Abstract
Aims
Protein kinase C (PKC) isozymes contribute to the development of heart failure through dysregulation of Ca2+ handling properties and disruption of contractile function in cardiomyocytes. However, the mechanisms by which PKC activation leads to Ca2+ dysfunction are incompletely understood.
Methods and Results
Shortly upon ventricular pressure overload in mice, we detected transient PKC activation that was associated with pulsed actin cytoskeletal rearrangement. In cultured cardiomyocytes, transient activation of PKC promoted long-term deleterious effects on the integrity of the transverse (T)-tubule system, resulting in a significant decrease in the amplitude and increase in the rising kinetics of Ca2+ transients. Treatment with a PKCα/β inhibitor restored the synchronization of Ca2+ transients and maintained T-tubule integrity in cultured cardiomyocytes. Supporting these data, PKCα/β inhibition protected against T-tubule remodeling and cardiac dysfunction in a mouse model of pressure overload-induced heart failure. Mechanistically, transient activation of PKC resulted in biphasic actin cytoskeletal rearrangement, consistent with in vivo observations in the pressure overloaded mouse model. Transient inhibition of actin polymerization or depolymerization resulted in severe T-tubule damage, recapitulating the T-tubule damage induced by PKC activation. Moreover, inhibition of stretch activated channels (SAC) protected against T-tubule remodeling and E-C coupling dysfunction induced by transient PKC activation and actin cytoskeletal rearrangement.
Conclusions
These data identify a key mechanistic link between transient PKC activation and long-term Ca2+ handling defects through PKC-induced actin cytoskeletal rearrangement and resultant T-tubule damage.
Keywords: Cardiomyocytes, protein kinase C, actin cytoskeleton, T-tubules, excitation-contraction coupling
Introduction
Heart failure is a pathologic state that is primarily characterized by a loss of cardiac contractile function. Contractile dysfunction is associated with dysregulation in Ca2+ handling, such as blunted systolic [Ca2+]i elevations, prolonged Ca2+ transient decay and decreased SR Ca2+ contents.[1–3] Hemodynamic and neurohormonal stresses can induce hypertrophy and heart failure via multiple pathways that modulate the biochemical status of Ca2+ handling proteins.[4, 5] Among the many regulators, protein kinase C (PKC) isoenzymes have been found to be crucial mediators of cardiac hypertrophy and heart failure.[6–9] Conventional PKC family members (α, β, γ) not only induce hypertrophic gene expression via phosphorylation-mediated histone deacetylase (HDAC) translocation,[10] but also directly contribute to Ca2+ handling dysfunction in cardiac hypertrophy and heart failure through several reported mechanisms.[11–14].
PKCα is the most abundantly expressed conventional PKC isoform in the myocardium. Long-term overexpression or activation of PKCα in transgenic mice decreases Ca2+ transients and cardiac contractility, thereby enhancing the development of cardiac hypertrophy and heart failure.[11, 12, 15–17] Silencing PKCα or overexpression of dominant negative PKCα or an inhibitor peptide enhances systolic Ca2+ elevation and contraction, which attenuates the development of heart failure.[11, 12, 15, 16] However, activation of PKC is likely a transient event given that diacyl glycerol (DAG), which is essential for the activation of conventional PKCs, is elevated transiently by cytokines and hormones and then returns to baseline[18, 19]. In addition, activation of PKC triggers its ubiquitination and degradation.[20, 21] Others have reported that transient activation of PKCα negatively regulates Ca2+ transients and cardiomyocyte contraction in vivo and in vitro.[11, 22, 23] However, these studies only examined the acute effects of PKCα activation, and it remains unclear whether transient activation of PKC produces long-lasting negative effects on contractile Ca2+ signaling in the heart.
In this study, we examined the long-term effects of transient PKC activation on cardiac function and Ca2+ handling properties. In addition to long-term suppression of Ca2+ transients, we found that transient activation of PKC induced severe T-tubule damage. Mechanistic analysis revealed that transient activation of PKC can lead to long-term detrimental effects on cardiac function through alterations in the actin cytoskeleton, T-tubule remodeling, and Ca2+ handling dysfunction.
Methods
Animal studies
Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 2011) and were approved by the Institutional Animal Care and Use Committee at the University of Iowa. C57Bl/6J mice were purchased from Jackson Laboratories. All experiments were performed in male mice aged 9–10 weeks. All mice were sacrificed by either cervical dislocation or by removal of heart after anesthetized with intraperitoneal (i. p) injection of ketamine/xylazine.
Transaortic Banding (TAB) Surgery
Mice were subjected to pressure overload by TAB surgery as described.[24] Briefly, mice were anesthetized with i.p. ketamine/xylazine (100 mg/kg/5 mg/kg), intubated with a 20-gauge tube and ventilated with a small rodent ventilator (Harvard Apparatus). A thoracotomy was created between the second and third intercostal space and aortic arch visualized. Aortic constriction was performed by tying a 7–0 nylon suture ligature against a 27-gauge needle to yield a narrowing 0.4 mm in diameter, with a reproducible TAB of 65–70%. In sham mice, aortic arch was visualized but not banded. The chest wall was then closed and the pneumothorax evacuated. Enzastaurin was administrated daily (i.p, 30 mg/kg/day) after TAB surgery.
Cardiac function by echocardiography
Transthoracic echocardiograms were performed at 5 weeks after TAB or sham surgery in the University of Iowa Cardiology Animal Phenotyping Core Laboratory using a Vevo 2100 Imager (VisualSonics) as described previously.[24, 25] Briefly, conscious sedation was achieved with midazolam (0.2–0.3 mg s.c.). 2D images were acquired in left ventricle (LV) short- and long-axis planes with a 40-MHz sector-array probe, yielding 100 frames per second. LV mass, volumes, and ejection fractions were calculated with the area-length method. Regions demonstrating akinesis or dyskinesis were visually identified, planimetered, and expressed as percentages of total LV end-diastolic silhouette.
PKC activity assay
At 0–35 days after TAB or sham surgery, mice were euthanized and PKC activity in left ventricular lysates was measured using PKC kinase activity kit from ENZO (ADI-EKS-420A) per the manufacturer’s instructions.
Adult cardiomyocyte culture and adenoviral infection
Isolation and culture of murine left ventricular cardiomyocytes were performed as described previously.[26] Briefly, mice were sacrificed by removing heart after anesthetized with i.p. ketamine/xylazine (100 mg/kg/5 mg/kg). The hearts were Langendorff perfused with Tyrode’s solution with collagenase II. The isolated single cardiomyocytes were plated on coverslips coated with laminin. Immediately after culturing cardiomyocytes, PMA (100 nM) was applied transiently for 30 min followed by medium change. Unless otherwise indicated, bisindolylmaleimide XI (Bis-XI, 5 µM), latrunculin A (1 µM), cytochalasin D (5 µM), Y-27632 (10 µM), Skf96365 (5 µM), or Gd3+ (10 µM) were applied to cells 30 min before addition of PMA and after PMA washout. Jasplakinolide (1 µM) was applied consistently. Empty adenovirus (Ad-empty) or adenoviruses carrying cDNA of rabbit PKCα or dominant negative PKCα (PKCα DN, both from Seven Hills Bioreagents) were applied to freshly isolated and cultured cardiomyocytes at a multiplicity of infection (MOI) of 100 for 40 hrs prior to 30 min application of PMA.
Western blot and immunostaining
Western blotting and immunostaining of left ventricular murine cardiomyocytes was performed as described previously [24, 26] using antibodies against PKCα (sc-208, Santa Cruz), JP2 (SC-51313, Santa Cruz), GAPDH (2118, Cell Signaling) and α-actinin (A7811, Sigma). Immunofluorescence imaging for α-actinin and JP2 was performed using a confocal microscope (Carl Zeiss LSM 510 MicroImaging) as previously described.[27] Cellular fractions of G-actin and F-actin were determined by the G-Actin/F-actin In Vivo Assay Biochem Kit (Cytoskeleton, Inc) per the manufacturer’s instructions.
