K, Kiyomatsu T, Morikawa T. et al. Prediction of pathological complete response using endoscopic findings and outcomes of patients who underwent watchful waiting after chemoradiotherapy for rectal cancer. Dis Colon Rectum. 2017;60:368–375. doi: 10.1097/DCR.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 12.Smith FM, Wiland H, Mace A, Pai RK, Kalady MF.. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014;57:311–315. doi: 10.1097/DCR.0b013e3182a84eba. [DOI] [PubMed] [Google Scholar]
- 13.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th. New York: John Wiley & Sons; 2009. [Google Scholar]
- 14.Dworak O, Keilholz L, Hoffmann A.. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorect Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 15.Martin ST, Heneghan HM, Winter DC.. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 16.Fraser MS, Rao C, Perez RO, Bujko K, Habr-Gama A, Faiz O.. Avoiding radical surgery improves early survival in elderly patients with rectal cancer, demonstrating complete clinical response after neoadjuvant therapy: results of a decision-analytic mode. Dis Colon Rectum. 2015;58:159–171. doi: 10.1097/DCR.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 17.Kuo LJ, Liu MC, Jian JJ, Horng CF, Cheng TI, Chen CM. et al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol. 2007;14:2766–72. doi: 10.1245/s10434-007-9471-z. [DOI] [PubMed] [Google Scholar]
- 18.Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS. et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895–903. doi: 10.1007/s10350-004-6325-7. [DOI] [PubMed] [Google Scholar]
- 19.Sprenger T, Rothe H, Conradi CR, Beissbarth T, Kauffels A, Kitz J. et al. Stage-dependent frequency of lymph node metastases in patients with rectal carcinoma after preoperative chemoradiation: results from the CAO/ARO/AIO-94 trial and from a comparative prospective evaluation with extensive pathological workup. Dis Colon Rectum. 2016;59:377–385. doi: 10.1097/DCR.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 20.Kong JC, Guerra RG, Warrier SK, Ramsay RG, Heriot AG.. Outcome and salvage surgery following “Watch and Wait” for rectal cancer after neoadjuvant therapy: a systematic review. Dis Colon Rectum. 2017;60:335–345. doi: 10.1097/DCR.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 21.Kim NK, Kim MS, Al-Asari SF.. Update and debate issues in surgical treatment of middle and low rectal cancer. J Korean Soc Coloproctol. 2012;28:230–240. doi: 10.1097/SLA.0b013e31829068c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maas M, Beets-Tan RG, Lanbregts DM, Lammering G, Nelemans PJ, Engelen SM. et al. Wait-and-see policy for clinical complete responders after chemoradioation for rectal cancer. J Clin Oncol. 2011;29:4633–40. doi: 10.1200/JCO.2011.37. [DOI] [PubMed] [Google Scholar]
- 23.Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG. et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 448 patients. J Am Coll Surg. 2002;194:131–5. doi: 10.1016/s1072-7515(01)01159-0. [DOI] [PubMed] [Google Scholar]
- 24.Chang KH, Smith MJ, McAnena OJ, Aprjanto AS, Dowdall JF.. Increased use of multidisciplinary treatment modalities adds little to the outcome of rectal cancer treated by optimal total mesorectal excision. Int J Colorectal Dis. 2012;27:1275–83. doi: 10.1007/s00384-012-1440-8. [DOI] [PubMed] [Google Scholar]
- 25.Loos M, Quentmeier P, Schuster T, Nitsche U, Gertler R, Keerl A. et al. Effect of preoperative radio(chemo)therapy on long term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1816–28. doi: 10.1245/s10434-012-2827-z. [DOI] [PubMed] [Google Scholar]