T-tubule imaging in cultured cells and in situ in whole hearts
Unless otherwise specified, 24 hours after culture, cardiomyocytes were incubated with Di-8-ANNEPES for 30 min to stain T-tubule membrane. For Langendorff-perfused hearts, MM 4–64 (AAT Bioquest) was used to stain T-tubules. Imaging of T-tubules in cultured cells and perfused hearts was performed as described previously.[24, 25, 28, 29] Confocal images were acquired using a 63×, 1.3 NA oil immersion objective mounted on a Zeiss LSM 510 confocal microscope. The regularity and density of T-tubules were analyzed using AutoTT.[28]
Confocal Ca2+ imaging
Unless otherwise specified, 24 hours after culture, cardiomyocytes were loaded with Fluo-4 AM (AAT BioQuest, CA) at 37°C for 30 min, followed by washing with Tyrode’s solution for 15 min before Ca2+ imaging.[26] Confocal images were acquired using a 63×, 1.3 NA oil immersion objective mounted on a Zeiss LSM 510 confocal microscope. Confocal line scanning was used to record Ca2+ signals. Steady state Ca2+ transients were recorded in Tyrode’s solution containing 1.8 mM Ca2+ under field stimulation of 1 Hz. At least 4 steady-state transients for each cell were analyzed and averaged to represent Ca2+ signals of the cell. Ca2+ imaging data were analyzed using CaTeasy.[30] The profile of local Ca2+ transient firing time was indicated by a red line overlapping on the Ca2+ image. The dyssynchrony index, defined by the mean absolute deviation of firing time of each scanning pixel (with every 8 pixels binned), was used to evaluate the dyssynchrony of Ca2+ transients.[30]
Statistics
Data are presented as mean±S.E. Analysis of variance (ANOVA), Student’s t test and mixed model were applied when appropriate. A p value of <0.05 was considered statistically significant.
Results
PKC inhibition attenuates pressure overload-induced T-tubule remodeling in mice
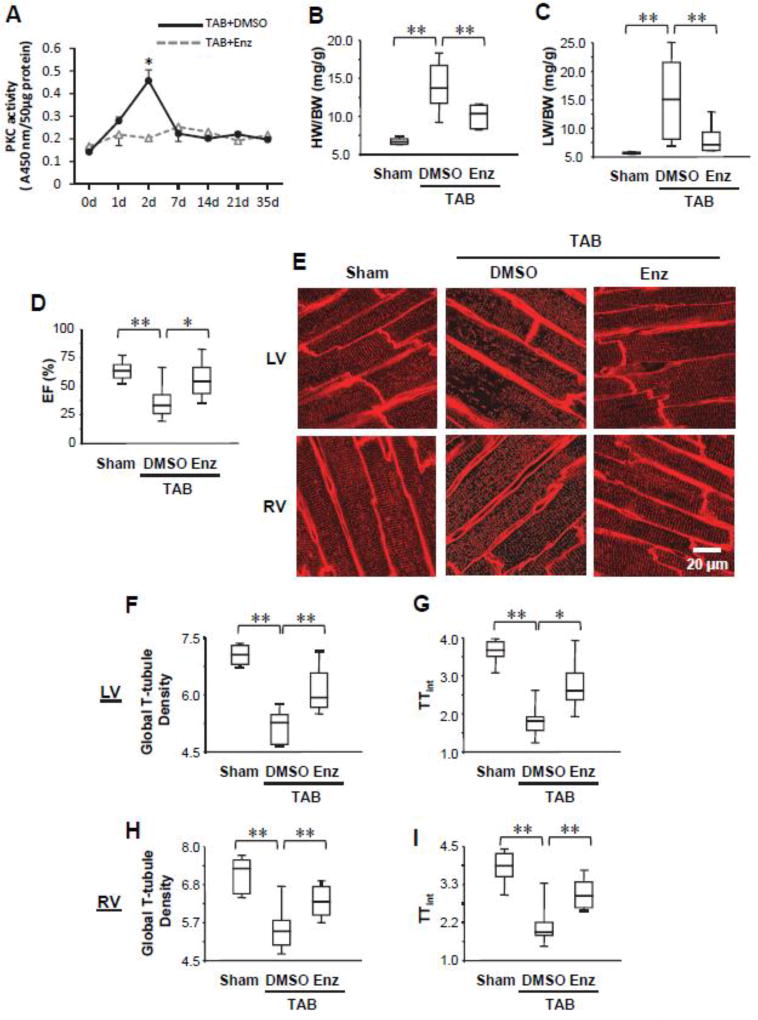
Studies in rat and pig models of heart failure have established that PKC inhibition improves cardiac function,[12, 31, 32] though the mechanism remains better understood. We first assessed the time course of PKC activation in left ventricular myocardium following pressure overload by transverse aortic banding (TAB) in mice. We detected a transient activation of PKC, whose activity peaked 2 days after TAB and then dramatically decreased to a lower level at Day 7 (Fig. 1A). Administration of PKC inhibitor enzastaurin (LY317615), which preferentially inhibits conventional PKC isozymes α and β,[33] abolished the transient activation of PKC in response to TAB (Fig. 1A). These data are consistent with reports by others that treatment with the potent PKC activator PMA results in rapid activation followed by depletion of phorbol ester-sensitive PKC isozymes.[20]
Figure 1. PKC inhibition protects against cardiac dysfunction and T-tubule remodeling following pressure overload.
A, Transient activation of PKC in mouse hearts induced by pressure overload. Administration of PKC inhibitor enzastaurin (Enz) abolished the transient activation of PKC. B–D, Summary data of heart weight/body weight ratio (HW/BW, B), lung weight/body weight ratio (LW/BW, C), and ejection fraction (EF, D) 5 wks after TAB or sham surgery. Some TAB mice were administered Enz, which attenuated the development of heart failure. n ≥ 6 mice/group. E, Representative left ventricular (LV) and right ventricular (RV) in situ confocal T-tubule images after staining with lipophilic marker MM4–64. F–I, Summary data in LV (F, G) and RV (H, I) of global T-tubule density (F, H) and the index of T-tubule integrity (TTint) (G, I). n=6, 10, 6 hearts for sham, TAB+DMSO and TAB+Enz, respectively. For each heart, 10 images were recorded from LV and RV, respectively. The analyzed outputs from the same heart were averaged to represent one heart. * p<0.05, ** p <0.01.
We then assessed whether inhibition of PKC can attenuate murine cardiomyocyte T-tubule remodeling following left ventricular pressure overload using enzastaurin. As compared to control mice, which developed severe heart failure (Figure 1B–D) and T-tubule damage as quantified by AutoTT [28] (Figure 1E–I), administration of the PKC inhibitor significantly attenuated the deterioration of cardiac morphology and function following pressure overload-induced cardiac stress (Figure 1B–D). This improvement in cardiac function was accompanied by protection against deleterious T-tubule remodeling as demonstrated by in situ confocal imaging (Figure 1E–I). These data indicate that activation of PKC in response to pressure overload may contribute to T-tubule damage, which we previously established as a key mediator in loss of Ca2+ handling properties and cardiac dysfunction.[24, 29, 30]
Transient activation of PKC has long-term effects on T-tubule integrity and Ca2+ release function
To investigate whether PKC signaling is directly involved in T-tubule structural remodeling, we performed studies in an in vitro cell culture model. We modeled the transient PKC activation using acute PMA treatment of isolated cardiomyocytes for 30 min, followed by washout of PMA (Figure 2A).31 We established that transient PKC activation with phorbol ester resulted in transient elevation of PKC activity, which decreased to baseline 24 hours after PMA treatment (Figure 2B). This increase in PKC activity is similar to results seen in vivo following TAB, where the peak PKC activity at 2 days after surgery was ~1.6-fold higher than at day 0 (Figure 1A).
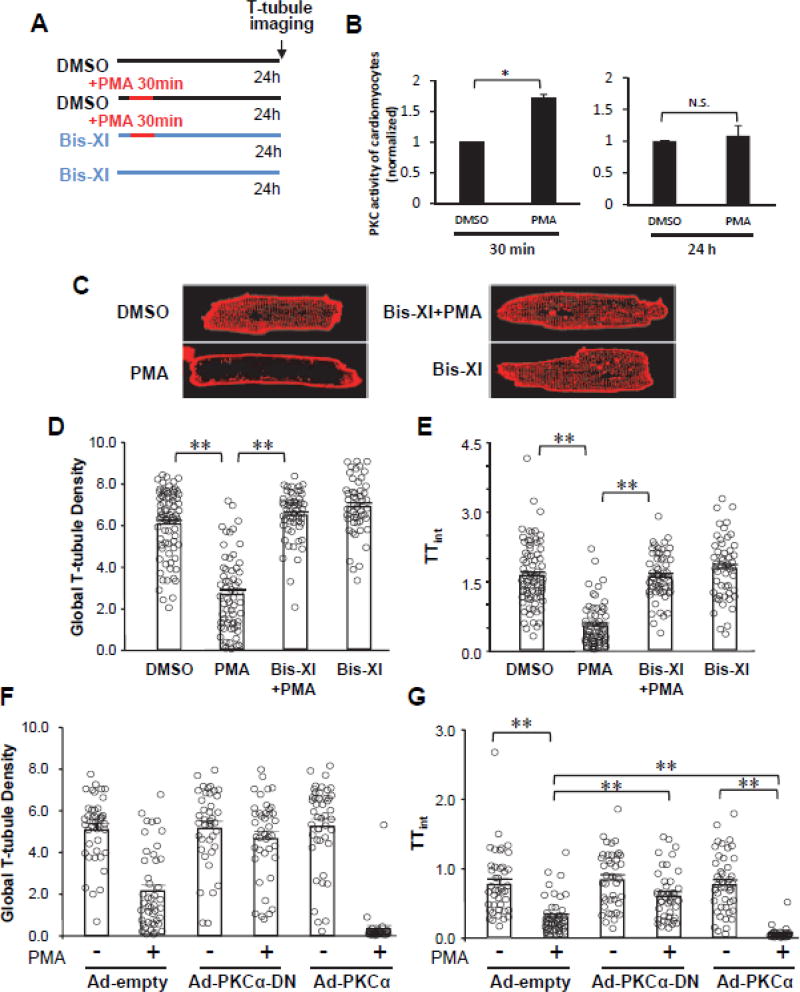
Figure 2. Long-lasting effects of transient PKC activation on T-tubule integrity.
A, Schematic of experimental protocol. B, Transient application of PMA induced pulsed activation of PKC. PKC activity returned to normal levels 24 hrs after transient PMA treatment. C, Representative confocal images of T-tubules in cultured cardiomyocytes after staining with lipophilic marker MM4–64. Cells were treated as diagrammed in (A). D–E, Summary data of global T-tubule density (D) and index of T-tubule integrity (TTint) (E). n=77, 62, 56 and 51 cells for DMSO, PMA, Bis-XI+PMA, Bis-XI respectively. F–G, Summary data for global T-tubule density (F) and TTint (G) in cultured cardiomyocytes infected with empty adenovirus (Ad-empty), adenovirus carrying WT PKC-α (Ad-PKC-α) or dominant negative PKCα (Ad-PKCα-DN). At 40 hrs after adenoviral infection, cardiomyocytes were treated with PMA for 30 min, followed by PMA washout and analysis of T-tubules 24 hrs later as diagrammed in (A). n=42, 45, 40, 42, 45 and 30 cells, respectively. ** p <0.01.
The transient PMA treatment resulted in severe disruption of T-tubule structure 24 hours later as compared to control cells (Figure 2C), while the cardiac sarcomere structure, as indicated by α-actinin, was not impaired (Supplemental Figure S1). Quantitative analysis of T-tubule architecture using AutoTT [28] showed that the global T-tubule density and the index of T-tubule integrity (TTint) were significantly reduced by PMA treatment (Figure 2D, E). The PMA-induced T-tubule damage observed at 24 hours is likely a chronic effect because the T-tubule structure was only mildly affected at 1 and 2 hours after PMA treatment (Supplemental Figure S2).
To determine if the effects of PMA on T-tubule integrity are due to activation of PKC, cardiomyocytes were pretreated with the PKC inhibitor Bisindolylmaleimide XI (Bis-XI) for 30 min prior to the addition of PMA, and then Bis-XI was replenished in the medium following PMA washout as diagrammed in Figure 2A. Pretreatment with Bis-XI rescued the T-tubule structure at 24 hours in PMA-treated cardiomyocytes (Figure 2C–E). These data demonstrate that transient activation of phorbol ester-sensitive PKC is sufficient to induce long-lasting T-tubule damage.
PKCα is a conventional PKC-isozyme that has been shown to play a crucial role in contractile dysfunction in failing hearts.[11, 12, 16, 17] We increased or decreased PKCα activity by overexpressing WT or dominant negative PKCα (dnPKCα), respectively, in cardiomyocytes via adenovirus. Expression of dnPKCα before PMA treatment significantly attenuated the PMA-induced reduction in T-tubule integrity and global T-tubule density, while overexpression of WT PKCα exacerbated PMA-induced T-tubule damage (Figure 2F, G). These data suggest that PKCα contributes, at least in part, to T-tubule remodeling following transient PMA treatment.
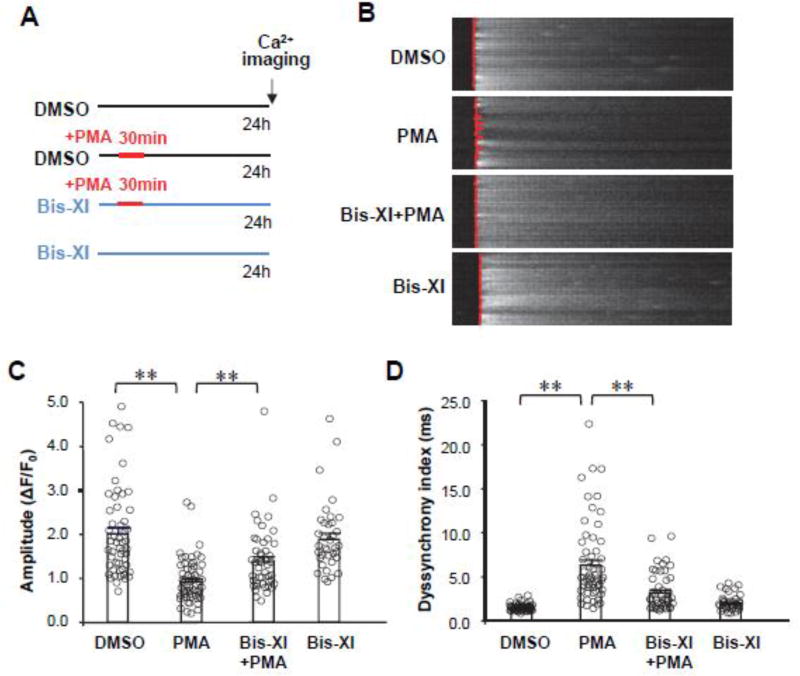
Since T-tubule structural integrity determines the synchronization of CICR, we next examined Ca2+ transients 24 hours after the short-term PMA treatment (Figure 3A). Under 1HZ field stimulation, the amplitude of Ca2+ transients was dramatically decreased in PMA-treated cardiomyocytes as compared to control cells (Figure 3B). Pretreatment with PKC inhibitor Bis-XI attenuated the PMA-mediated suppression of Ca2+ transients. To define the mechanism of this long-term effect, we analyzed the spatial-temporal synchrony of Ca2+ transients using our in-house software, CaTeasy.[30] The synchronization of the firing of Ca2+ transients was significantly decreased by PMA treatment and rescued by PKC inhibition (Figure 3C, D). These data indicate that transient activation of PKC has long-lasting negative effects on both T-tubule ultrastructure and systolic Ca2+ handling.
Figure 3. PKC activation disrupts Ca2+ handling properties in cardiomyocytes.
A, Schematic of experimental protocol. B, Representative cytosolic Ca2+ images under 1-Hz field in cultured cardiomyocytes after treatment as diagrammed in (A). The fluorescence intensity of Ca2+ imaging was normalized to baseline (F0). The red lines overlapping on Ca2+ images show the profile at the moment of Ca2+ transient firing on the scanning line in a point-by-point manner. A straighter line means better synchronization of the Ca2+ transients. C–D, Average data of the amplitude (C) and dyssynchronous index (D) of Ca2+ transients. n=53, 57, 46 and 36 cells, per group, respectively. ** p <0.01.
PKC-mediated T-tubule damage is mediated by biphasic actin cytoskeletal rearrangement
Next, we asked which pathways contribute to T-tubule damage and Ca2+ handling dysfunction induced by transient PMA treatment. Multiple studies from our group and others have established a critical role for the structural protein, junctophilin-2 (JP2), in maintenance of T-tubule integrity and normal cardiac function.[24, 25, 29, 30, 34, 35] JP2 dysregulation through either calpain-mediated proteolysis or microtubule-mediated redistribution to the cell periphery causes aberrant T-tubule remodeling.[27] However, we did not detect any evidence for JP2 downregulation or redistribution in response to acute PMA activation (Supplemental Figure S3). As another potential mechanism, PKC isozymes are important regulators of endocytosis, which could contribute to cell membrane dynamics and T-tubule remodeling. Treatment with the endocytosis inhibitor dynasore did not prevent PMA-induced T-tubule damage (Supplemental Figure S4). PKC isozymes contribute to development of cardiac hypertrophy by inducing phosphorylation dependent HDAC5 exportation.[10] To test whether the T-tubule remodeling induced by PKC activation is secondary to HDAC5 exportation induced hypertrophic responses, we overexpressed a HDAC5 mutant S259/498A, which is unable to be phosphorylated by PKC and stays in nucleus consistently.[10] T-tubule imaging analysis showed that neutralizing HDAC5 nuclear efflux by HDAC5 mutant S259/498A didn’t prevent T-tubule remodeling induced by PKC activation (Supplemental Figure S5), suggesting that PKC activation-induced T-tubule damage is not dependent on the effect of PKC on hypertrophic gene expression.
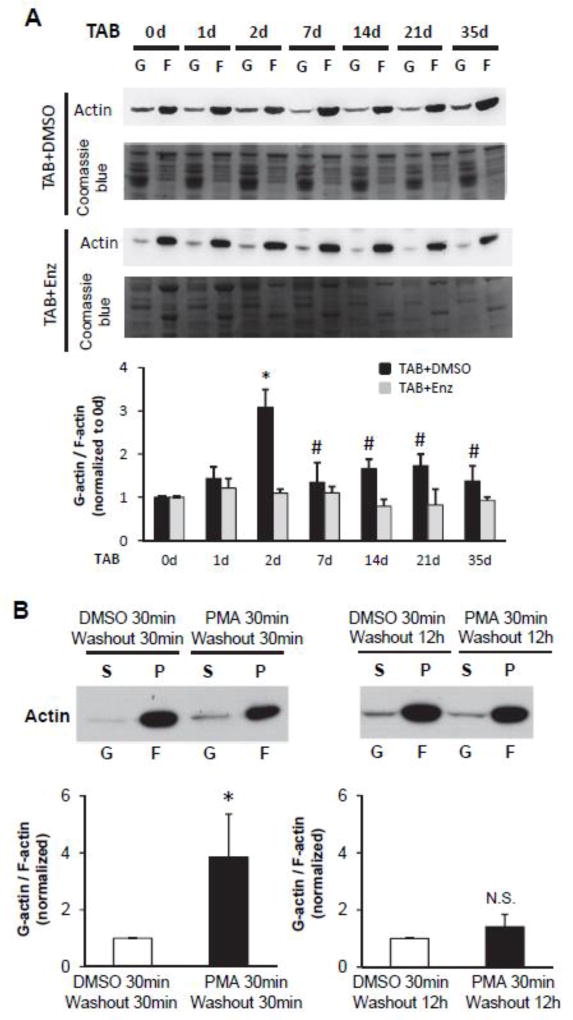
Since the actin cytoskeleton impacts the subcellular distribution of Ca2+ handling proteins and T-tubule ultrastructure,[36, 37] and PKCs have been reported to regulate the actin cytoskeleton network,[38] we hypothesized that a possible mechanism for PKC-mediated T-tubule remodeling is through reorganization of the actin cytoskeleton. We first examined actin cytoskeletal rearrangement (polymerization/depolymerization) during pressure overload-induced heart failure using cytoskeletal fractionation. Actin depolymerization was sharply increased upon pressure overload, as evidenced by an increase in the ratio of G-actin monomers to F-actin polymers on Day 2 after TAB (Figure 4A). The depolymerization ratio dropped to sham levels on Day 7 after TAB (Figure 4A), coinciding with the PKC activation pattern (Figure 1A). In addition, administration of PKC inhibitor abolished the transient actin depolymerization in response to TAB, indicating that this cytoskeletal rearrangement is PKC-dependent (Figure 4A).
Figure 4. PKC activation mediates a biphasic rearrangement of the actin cytoskeleton.
A, Western blot of G- and F-actin and summary data of the ratio of G-actin vs. F-actin in left ventricular myocardium after TAB in mice. PKC inhibitor enzastaurin (Enz) abolished actin depolymerization. n=3 hearts for each time point. * p<0.05 vs 0 d TAB+DMSO group; # p<0.05 vs 2d TAB+DMSO group. B, Western blot of G- and F-actin and summary data of the ratio of G-actin vs. F-actin in cultured cardiomyocytes 30 min or 12 hrs after transient treatment with PMA as in Figure 2 followed by cytoskeletal fractionation. The soluble actin in the supernatant (S) represents G-actin, and the insoluble actin in the pellet (P) corresponds with F-actin. n=3 batches of cells; * p < 0.05 vs. DMSO.
We then investigated whether this biphasic pattern of actin cytoskeletal rearrangement can be recapitulated in our cultured cardiomyocyte model with transient PKC activation. Short-term PMA treatment resulted in enhanced actin depolymerization at 1 hr after the start of 30-min PMA treatment (Figure 4B). This effect was normalized at 12 hours after PMA exposure, indicative of a biphasic rearrangement in the actin cytoskeleton in response to PMA treatment.
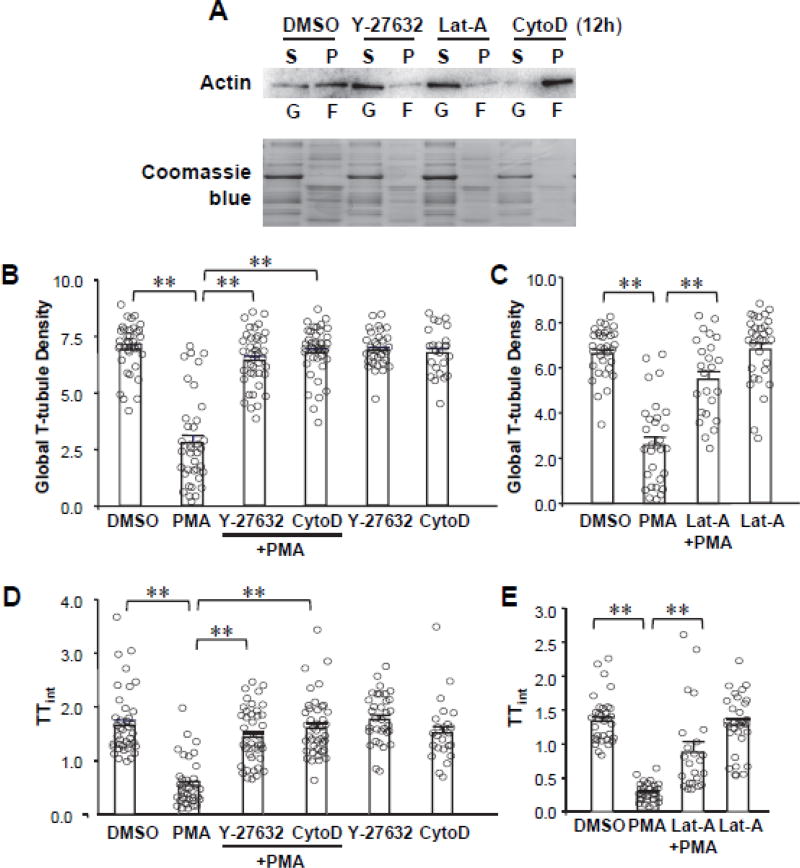
To investigate whether rearrangement of the actin cytoskeleton is involved in PKC-induced T-tubule remodeling, we applied the Rho-associated protein kinase (ROCK) inhibitor Y-27632 (which inhibits F-actin polymerization), the F-actin disruptor latrunculin A (Lat-A, chelates G-actin monomers), or the F-actin specific binding reagent cytochalasin D (Cyto-D) to cultured cardiomyocytes for 30 min prior to PMA treatment. After 30 min PMA treatment and washout, these reagents were maintained in the cell culture media (until T-tubule imaging was performed, i.e., at 24 hours). We first confirmed that treatment of cultured cardiomyocytes with these agents produced the anticipated effects on the G-actin/F-actin ratio (Figure 5A). Stabilization of F-actin with Cyto-D (for 24 hours) protected against T-tubule remodeling in response to transient PMA treatment (Figure 5B, D), consistent with a previous report.[37] Very surprisingly, treatment with F-actin disruptors Lat-A and Y-27632 (for 24 hours) also protected against PMA-induced T-tubule damage (Figure 5B–E).
Figure 5. Effect of PKC activation on cytoskeletal dynamics.
A, Western blot of G- and F-actin in cytoskeletal fractions confirms the effect of Y-27632, Latrunculin-A (Lat-A) and Cytochalasin D (CytoD) on F-actin dynamics. B–E, Summary data of global T-tubule density (B, C) and index of T-tubule integrity (TTint, D, E) following transient PKC activation with PMA as in Figure 2A in the presence (continuously, for 24 hours) or absence of cytoskeletal disrupting agents (Lat-A and Y-27632) or stabilizing agent (CytoD). In B and D, n=39, 37, 41, 41, 33 and 24 cells per group, respectively. In C and E, n= 33, 30, 24 and 31 cells per group, respectively.
We next examined whether the de novo formation of F-actin mediates T-tubule remodeling using Jasplakinolide (Jasp), which not only stabilizes F-actin but also promotes de novo F-actin polymerization by increasing the actin nucleation. Continuous treatment with Jasp severely damaged T-tubule integrity observed 24 hours later (Figure S6B–C), implicating excess de novo F-actin synthesis damages T-tubules.
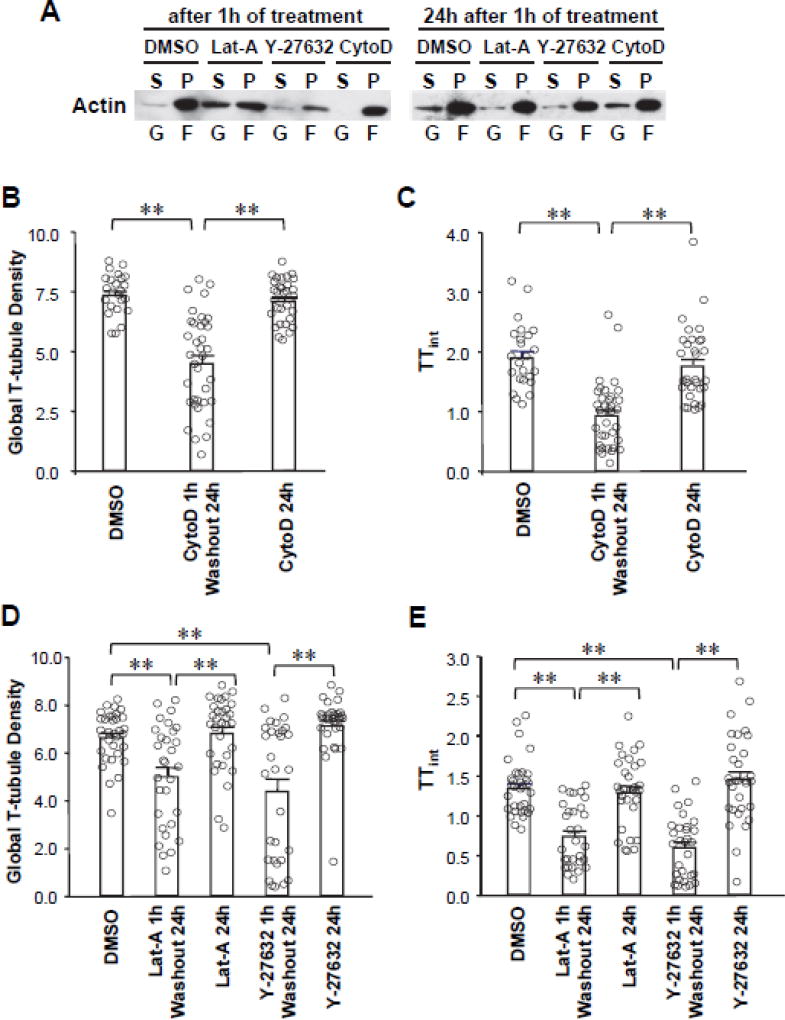
The paradoxical protective effect of the F-actin stabilizer and disruptors led us to hypothesize that abrupt rearrangement of the actin cytoskeleton is detrimental for the T-tubule ultrastructure following transient PKC activation. To test this hypothesis, we established a simplified model of F-actin biphasic rearrangement by transiently treating cardiomyocytes with F-actin stabilizer (Cyto-D) or disruptors (Lat-A and Y-27632) in the absence of PMA. Cytoskeletal fractionation demonstrated that transient treatment with Lat-A, Y-27632 or Cyto-D for 1 hour resulted in biphasic F-actin rearrangement (Figure 6A) and subsequent T-tubule remodeling observed 24 hours later (Figure 6B–E). Importantly, the degree of T-tubule damage with these agents (in the absence of PMA) was similar to the long-term T-tubule damage observed with transient PMA treatment. In contrast, chronical treatment with cytoskeletal stabilizer CytoD (B–C) or disrupters (Lat-A, Y-27632, D–E) had no effects on T-tubule integrity. Thus, these data suggest that PKC-induced T-tubule damage is dependent on the dynamic F-actin rearrangement.
Figure 6. PKC activation mediates T-tubule remodeling through biphasic cytoskeletal rearrangement.
A, Analysis of F-actin biphasic rearrangement by transiently treating cardiomyocytes with F-actin disruptors (Lat-A and Y-27632) or stabilizer (Cyto-D). Left blot demonstrates that a change in the G-actin/F-actin ratio at 1 hr after treatment. Right blot demonstrates that, 24 hours after washout of the reagents, the G-actin/F-actin ratio returned to baseline. B–E, Summary data of global T-tubule density (B, D) and index of T-tubule integrity (TTint, C, E) in cardiomyocytes transiently (1h treatment, 24h washout) or chronically (24h treatment) treated with cytoskeletal stabilizer CytoD (B, C) or disrupters (Lat-A or Y-27632, D, E). In B and C, n=24, 36 and 31 cells per group, respectively. In D and E, n= 33, 30, 31, 30 and 31 cells per group, respectively. ** p <0.01.
Blocking Stretch Activated Channels (SACs) attenuates PKC-induced T-tubule damage
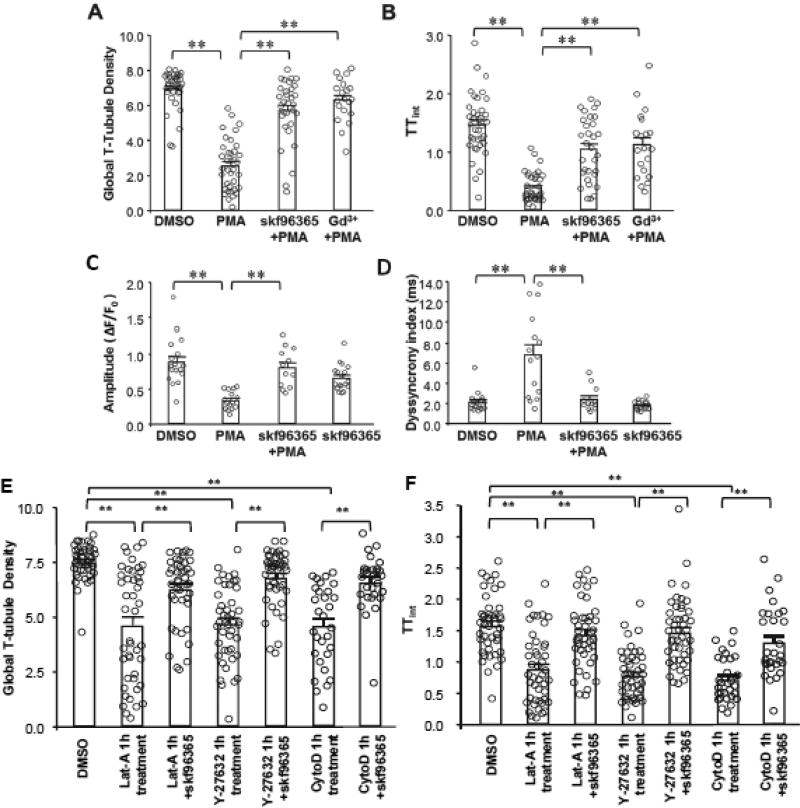
Next we investigated the mechanisms underlying T-tubule damage induced by actin rearrangement. Since the actin cytoskeleton determines the cell’s morphology and surface tension, we tested the hypothesis that actin rearrangement may alter the plasma membrane shearing force, which could trigger T-tubule damage. Given the stretch activated channels (SACs) serve as mechanical sensors on cell membrane, we tested whether these channels are involved in T-tubule damage induced by transient PKC activation or cytoskeletal perturbation. Treatment with the widely-used SAC inhibitors SKF96365 or gadolinium (Gd3+) significantly attenuated long-term T-tubule damage (at 24 hours) induced by transient PMA treatment (Figure 7A, B). Consistent with the improved T-tubule integrity, SKF96365 also improved the amplitude and synchronization of Ca2+ transients in cardiomyocytes treated with PMA (Figure 7C, D). SKF96365 also prevented T-tubule damage induced by transient application of F-actin disruptors (Lat-A and Y-27632) or stabilizer (Cyto-D), as well as continuous application of activator of de novo F-actin growth (Jasp, 24 hours) (Figure S6B–C). Taken together, these data implicate SAC activity in the mechanism of PKC-mediated T-tubule remodeling and Ca2+ handling dysfunction.
Figure 7. Stretch-activated channel activity is essential for biphasic cytoskeletal rearrangement-induced loss of T-tubule integrity.
A–B, Protective effect of SAC inhibitor skf96365 and TRP channel inhibitor gadolinium (Gd3+) on PMA (30 min)-induced T-tubule disruption as measured by global T-tubule density (A) and TTint (B). skf96365 / Gd3+ were incubated in cultured cardiomyocytes continuously. PMA were applied 30 mins after skf96365 / Gd3+ addition, for only 30 mins. T-tubule images were acquired at 24 hours. n=38, 37, 31 and 20 cells per group, respectively. C–D, Protective effect of skf96365 on PMA-induced disruption of the amplitude (C) and synchrony (D) of Ca2+ transients. n=17, 15, 12 and 18 cells per group, respectively. E–F, Protective effect of SAC inhibitor skf96365 on biphasic F-actin remodeling induced T-tubule disruption. skf96365 were incubated in cultured cardiomyocytes continuously. F-actin modulators (Lat-A, Y-27632 and Cyto-D) were started 30 min after skf96365 addition, for only 1 hour. T-tubule images were acquired at 24 hours. E: Summary data of global T-tubule density; F: Summary data of TTint. In G and H, n= 45, 45, 44, 45, 46, 30 and 29 cells per group, respectively. ** p <0.01.
Discussion
Studies using pharmacologic or genetic manipulation of PKC expression/activation have provided clear evidence for the role of PKC in maintaining cardiac function. These data suggest that PKC inhibitors may have a place in the armamentarium of heart failure therapeutics, though mechanistic insight is lacking. Our objective in this study was to define the molecular events whereby PKC activation results in long-lasting negative effects on cardiac function. Our data provide a novel mechanism by which PKC mediates Ca2+ handling dysfunction and heart failure. First we identified a link between PKC activation and T-tubule remodeling, which we have previously established is a mediator of E-C coupling dysfunction and heart failure. Second, our data revealed that T-tubule organization is likely stabilized by the actin cytoskeletal network. Finally, inhibition of SAC prevented PMA-induced T-tubule damage and Ca2+ handling dysfunction. Taken together, this study provides unique insights into how transient activation of PKC results in long-term detrimental effects in the heart and identifies additional potential therapeutic opportunities in addition to direct inhibition of PKC.
Numerous studies have implicated increased expression and activity of PKC in loss of cardiac function and Ca2+ handling properties, yet the molecular and cellular pathways have remained poorly defined. Previous studies suggested that PKC-mediated phosphorylation of phosphatase inhibitor 1 results in activation of protein phosphatase 1 and subsequent dephophorylation of phospholamban. Dephosphorylation of phospholamban inhibits SERCA, leading to attenuated SR Ca2+ restoration and decreased systolic Ca2+ release.[11] Based on this reported mechanism, the transient activation of PKC, which we observed in pressure overload-induced cardiac hypertrophy and heart failure model in the present study, should not have long-term effects on the Ca2+ transients, e.g., after PKC activity is reduced. However, we found that transient activation of PKC resulted in a dramatic decrease in the amplitude of Ca2+ transients at the 24-hr time point in culture condition. These data suggest that another mechanism contributes to Ca2+ handling dysfunction, in particular in response to transient PKC activation. We found that T-tubule damage occurs in response to transient PKC activation, which provides an underlying mechanism for E-C coupling dysfunction since T-tubule integrity is essential for normal CICR. This discovery is especially important for understanding the process of heart failure given that production of DAG, which activates conventional PKCs, is usually transient due to DAG metabolism.[18, 19] In the scenario of failing hearts, PKC-induced T-tubule damage as well as SR Ca2+ content depletion may represent parallel pathways that contribute to loss of normal CICR.
Mechanistically, we found that transient PKC activation is associated with a bimodal shift in F-actin homeostasis, which could consequentially induce T-tubule damage. It is well known that actin cytoskeleton determines the morphology of cells. [39, 40]. Actin cytoskeleton participates in maintenance of membrane subdomains such as intercalated discs, T-tubules, caveolae as well as in regulating microtubule-based targeted delivery of trafficking (e.g., Cx43, Bin1).[37, 41–43] Our study substantiates previous findings by demonstrating that the consistent presence of cytoskeletal disrupters or stabilizers preserved T-tubule integrity following transient PKC activation. However, we also found that short-term treatment with these reagents followed by washout induced T-tubule disruption, phenocopying transient PKC activation. This was true for both stabilizers and disrupters, suggesting a role for dynamic actin rearrangement in maintaining T-tubule integrity. Our data with Jasplakinolide (Jasp), which is a cytoskeletal stabilizer as well as an inducer of de novo F-actin formation, suggest that enhanced de novo F-actin formation promotes T-tubule remodeling. It is important to note that Cyto-D is typically considered to be an F-actin disruptor, though our data and published reports in cardiomyocytes suggest that Cyto-D stabilizes F-actin.[37] Finally, our data indicate that the role of the actin cytoskeleton in T-tubule structure is more complex than previously suggested.
A number of studies have linked elevated myocardial workload to T-tubule remodeling,[25, 44–46] indicating a causative role of mechanical overload in T-tubule damage. Cardiomyocyte cytoskeleton allows an integrative connection to the extracellular matrix and thereby senses biomechanical stress.[47] In response to mechanical overload, cytoskeleton translates the abnormal workload into a number of adaptive or maladaptive cellular events, including changes in cell stiffness and T-tubule structure. There is growing evidence suggesting that the stiffness of cardiomyocytes, which is determined by density and biomechanical properties of cytoskeleton, contributes to the transition from adaptive to maladaptive hypertrophy.[48–53] Sustained pressure overload and wall stress induce a substantial increase of microtubule cytoskeleton density,[53] which increases myocyte stiffness and disrupts T-tubule structure as shown in our previous study,[27] consequently impairing contractility.[51] In the present study, we observed a PKC-dependent actin cytoskeleton disassembly occurs shortly upon pressure overload (Figure 1). We consider this as an immediate adaptive cellular event, by which cardiomyocyte reduces its stiffness in order to avoid mechanical injury when workload suddenly rises. However, coinciding with the transient activation of PKC, the disassembly of F-actin is not sustainable. F-actin reassembly ensues and results in a fluctuation in F-actin which likely contributes, at least in part, to the maladaptive T-tubule remodeling.
Our data also provide the first evidence linking SAC activity with T-tubule structure. SACs are ion channels that respond to mechanical deformation by changes in open probability. It is well-established that myocardial stretch can modulate the excitation of cardiomyocytes by alterations in the action potential and Ca2+ transients.[54, 55] This modulation is referred to as a mechano-electric feedback, and SACs have been implicated as possible mechano-transducers in this process. The actin cytoskeleton determines the mechanical tension of cell membrane, and several studies indicate that SACs can be modulated by actin rearrangement.[56, 57] We found that inhibition of SACs prevents F-actin remodeling-induced T-tubule damage, raising the possibility that activation of SACs in response to actin cytoskeletal rearrangement may induce signals, such as local Ca2+ changes, that trigger downstream pathways that directly impact on T-tubule integrity. The mechanisms by which SACs mediate T-tubule damage remain unclear and warrant further study.
PKC activation has been shown to be responsible for the positive inotropic effect of receptor activation in cardiomyocytes.[22] While our interpretation of our data is that transient PKC activation triggers a signaling cascade that results in long-term deleterious effects, it is possible that moderate PKC activation plays a beneficial role in the short-term. We found that 1–2 hours of PMA treatment had no significant effect on T-tubule integrity. While we interpret these findings to mean that the deleterious effects occur in the long-term, it is possible that a beneficial mechanism is activated in the short-term yet offset by progressive T-tubule damage over time.
T-tubule remodeling is a common phenotype observed in almost all cardiomyopathies and contributes to cardiac dysfunction via disruption of E-C coupling.[58, 59] Accordingly, therapeutic strategies that preserve the T-tubule network could improve cardiac function and attenuate the development of cardiac diseases.[60, 61] In this study, we identified three potential approaches to protect against T-tubule remodeling in cardiomyocytes: 1) inhibition of PKC activity; 2) inhibition of actin rearrangement via cytoskeletal disruptors or stabilizers; and 3) inhibition of SACs. While specific inhibitors of SAC have yet to be identified, several inhibitors of PKC, including PKCα/β inhibitors ruboxistaurin (LY333531) and enzostaurin (LY317615), are currently under development as anti-cancer agents.[62, 63] Moreover, these agents have proven effective in pig and rat models of heart failure.[31, 32] Our data add to these studies by demonstrating that enzastaurin protects against T-tubule remodeling and cardiac dysfunction following pressure overload-induced cardiac stress in mice, setting the stage for use of PKC inhibitors to treat heart failure in humans.
Supplementary Material
Highlights.
Following pressure overload, PKC is transiently activated (peak at Day 2);
PKC transient activation induces long term detrimental effects on T-tubule integrity and E-C coupling function in cardiomyocytes through PKC-induced actin cytoskeletal rearrangement;
Inhibiting stretch activated channels protect against T-tubule remodeling and E-C coupling dysfunction induced by transient PKC activation and actin cytoskeletal rearrangement.
PKC inhibition protects against T-tubule remodeling and cardiac dysfunction in pressure overload-induced heart failure model.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [R01 HL090905 & HL130346]; the United States Department of Veterans Affairs Biomedical Laboratory Research [Merit Review Award I01BX002334]; the American Heart Association [Scientific Development Award 16SDG30820003]; and China Natural Science Foundation [NSF 81770395, 57201701 and 81570293]. The contents do not represent the views of the United States Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Conflict of interest: none declared.
References
- 1.Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92(6):651–8. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 2.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103(11):4305–10. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, Takano H, Hiroi Y, Ueki K, Tobe K, et al. Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J Clin Invest. 1995;96(1):438–46. doi: 10.1172/JCI118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–71. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 6.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99(3):384–91. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 7.Dorn GW, 2nd, Tepe NM, Wu G, Yatani A, Liggett SB. Mechanisms of impaired beta-adrenergic receptor signaling in G(alphaq)-mediated cardiac hypertrophy and ventricular dysfunction. Mol Pharmacol. 2000;57(2):278–87. [PubMed] [Google Scholar]
- 8.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, Walsh RA, King GL. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A. 1997;94(17):9320–5. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115(3):527–37. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24(19):8374–85. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10(3):248–54. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 12.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, Kimball TF, Hewett TE, Dorn GW, 2nd, Koch WJ, Molkentin JD. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114(6):574–82. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87(12):1095–102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280(1):207–14. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 15.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW., 2nd Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res. 2003;93(11):1111–9. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- 16.Hambleton M, York A, Sargent MA, Kaiser RA, Lorenz JN, Robbins J, Molkentin JD. Inducible and myocyte-specific inhibition of PKC-alpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293(6):H3768–71. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Chen X, Macdonnell SM, Kranias EG, Lorenz JN, Leitges M, Houser SR, Molkentin JD. Protein kinase C{alpha}, but not PKC{beta} or PKC{gamma}, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res. 2009;105(2):194–200. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habenicht AJ, Glomset JA, King WC, Nist C, Mitchell CD, Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981;256(23):12329–35. [PubMed] [Google Scholar]
- 19.Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986;261(19):8597–600. [PubMed] [Google Scholar]
- 20.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18(2):839–45. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang BS, French OG, Sando JJ, Hahn CS. Activation-dependent degradation of protein kinase C eta. Oncogene. 2000;19(37):4263–72. doi: 10.1038/sj.onc.1203779. [DOI] [PubMed] [Google Scholar]
- 22.Capogrossi MC, Kaku T, Filburn CR, Pelto DJ, Hansford RG, Spurgeon HA, Lakatta EG. Phorbol ester and dioctanoylglycerol stimulate membrane association of protein kinase C and have a negative inotropic effect mediated by changes in cytosolic Ca2+ in adult rat cardiac myocytes. Circ Res. 1990;66(4):1143–55. doi: 10.1161/01.res.66.4.1143. [DOI] [PubMed] [Google Scholar]
- 23.Capogrossi MC, Kachadorian WA, Gambassi G, Spurgeon HA, Lakatta EG. Ca2+ dependence of alpha-adrenergic effects on the contractile properties and Ca2+ homeostasis of cardiac myocytes. Circ Res. 1991;69(2):540–50. doi: 10.1161/01.res.69.2.540. [DOI] [PubMed] [Google Scholar]
- 24.Guo A, Zhang X, Iyer VR, Chen B, Zhang C, Kutschke WJ, Weiss RM, Franzini-Armstrong C, Song LS. Overexpression of junctophilin-2 does not enhance baseline function but attenuates heart failure development after cardiac stress. Proc Natl Acad Sci U S A. 2014;111(33):12240–5. doi: 10.1073/pnas.1412729111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107(4):520–31. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo A, Cala SE, Song LS. Calsequestrin accumulation in rough endoplasmic reticulum promotes perinuclear Ca2+ release. J Biol Chem. 2012;287(20):16670–80. doi: 10.1074/jbc.M112.340927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, Yuan C, Kutschke W, Zimmerman K, Weiss RM, Wehrens XH, Hong J, Johnson FL, Santana LF, Anderson ME, Song LS. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation. 2014;129(17):1742–50. doi: 10.1161/CIRCULATIONAHA.113.008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo A, Song LS. AutoTT: automated detection and analysis of T-tubule architecture in cardiomyocytes. Biophys J. 2014;106(12):2729–36. doi: 10.1016/j.bpj.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, Kutschke W, Zimmerman K, Weiss RM, Zingman L, Anderson ME, Wehrens XH, Song LS. Critical roles of Junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovasc Res. 2013 doi: 10.1093/cvr/cvt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo A, Hall D, Zhang C, Peng T, Miller JD, Kutschke W, Grueter CE, Johnson FL, Lin RZ, Song LS. Molecular Determinants of Calpain-dependent Cleavage of Junctophilin-2 Protein in Cardiomyocytes. J Biol Chem. 2015;290(29):17946–55. doi: 10.1074/jbc.M115.652396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyle AJ, Kelly DJ, Zhang Y, Cox AJ, Gow RM, Way K, Itescu S, Krum H, Gilbert RE. Inhibition of protein kinase C reduces left ventricular fibrosis and dysfunction following myocardial infarction. J Mol Cell Cardiol. 2005;39(2):213–21. doi: 10.1016/j.yjmcc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Ladage D, Tilemann L, Ishikawa K, Correll RN, Kawase Y, Houser SR, Molkentin JD, Hajjar RJ. Inhibition of PKCalpha/beta with ruboxistaurin antagonizes heart failure in pigs after myocardial infarction injury. Circ Res. 2011;109(12):1396–400. doi: 10.1161/CIRCRESAHA.111.255687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, Banks C, Capen A, Goode R, Lewis JE, Sams L, Huss KL, Campbell RM, Iversen PW, Neubauer BL, Brown TJ, Musib L, Geeganage S, Thornton D. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65(16):7462–9. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 34.Guo Y, VanDusen NJ, Zhang L, Gu W, Sethi I, Guatimosim S, Ma Q, Jardin BD, Ai Y, Zhang D, Chen B, Guo A, Yuan GC, Song LS, Pu WT. Analysis of Cardiac Myocyte Maturation Using CASAAV, a Platform for Rapid Dissection of Cardiac Myocyte Gene Function In Vivo. Circ Res. 2017;120(12):1874–1888. doi: 10.1161/CIRCRESAHA.116.310283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123(9):979–88. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium. 2005;38(5):515–26. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Tian Q, Pahlavan S, Oleinikow K, Jung J, Ruppenthal S, Scholz A, Schumann C, Kraegeloh A, Oberhofer M, Lipp P, Kaestner L. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. J Mol Cell Cardiol. 2012;52(1):113–24. doi: 10.1016/j.yjmcc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18(3):276–84. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Lacayo CI, Pincus Z, VanDuijn MM, Wilson CA, Fletcher DA, Gertler FB, Mogilner A, Theriot JA. Emergence of large-scale cell morphology and movement from local actin filament growth dynamics. PLoS Biol. 2007;5(9):e233. doi: 10.1371/journal.pbio.0050233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee K, Ishii K, Pillalamarri V, Kammin T, Atkin JF, Hickey SE, Xi QJ, Zepeda CJ, Gusella JF, Talkowski ME, Morton CC, Maas RL, Liao EC. Actin capping protein CAPZB regulates cell morphology, differentiation, and neural crest migration in craniofacial morphogenesisdagger. Hum Mol Genet. 2016;25(7):1255–70. doi: 10.1093/hmg/ddw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epifantseva I, Shaw RM. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim Biophys Acta. 2017 doi: 10.1016/j.bbamem.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basheer WA, Xiao S, Epifantseva I, Fu Y, Kleber AG, Hong T, Shaw RM. GJA1-20k Arranges Actin to Guide Cx43 Delivery to Cardiac Intercalated Discs. Circ Res. 2017;121(9):1069–1080. doi: 10.1161/CIRCRESAHA.117.311955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong T, Shaw RM. Cardiac T-Tubule Microanatomy and Function. Physiol Rev. 2017;97(1):227–252. doi: 10.1152/physrev.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frisk M, Ruud M, Espe EK, Aronsen JM, Roe AT, Zhang L, Norseng PA, Sejersted OM, Christensen GA, Sjaastad I, Louch WE. Elevated ventricular wall stress disrupts cardiomyocyte t-tubule structure and calcium homeostasis. Cardiovasc Res. 2016;112(1):443–51. doi: 10.1093/cvr/cvw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrahim M, Kukadia P, Siedlecka U, Cartledge JE, Navaratnarajah M, Tokar S, Van Doorn C, Tsang VT, Gorelik J, Yacoub MH, Terracciano CM. Cardiomyocyte Ca2+ handling and structure is regulated by degree and duration of mechanical load variation. J Cell Mol Med. 2012;16(12):2910–8. doi: 10.1111/j.1582-4934.2012.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahim M, Terracciano CM. Reversibility of T-tubule remodelling in heart failure: mechanical load as a dynamic regulator of the T-tubules. Cardiovasc Res. 2013;98(2):225–32. doi: 10.1093/cvr/cvt016. [DOI] [PubMed] [Google Scholar]
- 47.Sequeira V, Nijenkamp LL, Regan JA, van der Velden J. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochim Biophys Acta. 2014;1838(2):700–22. doi: 10.1016/j.bbamem.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Roe AT, Aronsen JM, Skardal K, Hamdani N, Linke WA, Danielsen HE, Sejersted OM, Sjaastad I, Louch WE. Increased passive stiffness promotes diastolic dysfunction despite improved Ca2+ handling during left ventricular concentric hypertrophy. Cardiovasc Res. 2017;113(10):1161–1172. doi: 10.1093/cvr/cvx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111(6):774–81. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 50.Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45(2):273–8. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 51.Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science. 2016;352(6284):aaf0659. doi: 10.1126/science.aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper Gt. Cardiocyte cytoskeleton in hypertrophied myocardium. Heart Fail Rev. 2000;5(3):187–201. doi: 10.1023/A:1009836918377. [DOI] [PubMed] [Google Scholar]
- 53.Cooper Gt. Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2006;291(3):H1003–14. doi: 10.1152/ajpheart.00132.2006. [DOI] [PubMed] [Google Scholar]
- 54.Peyronnet R, Nerbonne JM, Kohl P. Cardiac Mechano-Gated Ion Channels and Arrhythmias. Circ Res. 2016;118(2):311–29. doi: 10.1161/CIRCRESAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res. 2009;104(6):787–95. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staruschenko A, Negulyaev YA, Morachevskaya EA. Actin cytoskeleton disassembly affects conductive properties of stretch-activated cation channels in leukaemia cells. Biochim Biophys Acta. 2005;1669(1):53–60. doi: 10.1016/j.bbamem.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Ito S, Suki B, Kume H, Numaguchi Y, Ishii M, Iwaki M, Kondo M, Naruse K, Hasegawa Y, Sokabe M. Actin cytoskeleton regulates stretch-activated Ca2+ influx in human pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol. 2010;43(1):26–34. doi: 10.1165/rcmb.2009-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo A, Zhang C, Wei S, Chen B, Song LS. Emerging mechanisms of T-tubule remodelling in heart failure. Cardiovasc Res. 2013;98(2):204–15. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crocini C, Ferrantini C, Scardigli M, Coppini R, Mazzoni L, Lazzeri E, Pioner JM, Scellini B, Guo A, Song LS, Yan P, Loew LM, Tardiff J, Tesi C, Vanzi F, Cerbai E, Pavone FS, Sacconi L, Poggesi C. Novel insights on the relationship between T-tubular defects and contractile dysfunction in a mouse model of hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2016;91:42–51. doi: 10.1016/j.yjmcc.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie YP, Chen B, Sanders P, Guo A, Li Y, Zimmerman K, Wang LC, Weiss RM, Grumbach IM, Anderson ME, Song LS. Sildenafil prevents and reverses transverse-tubule remodeling and Ca(2+) handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension. 2012;59(2):355–62. doi: 10.1161/HYPERTENSIONAHA.111.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen B, Li Y, Jiang S, Xie YP, Guo A, Kutschke W, Zimmerman K, Weiss RM, Miller FJ, Anderson ME, Song LS. beta-Adrenergic receptor antagonists ameliorate myocyte T-tubule remodeling following myocardial infarction. FASEB J. 2012;26(6):2531–7. doi: 10.1096/fj.11-199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konopatskaya O, Poole AW. Protein kinase Calpha: disease regulator and therapeutic target. Trends Pharmacol Sci. 2010;31(1):8–14. doi: 10.1016/j.tips.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7(7):554–62. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.